Abstract
Vagus nerve stimulation exerts protective effects against ischemic brain injury; however, the underlying mechanisms remain unclear. In this study, a rat model of focal cerebral ischemia was established using the occlusion method, and the right vagus nerve was given electrical stimulation (constant current of 0.5 mA; pulse width, 0.5 ms; frequency, 20 Hz; duration, 30 seconds; every 5 minutes for a total of 60 minutes) 30 minutes, 12 hours, and 1, 2, 3, 7 and 14 days after surgery. Electrical stimulation of the vagus nerve substantially reduced infarct volume, improved neurological function, and decreased the expression levels of tumor necrosis factor-α and interleukin-6 in rats with focal cerebral ischemia. The experimental findings indicate that the neuroprotective effect of vagus nerve stimulation following cerebral ischemia may be associated with the inhibition of tumor necrosis factor-α and interleukin-6 expression.
Keywords: nerve regeneration, vagus nerve stimulation, cerebral ischemia, inflammatory cytokines, infarct volume, neurological function, NSFC grants, neural regeneration
Introduction
Ischemic strokes, caused by cerebral artery stenosis or occlusion, lead to a reduction or abrupt interruption of local cerebral blood flow, resulting in anoxia in the arterial territory, secondary vascular endothelial injury, and the initiation of various pathological conditions (Jian et al., 2013; Kim et al., 2013; Wang et al., 2014). With technological advances, the clinical diagnosis of stroke has improved (Hofmann et al., 2012; Ii and Tomimoto, 2013), but therapies are only partially effective. Inflammation is one of the most important pathophysiological processes following ischemic stroke. Pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) can induce inflammation, promote the secretion of other inflammatory mediators, and aggravate ischemic neuronal damage (Mohagheghi et al., 2013).
Vagus nerve stimulation (VNS) has long been used to treat various conditions. It was initially used in anti-epileptic therapy, and then for the treatment of Alzheimer's disease, migraine, traumatic brain injury and neuropathic pain (George et al., 2002; Sjögren et al., 2002; Bohotin et al., 2003; Mauskop, 2005). Recent studies have shown that VNS may also have a neuroprotective effect in ischemic brain injury (Masada et al., 1996b; Miyamoto et al., 2003; Tracey, 2007; Sun et al., 2012). The vagus nerve mediates communication between the central nervous system and the immune system, and activation of the vagus nerve inhibits inflammation (Goehler et al., 1999; Mravec, 2011; Picq et al., 2013). Thus, the vagus nerve is a potential therapeutic target for protecting the brain against damage caused by ischemic stroke. In the present study, we examined the neuroprotective effects of VNS and its impact on inflammatory cytokines in a rat model of cerebral ischemia.
Materials and Methods
Animals
One hundred and eight healthy adult male Wistar rats of specific pathogen-free grade, 3 months of age, weighing 280 ± 20 g, were provided by the Laboratory Animal Center, Academy of Military Medical Sciences, Beijing, China (license No. SCXK (army) 2010-003). Rats were maintained in an artificially illuminated environment, with a 12-hour day/night cycle, at a controlled temperature of 24°C and a humidity of 50%. Procedures for the use of laboratory animals were approved by the Institutional Animal Care and Use Committee of Chinese PLA General Hospital in China. Rats were divided into sham (n = 36), model (n = 36) and VNS-treated (n = 36) groups.
Establishment of cerebral ischemia model in rats
Rats in the model and VNS-treated groups were anesthetized with 10% chloral hydrate (3.5 mL/kg) and placed supine in a stereotactic instrument, exposing the neck. Focal cerebral ischemia was produced according to the method of Zea Longa (Longa et al., 1989; Luckl et al., 2008). The right common carotid, external carotid and internal carotid arteries were separated along the inner border of the sternocleidomastoid muscle. Afterwards, the proximal parts of the common carotid and external carotid arteries were individually ligated. A small hole was made 5 mm lateral to the carotid bifurcation, and a wire was inserted into the internal carotid artery using ophthalmic tweezers. After the wire was inserted to a distance of 18 mm, resistance was felt. The occluding suture remained in place for 2 hours and then was removed. A heat lamp was used during and following surgery to keep the animal warm until it was able to regulate its own body temperature. Animals were placed in separate cages to prevent biting of the exposed electrode on other animals. Animals that died or had surgical complications such as subarachnoid hemorrhage were replaced with new rats. The sham group also underwent the procedure, but no vascular occlusion was performed.
VNS procedure
The right vagus nerve of rats in the VNS-treated group was exposed and stimulated using solid stimulating electrodes, which were anchored to the sternocleidomastoid muscle. Placement of the stimulating electrodes is shown in Figure 1. In the VNS-treated group, stimulation was performed 30 minutes, 12 hours, and 1, 2, 3, 7 and 14 days after surgery. Square pulses were delivered at a constant current of 0.5 mA, pulse width of 0.5 ms and frequency of 20 Hz every 5 minutes, for a total period of 60 minutes as previously described (Ay et al., 2009). A schematic of the VNS protocol is shown in Figure 2.
Figure 1.
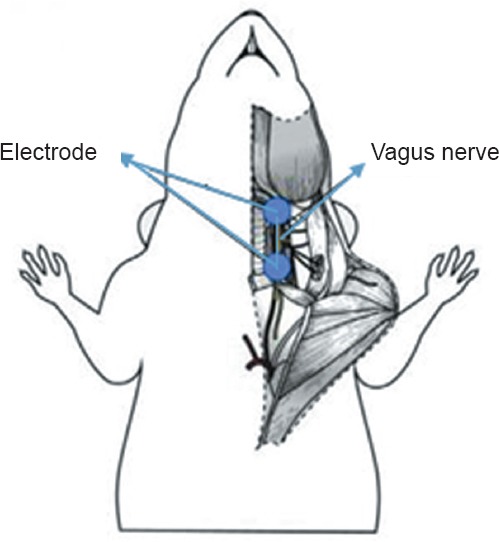
Placement of the electrode for vagus nerve stimulation.
Figure 2.
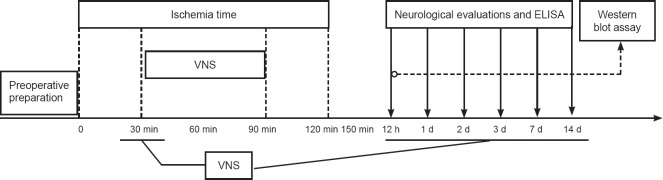
Flow chart showing the timing of vagus nerve stimulation (VNS) and subsequent evaluation and analyses.
min: Minutes; h: hours; d: day(s).
Neurological evaluation
Neurological evaluation was performed according to the scoring criteria used by De Ryck et al. (1989). Animals were scored at 12 hours and 1, 2, 3, 7 and 14 days after surgery. The total possible score was 12 points on this scale. Higher scores indicate more severe neurological dysfunction.
Measurement of infarct volume
At 12 hours and 1, 2, 3, 7 and 14 days after surgery, six rats in each group were selected for analysis. After the rat was sacrificed, the brain was removed and washed with saline, and then cut into coronal slices (2 mm thickness). Slices were stained with a 2% triphenyltetrazolium chloride (TTC) solution, washed for 20 minutes in a water bath, and fixed with 4% paraformaldehyde. Sections were photographed. Image-Pro Plus 6 (East Digital Technology Company, Nanjing, Jiangsu Province, China) was used to estimate infarct size. Infarct size was calculated according to the following formula: Infarct size = ischemic area of the contralateral hemisphere − non-infarcted area of the ischemic area. The infarct volume was calculated as infarct size multiplied by the thickness of the slice, and infarct volumes of individual slices were added.
Detection of inflammatory cytokines in the ischemic brain
After the neurological evaluation was completed, ischemic brain tissues were removed and prepared for detection of TNF-α and IL-6 expression either by ELISA kit (Nanjing Jiancheng Bio-engineering Company, Nanjing, Jiangsu Province, China) or western blot assay. Ischemic brain tissues were removed using craniotomy, rinsed, dried with filter paper, and weighed. Subsequently, brain tissues were placed into the homogenizer to prepare a homogenate, and centrifuged for 15 minutes. The supernatant was kept in a refrigerator at −70°C. ELISA detection was performed according to the manufacturer's instructions. Quantitation was performed using standard samples.
For western blot assay, blots were incubated with rabbit anti-rat GAPDH monoclonal antibody (1:2,000; Abcam, Cambridge, MA, USA), rabbit anti-rat IL-6 monoclonal antibody (1:2,000; Abcam) or mouse anti-rat TNF-α monoclonal antibody (1:500; Abcam) at 4°C for 8 hours, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000; Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China) or horseradish peroxidase-conjugated goat anti-mouse IgG (1:1,000; Beijing Zhongshan Golden Bridge Biotechnology Company) at 37°C for 1 hour. Western blot images were analyzed using IPP software (East Digital Technology Company, Nanjing, Jiangsu Province, China). The results were expressed as the ratio of target proteins to GAPDH optical density.
Statistical analysis
SPSS 16.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. All data are expressed as the mean ± SD. The Wilcoxon signed rank test was used for analysis of neurological evaluation scores. One-way analysis of variance for between group comparisons was used to test for differences in infarct volume and levels of pro-inflammatory cytokines. P < 0.05 was considered statistically significant.
Results
Effect of VNS on neurological function in rats with cerebral ischemia
As shown in Figure 3, the neurological function score was improved in both the model and VNS-treated groups (P < 0.05), and the score was higher in the VNS-treated group than in the model group (P < 0.05).
Figure 3.
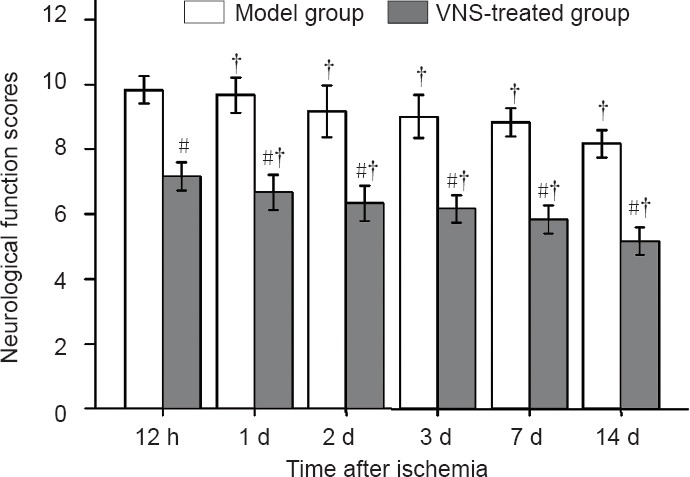
Neurological function scores in the model and VNS-treated groups at different time points following middle cerebral artery occlusion.
A lower score indicates better neurological function in this testing protocol. Data are expressed as the mean ± SD, with six rats in each group. The Wilcoxon signed rank test was used for analysis of neurological evaluation scores. #P < 0.05, vs. model group; †P < 0.05, vs. previous time point. VNS: Vagus nerve stimulation. h: Hours; d: day(s).
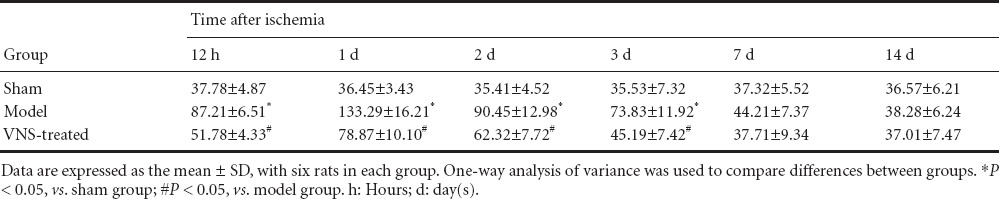
Effect of VNS on infarct volume in rats with cerebral ischemia
TTC staining showed that there was no infarction in the brain in the sham group. The cerebral infarct sizes were larger in the model group than in the sham group (P < 0.05), and infarct sizes were significantly smaller in the VNS-treated group than in the model group (P < 0.05; Figure 4, Table 1).
Figure 4.
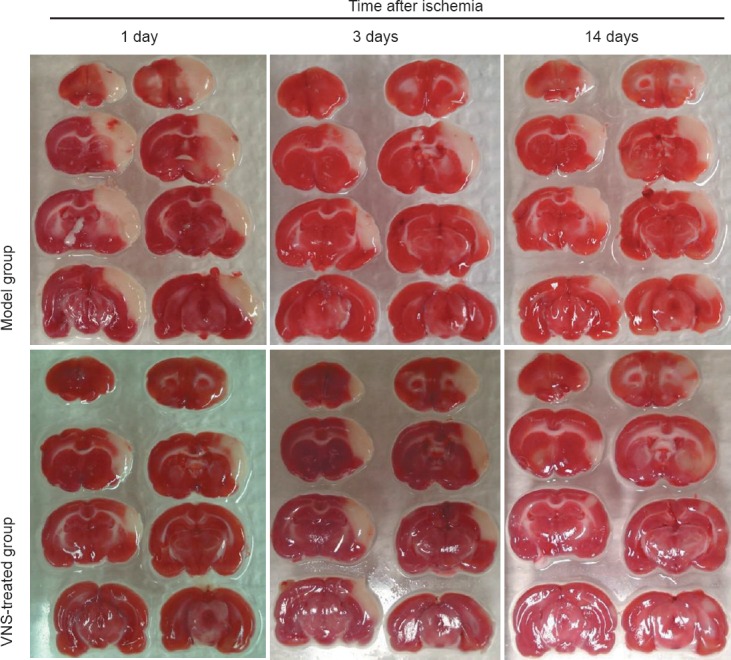
Effect of vagus nerve stimulation (VNS) on cerebral infarct volume in rats with cerebral ischemia (TTC staining).
Red areas indicate non-infarcted viable tissue, while uncolored areas indicate infarcts (or non-staining structures such as the corpus callosum).
Table 1.
Effect of vagus nerve stimulation (VNS) on cerebral infarct volume (mm3) in rats with cerebral ischemia
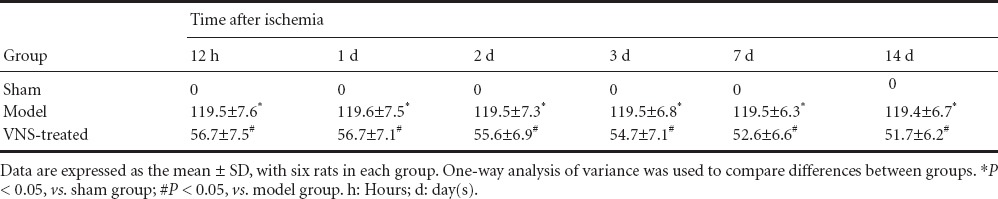
Effect of VNS on TNF-α and IL-6 expression in the brain of rats with cerebral ischemia
ELISA showed that expression of TNF-α and IL-6 in the brain was significantly higher in the model group than in the sham group (P < 0.05). TNF-α and IL-6 expression levels peaked at 1 day, then gradually decreased to normal levels at 7 and 14 days. The expression of TNF-α and IL-6 was significantly lower in the VNS-treated group than in the model group (P < 0.05; Tables 2, 3).
Table 2.
Effect of vagus nerve stimulation (VNS) on interleukin-6 level (ng/mL) in the brain of rats with cerebral ischemia
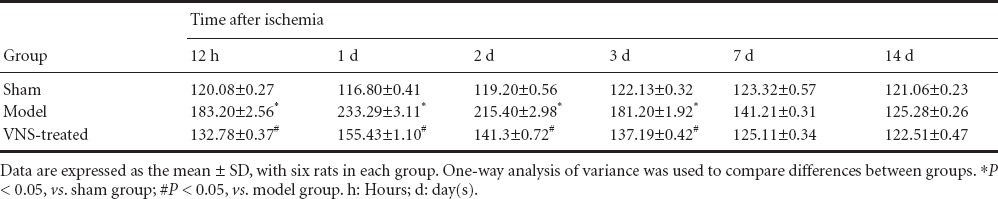
Table 3.
Effect of vagus nerve stimulation (VNS) on tumor necrosis factor-α levels (ng/mL) in the brain of rats with cerebral ischemia
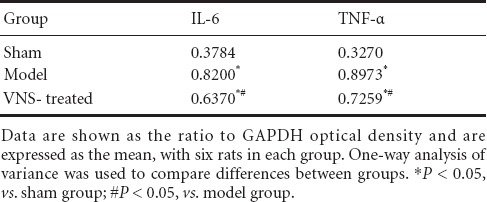
Western blot assay showed that brain expression levels of TNF-α and IL-6 were increased in the model group compared with the sham group 1 day post-surgery (P < 0.05). VNS reduced TNF-α and IL-6 expression in the brain following occlusion (P < 0.05; Figure 5, Table 4).
Figure 5.
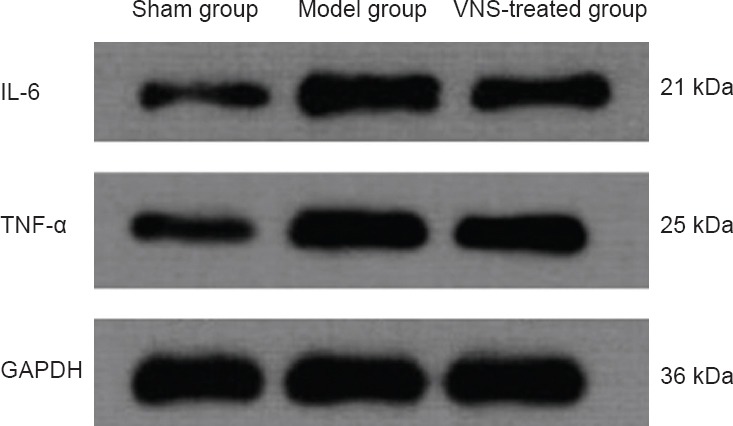
Effect of vagus nerve stimulation (VNS) on tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) expression in the brain of rats at 1 day after ischemia (western blot assay).
GAPDH was used as an internal control.
Table 4.
Effect of vagus nerve stimulation (VNS) on tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) expression in the brain of rats with cerebral ischemia at 1 day after ischemia (western blot assay)
Discussion
Ischemic stroke is caused by the blockage of blood vessels in brain tissue, leading to cellular dysfunction and severe reperfusion injury (Sun et al., 2014; Tan et al., 2014). This secondary injury may continue for days or even weeks. While the mechanisms underlying reperfusion injury are well known, increasing evidence suggests that cell death may be exacerbated by activation of inflammatory response pathways. These pathways may represent potential therapeutic targets for future studies.
Ischemic injury and pro-inflammatory cytokines
Inflammation plays an important role in the pathophysiology of ischemic stroke. Previous studies have shown that leukocyte infiltration in the brain parenchyma and endogenous microglia play a leading role in reperfusion injury following stroke (Wong and Crack, 2008). Pro-inflammatory cytokines are a group of immunoreactive molecules derived from macrophages, lymphocytes and endothelial cells. Following an infarction, reperfusion may lead to an increase in oxygen free radicals, triggering an inflammatory cascade and promoting local secretion of inflammatory cytokines such as IL-1, IL-6 and TNF-α, as well as chemokines. During the inflammatory response, TNF-α stimulates the expression of IL-6, causing an inflammatory cascade. High levels of TNF-α also have neurotoxic effects and hasten neuronal cell death. Many cells in the nervous system, such as neurons, microglia and endothelial cells, can produce TNF-α. Neutrophil activation is also important during inflammation (Czura and Tracey, 2005). Huang et al. (2006) found that elevated IL-1 and IL-6 levels induced ischemic vascular endothelial cells to express intercellular adhesion molecule-1 and mediate neutrophil accumulation in the ischemic area, causing damage to the brain tissue, which can be detected up to 10 days later. A previous study (Karki et al., 2010) found that ischemic stroke damage continues for at least 1 year, while another group found that cell death reached a peak 24–48 hours after injury and probably stopped in the third week (Li et al., 1995). In this study, we found elevated levels of IL-6 at 7 and 14 days, although inflammatory cytokine levels peaked at 1 day. Therefore, early inhibition of the inflammatory cascade may be particularly important in limiting the extent of brain damage due to stroke.
Immunomodulatory effects of VNS
The vagus nerve originates in the medulla oblongata and is an important part of the parasympathetic nervous system (da Silva and Dorsher, 2013). It has recently become clear that the vagus nerve has important immunomodulatory functions and can suppress the inflammatory response (Czura and Tracey, 2005; Gallowitsch-Puerta and Pavlov, 2007; Tracey, 2007). The generation of peripheral inflammatory factors can be blocked by the vagus nerve, suggesting that a signaling mechanism exists between the vagus nerve and immune cells. Indeed, there is a neuro-immunoregulatory pathway (Ulloa, 2005) between the central nervous system and the immune system, called the cholinergic anti-inflammatory pathway (Borovikova et al., 2000). This pathway can reduce the release of pro-inflammatory cytokines and inhibit inflammation. This pathway is mainly mediated through the vagus nerve and the neurotransmitter acetylcholine. Kawashima et al. (2007) demonstrated that the molecular conduit between the nervous system and the immune system is an α-bungarotoxin-sensitive nicotinic acetylcholine receptor (α7nAChR) that is located on macrophages. The α7nAChR regulates cytokine production during the inflammatory response.
VNS
VNS has been widely used for therapy in several diseases, but insight into the neuroprotective effects of VNS in ischemic cerebral injury is limited. Furthermore, numerous mechanisms have been suggested to underlie the neuroprotective effects of VNS. Potential mechanisms include the reduction of excitatory amino acid levels (Miyamoto et al., 2003), an increase in the inhibitory amino acid GABA (Neese et al., 2007), the induction of a neurological stress response (Zagon and Kemeny, 2000), an increase in brain-derived neurotrophic factor expression (Schabitz et al., 1997), and slowing of the ischemia-mediated inflammatory response (Borovikova et al., 2000).
It should be noted that ischemic stroke has functional consequences, e.g., contralateral limb dysfunction (Deb et al., 2010). Reports show that about 75% of patients who survive after cerebral ischemic stroke have limb dysfunction (Kwakkel, 2009; Levine and Greenwald, 2009). Of relevance to the current study, Khodaparast et al. (2013) found that VNS can significantly improve functional capacity following stroke.
Henninger and Fisher (2007) proposed that the sphenopalatine ganglion has a role in protecting against cerebral ischemic injury. There is evidence that VNS can exert neuroprotective effects on cerebral ischemia by regulating the activity of the sphenopalatine ganglion, but were unable to make a positive correlation (Ay and Ay, 2013). In an earlier study (Masada et al., 1996a), VNS had a neuroprotective effect against transient ischemia, resulting in a nearly 50% reduction in ischemic infarct volume after 24 hours, and animals receiving VNS had significantly higher neurological scores than animals that did not receive VNS (Ay et al., 2009; Sun et al., 2012; Ay and Ay, 2013). Sun et al. (2012) demonstrated that electrical stimulation of the vagus nerve has a neuroprotective effect and reduces cerebral infarct area by more than 50%. While studies have shown that VNS does not provide neuroprotection in the brain by altering cerebral blood flow (Ay et al., 2011; Sun et al., 2012), cerebral blood flow was not monitored during our study.
Through the use of a transient cerebral ischemia model in rats and VNS at different time points in each subgroup, we found that infarct volumes were significantly reduced, by more than 50%, in the VNS-treated group compared to the model group, which is consistent with previous studies (Sun et al., 2012). VNS significantly reduced infarct volume in both the short (30 minutes) and long (14 days) periods. Corresponding with this reduction in infarct volume, an improvement in neurological function scores was detected. We performed neurological evaluations of all animals at the various time points following middle cerebral artery occlusion and found that VNS can significantly improve neurological scores, which is consistent with previous findings (Ulloa, 2005; Gallowitsch-Puerta and Pavlov, 2007; Hofmann et al., 2012). While the functional score in both treated and untreated groups improved over the observation period, there was a statistically significant improvement due to VNS treatment compared to animals in the model group, which is in agreement with the findings of Hiraki et al. (2012).
Numerous studies have shown that electrical stimulation of the vagus nerve can reduce the production of TNF-α and other cytokines in experimental models of endotoxemia and hemorrhagic shock (Bernik et al., 2002; Guarini et al., 2003; Wang et al., 2003; Huston et al., 2006). Another study found that direct stimulation of the efferent branch of the vagus nerve can inhibit inflammation and decrease pro-inflammatory cytokine levels in serum and tissue, while promoting the release of anti-inflammatory hormones and cytokines (Czura et al., 2003). The researchers also found that vagotomy led to greater inflammation (Kotecha and Toledo-Pereyra, 2013; Hagberg and Mallard, 2005; Kox et al., 2012).
Previous studies have proposed various mechanisms whereby the vagus modulates the immune response and provides neuroprotection. One potential neuroprotective mechanism involves the afferent vagal nerve pathway, whose activity is relayed to the solitary nucleus and projects to the locus coeruleus (Fahy, 2010). This pathway may produce anti-inflammatory effects through the release of norepinephrine and serotonin (5-HT) (Cheyuo et al., 2011). Activation of the afferent vagal nerve by VNS could also have anti-inflammatory effects by stimulating the solitary nucleus, which can reduce glutamate release to play a protective role in the process of ischemic brain injury (Marcoli et al., 2004; Manta et al., 2009). No matter what mechanism is at work, both studies revealed that VNS has anti-inflammatory effects. Related studies found that, in addition to the peripheral component, the cholinergic anti-inflammatory pathway represents a potential central pathway for suppression of the inflammatory response through the activation of a cholinergic pathway (Wang et al., 2003; Shytle et al., 2004). Other researchers (Shytle et al., 2004; Kawashima et al., 2007) have found that astroglial cells also express α7nAchR, and when VNS is performed, activation of efferent fibers leads to acetylcholine release in peripheral nerve endings, which via α7nAchR on immune cells, prevents the release of the pro-inflammatory cytokines TNF-α and IL-6 to regulate the inflammatory response (Borovikova et al., 2000; Pavlov et al., 2003).
In the present study, cytokines associated with inflammation were specifically measured in a model of cerebral ischemia, and we found that VNS significantly reduced the levels of two important cytokines, which is consistent with previous studies (Ay et al., 2009, 2011; Sun et al., 2012; Ay and Ay, 2013). ELISA for TNF-α and IL-6 revealed that VNS significantly reduced their levels compared with the model group, and that the highest cytokine levels were present 1–2 days following occlusion. At this time point, VNS had the greatest effect. At later time points, VNS was still effective, but its impact gradually weakened. Using western blot analysis to detect TNF-α and IL-6 expression at 1 day post-occlusion, we found that expression of both cytokines was increased in the model group compared to the sham group, and this increase was significantly attenuated by VNS.
We conclude that the VNS substantially protects against cerebral ischemia, and that its neuroprotective effect is associated with the inhibition of expression of TNF-α and IL-6. Martlé et al. (2014) found that the long-term effects of VNS may be better evaluated using the dog model. For differing degrees of ischemia and inflammation, a constant stimulation parameter is not sufficient to evaluate the full potential of VNS. In future studies, VAS parameters should be varied to more comprehensively evaluate its impact on immune function.
Direct VNS is an invasive procedure which has certain risks and complications that may not be acceptable clinically. Therefore, non-invasive approaches need to be explored. Zobel et al. (2005) found that transcutaneous VNS had similar effects to direct VNS. Although transcutaneous VNS is slightly inferior to VNS, treatment can significantly improve the quality of life of patients. Furthermore, because of its safety and reasonable price, it can be widely applied (Zobel et al., 2005). Transcutaneous VNS is therefore clinically promising for the treatment of cerebral ischemia and merits further research.
Footnotes
Funding: This study was supported by the Beijing Natural Science Foundation of China, No. 7122164.
Conflicts of interest: None declared.
Copyedited by Patel B, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Ay I, Ay H. Ablation of the sphenopalatine ganglion does not attenuate the infarct reducing effect of vagus nerve stimulation. Auton Neurosci. 2013;174:31–35. doi: 10.1016/j.autneu.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–115. doi: 10.1016/j.brainres.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay I, Lu J, Ay H, Gregory Sorensen A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–151. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- Bohotin C, Scholsem M, Multon S, Martin D, Bohotin V, Schoenen J. Vagus nerve stimulation in awake rats reduces formalin-induced nociceptive behaviour and fos-immunoreactivity in trigeminal nucleus caudalis. Pain. 2003;101:3–12. doi: 10.1016/s0304-3959(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Cheyuo C, Jacob A, Wu R, Zhou M, Coppa GF, Wang P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31:1187–1195. doi: 10.1038/jcbfm.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med. 2005;257:156–166. doi: 10.1111/j.1365-2796.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- Czura CJ, Friedman SG, Tracey KJ. Neural inhibition of inflammation: the cholinergic anti-inflammatory pathway. J Endotoxin Res. 2003;9:409–413. doi: 10.1179/096805103225002755. [DOI] [PubMed] [Google Scholar]
- da Silva MAH, Dorsher PT. Neuroanatomic and clinical correspondences: acupuncture and vagus nerve stimulation. J Altern Complement Med. 2013;20:233–240. doi: 10.1089/acm.2012.1022. [DOI] [PubMed] [Google Scholar]
- De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20:1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Fahy BG. Intraoperative and perioperative complications with a vagus nerve stimulation device. J Clin Anesth. 2010;22:213–222. doi: 10.1016/j.jclinane.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Gallowitsch-Puerta M, Pavlov VA. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007;80:2325–2329. doi: 10.1016/j.lfs.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Nahas Z, Bohning DE, Kozel FA, Anderson B, Chae JH, Lomarev M, Denslow S, Li X, Mu C. Vagus nerve stimulation therapy: a research update. Neurology. 2002;59:S56–61. doi: 10.1212/wnl.59.6_suppl_4.s56. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarini S, Altavilla D, Cainazzo M-M, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005;18:117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- Henninger N, Fisher M. Stimulating circle of Willis nerve fibers preserves the diffusion-perfusion mismatch in experimental stroke. Stroke. 2007;38:2779–2786. doi: 10.1161/STROKEAHA.107.485581. [DOI] [PubMed] [Google Scholar]
- Hiraki T, Baker W, Greenberg JH. The effect of vagus nerve stimulation during transient focal cerebral ischemia on chronic outcome in rats. J Neurosci Res. 2012;90:887–894. doi: 10.1002/jnr.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann BM, Zikeli U, Ringelstein EB. Act on Stroke-Optimization of clinical processes and workflow for stroke diagnosis and treatment. Perspect Med. 2012;1:73–76. [Google Scholar]
- Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii Y, Tomimoto H. The clinical diagnosis of ischemic stroke: an update. Brain Nerve. 2013;65:739–751. [PubMed] [Google Scholar]
- Jian H, Yi-Fang W, Qi L, Xiao-Song H, Gui-Yun Z. Cerebral blood flow and metabolic changes in hippocampal regions of a modified rat model with chronic cerebral hypoperfusion. Acta Neurol Belg. 2013;113:313–317. doi: 10.1007/s13760-012-0154-6. [DOI] [PubMed] [Google Scholar]
- Karki K, Knight RA, Shen LH, Kapke A, Lu M, Li Y, Chopp M. Chronic brain tissue remodeling after stroke in rat: a 1-year multiparametric magnetic resonance imaging study. Brain Res. 2010;1360:168–176. doi: 10.1016/j.brainres.2010.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–2319. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker Ii RL, Kilgard MP. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60:80–88. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi KH, Jang YJ, Bae SS, Shin BC, Choi BT, Shin HK. Electroacupuncture acutely improves cerebral blood flow and attenuates moderate ischemic injury via an endothelial mechanism in mice. PLoS One. 2013;8:e56736. doi: 10.1371/journal.pone.0056736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha R, Toledo-Pereyra LH. Oxygen treatment attenuates systemic inflammation via cholinergic pathways. J Surg Res. 2013;181:71–73. doi: 10.1016/j.jss.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Kox M, Vaneker M, van der Hoeven JG, Scheffer GJ, Hoedemaekers CW, Pickkers P. Effects of vagus nerve stimulation and vagotomy on systemic and pulmonary inflammation in a two-hit model in rats. PLoS One. 2012;7:e34431. doi: 10.1371/journal.pone.0034431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G. Towards integrative neurorehabilitation science. Physiother Res Int. 2009;14:137–146. doi: 10.1002/pri.446. [DOI] [PubMed] [Google Scholar]
- Levine J, Greenwald BD. Fatigue in Parkinson disease, stroke, and traumatic brain injury. Phys Med Rehabil Clin N Am. 2009;20:347–361. doi: 10.1016/j.pmr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Jiang N, Yao F, Zaloga C. Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1995;15:389–397. doi: 10.1038/jcbfm.1995.49. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci. 2009;34:272–280. [PMC free article] [PubMed] [Google Scholar]
- Marcoli M, Cervetto C, Castagnetta M, Sbaffi P, Maura G. 5-HT control of ischemia-evoked glutamate efflux from human cerebrocortical slices. Neurochem Int. 2004;45:687–691. doi: 10.1016/j.neuint.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Martlé V, Peremans K, Raedt R, Vermeire S, Vonck K, Boon P, Van Ham L, Tshamala M, Caemaert J, Dobbeleir A, Duchateau L, Waelbers T, Gielen I, Bhatti S. Regional brain perfusion changes during standard and microburst vagus nerve stimulation in dogs. Epilepsy Res. 2014;108:616–622. doi: 10.1016/j.eplepsyres.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Masada T, Itano T, Fujisawa M, Miyamoto O, Tokuda M, Matsui H, Nagao S, Hatase O. Protective effect of vagus nerve stimulation on forebrain ischaemia in gerbil hippocampus. Neuroreport. 1996a;7:446–448. doi: 10.1097/00001756-199601310-00017. [DOI] [PubMed] [Google Scholar]
- Masada T, Itano T, Fujisawa M, Miyamoto O, Tokuda M, Matsui H, Nagao S, Hatase O. Protective effect of vagus nerve stimulation on forebrain ischaemia in gerbil hippocampus. Neuroreport. 1996b;31:446–448. doi: 10.1097/00001756-199601310-00017. [DOI] [PubMed] [Google Scholar]
- Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82–86. doi: 10.1111/j.1468-2982.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto O, Pang J, Sumitani K, Negi T, Hayashida Y, Itano T. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. Neuroreport. 2003;14:1971–1974. doi: 10.1097/00001756-200310270-00018. [DOI] [PubMed] [Google Scholar]
- Mohagheghi F, Khalaj L, Ahmadiani A, Rahmani B. Gemfibrozil pretreatment affecting antioxidant defense system and inflammatory, but not Nrf-2 signaling pathways resulted in female neuroprotection and male neurotoxicity in the rat models of global cerebral ischemia-reperfusion. Neurotox Res. 2013;23:225–237. doi: 10.1007/s12640-012-9338-3. [DOI] [PubMed] [Google Scholar]
- Mravec B. The inflammatory reflex: the role of the vagus nerve in regulation of immune functions. Cesk Fysiol. 2011;60:57–69. [PubMed] [Google Scholar]
- Neese SL, Sherill LK, Tan AA, Roosevelt RW, Browning RA, Smith DC, Duke A, Clough RW. Vagus nerve stimulation may protect GABAergic neurons following traumatic brain injury in rats: an immunocytochemical study. Brain Res. 2007;1128:157–163. doi: 10.1016/j.brainres.2006.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- Picq CA, Clarençon D, Sinniger VE, Bonaz BL, Mayol JF. Impact of anesthetics on immune functions in a rat model of vagus nerve stimulation. PLoS One. 2013;8:e67086. doi: 10.1371/journal.pone.0067086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabitz WR, Schwab S, Spranger M, Hacke W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997;17:500–506. doi: 10.1097/00004647-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Sjögren MJ, Hellström PT, Jonsson MA, Runnerstam M, Silander HC, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer's disease: a pilot study. J Clin Psychiatry. 2002;63:972–980. doi: 10.4088/jcp.v63n1103. [DOI] [PubMed] [Google Scholar]
- Sun NX, Gao WJ, Yi ZL. Improved suture occlusion method to prepare focal cerebral ischemia reperfusion models in Sprague-Dawley rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:225–230. [Google Scholar]
- Sun Z, Baker W, Hiraki T, Greenberg JH. The effect of right vagus nerve stimulation on focal cerebral ischemia: an experimental study in the rat. Brain Stimul. 2012;5:1–10. doi: 10.1016/j.brs.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RF, Xia AH, Wu XG, Cao NN, Li MM, Zhang TG, Wang YR, Yue ZL. Total Flavone of Hawthorn Leaf inhibits neuronal apoptosis in brain tissue of rat models of chronic cerebral ischemia. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7879–7883. [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang YR, Mao HF, Chen JQ. Effects of huwentoxin on tumor necrosis factor apoptotic pathway in the hippocampus of a rat model of cerebral ischemia. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:5813–5818. [Google Scholar]
- Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- Zagon A, Kemeny AA. Slow hyperpolarization in cortical neurons: a possible mechanism behind vagus nerve simulation therapy for refractory epilepsy? Epilepsia. 2000;41:1382–1389. doi: 10.1111/j.1528-1157.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Zobel A, Joe A, Freymann N, Clusmann H, Schramm J, Reinhardt M, Biersack HJ, Maier W, Broich K. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: An exploratory approach. Psychiatry Res. 2005;139:165–179. doi: 10.1016/j.pscychresns.2005.02.010. [DOI] [PubMed] [Google Scholar]


