Abstract
In this study, rats were put into traumatic brain injury-induced coma and treated with median nerve electrical stimulation. We explored the wake-promoting effect, and possible mechanisms, of median nerve electrical stimulation. Electrical stimulation upregulated the expression levels of orexin-A and its receptor OX1R in the rat prefrontal cortex. Orexin-A expression gradually increased with increasing stimulation, while OX1R expression reached a peak at 12 hours and then decreased. In addition, after the OX1R antagonist, SB334867, was injected into the brain of rats after traumatic brain injury, fewer rats were restored to consciousness, and orexin-A and OXIR expression in the prefrontal cortex was downregulated. Our findings indicate that median nerve electrical stimulation induced an up-regulation of orexin-A and OX1R expression in the prefrontal cortex of traumatic brain injury-induced coma rats, which may be a potential mechanism involved in the wake-promoting effects of median nerve electrical stimulation.
Keywords: nerve regeneration, traumatic brain injury, coma, median nerve electrical stimulation, wake-promoting, orexin-A, OX1R, NSFC grants, neural regeneration
Introduction
The incidence of traumatic brain injury is increasing rapidly in China. Recent progress in traumatic brain injury treatment technology has resulted in a sharp decrease in traumatic brain injury patient's mortality. However, approximately 14% of traumatic brain injury patients remain in long-term coma or a vegetative state after rescue, and this percentage is gradually increasing (Harvey, 2013). Preliminary research has shown that median nerve electrical stimulation is associated with an arousal-promoting effect in comatose humans (Cooper et al., 1999), but the exact mechanisms remain unclear.
Orexin-A is a hypothalamic neuropeptide, which is involved in a number of physiological functions such as feeding, energy homeostasis, and wakefulness (Gao and Wang, 2010). Orexin receptor-1 (OX1R) is expressed in many brain regions, including the prefrontal cortex, cerebral cortex, ventromedial hypothalamus nucleus, and locus coeruleus (Sakurai and Mieda, 2011). Orexin-A is one of the most important neurotransmitters in the ascending reticular activating system, and contributes to awareness and/or the sleep-wake cycle (Feeney et al., 1981; Sakurai and Mieda, 2011). Thus, we hypothesized that orexin-A and OX1R play an essential role in the mechanism of the wake-enhancing effect of median nerve electrical stimulation. The present study aimed to verify this hypothesis.
Materials and Methods
Establishment of traumatic brain injury-induced coma model
A total of 120 male/female adult Sprague-Dawley rats, of specific pathogen free grade, weighing 250−300 g, were used in this study. Rats were obtained from the Institute of Laboratory Animals of Nanchang University (Nanchang, Jiangxi Province, China). All rats were housed in the Laboratory Animal Center of the First Affiliated Hospital of Nanchang University (Nanchang, Jiangxi Province, China). All rats were maintained in an air-conditioned room with a 12-hour light/dark cycle, standard diet and water were available ad libitum. Ethical approval for this study was obtained from the Ethics Committee of Nanchang University (Nanchang, Jiangxi Province, China). Animals were divided into four groups: control, model, stimulated, and antagonist groups. Each group comprised 30 rats.
Rats in the model, stimulated, and antagonist groups were anesthetized using diethyl ether inhalation and allowed to breathe air spontaneously. After anesthesia, the skull was exposed via a 5 mm vertical incision using conventional surgical techniques. A cross hit point was marked by a syringe needle at 2 mm adjacent to the left midline and 1 mm anterior to the coronal suture. A cylindrical impact hammer weighing 400 g was dropped at a vertical height of 40−44 cm along a vertical metal bar to hit the plastic spacer on the hit-point marked previously, resulting in a concave fracture of the skull (Feeney et al., 1981). After injury, the incision was closed, and animals were disinfected and removed to a cage. 1 hour later, we classified consciousness into I−VI degrees according to sensory and motor functions (Stephens and Levy, 1994). Degree I: Normal activity freely engaged in cages; degree II: decreased activity; degree III: decreased activity with motor incoordination; degree IV: righting reflex could be elicited; animals could stand up; degree V: righting reflex disappeared; animals could react to pain; degree VI: animals have no reaction to pain. We defined rats in degrees V and VI, which lasted at least 30 minutes, as rats in a coma state and suitable for the following procedures.
Intracerebroventricular injection of OX1R antagonist SB334867
Under sterile conditions, an injection catheter was inserted into the left cerebral ventricle of rats in the antagonist group. One hour before surgery, each rat received pre-treatment with gentamicin (0.1 mL/100 g, intramuscular injection), and was anesthetized with 10% chloral hydrate (0.3 mL/100 g, intraperitoneal injection). Rats were positioned in a stereotaxic frame (ZS-B/S, Beijing Zhongshi Dichuang Science and Technology Development Co., Ltd., Beijing, China), and the co-ordinates used to map the guide cannula were as follows: 1.0 mm posterior to bregma, 1.5 mm lateral to the midline, and 4.5 mm ventral to the skull surface, with the incisor bar 3.2 mm below the interauricular line. Following surgery, rats received an injection of SB334867 (Tocris Bioscience, Ellisville, MO, USA), 10 mg/kg dissolved in 5 μL of 60:40 dimethyl sulfoxide solution. After rats awoke from anesthesia, they were prepared for median nerve electrical stimulation treatment.
Median nerve electrical stimulation
Rats in the stimulated and antagonist groups were treated with median nerve electrical stimulation following traumatic brain injury, after evaluation of consciousness, using a low frequency electrical stimulator (ES-420, ITO Physiotherapy & Rehabilitation, Tokyo, Japan). An acupuncture needle was inserted (depth: 1 mm, angle: 45°) 5 mm lateral to the midpoint of the volar wrist crease, between the two tendons and was connected to the stimulator. Stimulation parameters were as follows: frequency, 30 Hz; pulse width, 0.5 ms; electrical current, 1.0 mA; total stimulation time, 15 minutes. According to the assessment criteria of consciousness specified above, we observed and evaluated animals’ behavior and consciousness again as soon as median nerve electrical stimulation intervention was completed. Finally, rats were returned to cages, and fed with sufficient food and water until euthanization (Okazaki et al., 2008).
Tissue extraction
Rats in the stimulated and antagonist groups were euthanized with 10% chloral hydrate at 6, 12, and 24 hours after median nerve electrical stimulation, respectively, along with a corresponding control and traumatic brain injury-induced coma rat simultaneously. Next, prefrontal cortex tissues (within the frontal lobe) were extracted (Yeterian et al., 2012), and expression of orexin-A and OX1R was detected using immunohistochemistry, western blot analysis, and enzyme-linked immunosorbent assay.
Western blot assay
The tissue samples obtained were homogenized using a Tissue Protein Extraction Kit (CW0891, Beijing Kangwei Biotechnology Co., Ltd., Beijing, China). Homogenates were centrifuged at 12,000 × g for 10 minutes at 4°C. Supernatants were divided into small aliquots and stored at −80°C. The amount of total protein in each sample was determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA, USA). Homogenated samples containing equal amounts of protein and loading buffer were boiled for 5 minutes in water and run on a 10% sodium dodecyl sulfate/polyacrylamide gel. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes. Membranes were blocked for 2 hours at room temperature with TBS-T buffer (150 mM NaCl, 20 mM Tris HCl, pH 7.4, 0.1% Tween 20) containing 5% milk. The blots were incubated overnight with rabbit anti-OX1R polyclonal antibody (1:200; ab68718, Abcam, New Territories, HK Co., Ltd., China) and rabbit anti-rat β-actin monoclonal antibody (1:400; CW0096, Beijing Kangwei, Beijing, China) at 4°C overnight. After incubation, the membranes were washed three times with TBS-T and incubated for 2 hours with horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) (ZB-2301, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) diluted 1:3,000 in TBS-T containing 5% milk at room temperature for 1 hour. The concentration of proteins was detected using the bicinchoninic acid protein assay. To monitor loading of gel lanes, the blots were stripped by incubation for 30 minutes at 70°C with a solution containing 2% sodium dodecyl sulfate, 100 mM β-mercaptoethanol in 62.5 mM Tris-HCl, pH 6.8, and re-probed using rabbit anti-β-actin monoclonal antibody (CW0096, Beijing Kangwei). Then, blots were incubated with a chemiluminescence substrate (32109, ECL Plus, Amersham Biosciences, NJ, USA) and quantified using Quantity One software (Bio-Rad). Western blot analysis of OX1R in rat prefrontal cortex tissues was performed at 6, 12, and 24 hours. The data generated represent optical density measurements of individual bands from western blot normalized to β-actin.
Enzyme-linked immunosorbent assay
Prepared tissue samples were tested using an enzyme-linked immunosorbent assay kit for orexin-A (cE90607a 96 Tests, Uscn Life Science Inc., Wuhan, Hubei Province, China). After reagent preparation, wells for diluted standard, blanks, and samples were set up. Then, the standard and sample were diluted. When the reaction was completed, plates were read using a microplate reader (Model 680, Bio-Rad) and measured at optical density of 450 nm immediately. The optical density of the standard (X-axis) was plotted against the log of concentration of the standard (Y-axis) and the concentration of orexin-A was calculated.
Statistical analysis
Data are shown as the mean ± SD, and one-way analysis of variance was performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). P < 0.05 was accepted as statistically significant.
Results
Median nerve electrical stimulation promoted re-awakening of traumatic brain injury-induced coma rats
Only 5 of 30 rats re-awakened in the model group (degree V: 10 rats’ righting reflex disappeared; degree VI: 15 rats had no reaction to pain stimuli). In the stimulated group, 22 rats re-awakened (degree V: 5 rats’ righting reflex disappeared; degree VI: 3 rats had no reaction to pain stimuli), 8 rats remained in a stuporous state. In the antagonist group, 19 rats returned to consciousness from a comatose state (degree V: 6 rats’ righting reflex disappeared; degree VI: 5 rats had no reaction to pain stimuli).
Median nerve electrical stimulation increased orexin-A expression in the prefrontal cortex of traumatic brain injury-induced coma rats
Enzyme-linked immunosorbent assay results showed that the expression of orexin-A in different groups and/or time points was consistent. The concentration of orexin-A in the different groups showed the following trend: antagonist group < control group < model group < stimulated group at 6, 12, and 24 hours (P < 0.05). Comparison within the groups showed that the orexin-A expression level was as follows: 6 hours < 12 hours < 24 hours in the control, model, stimulated, and antagonist groups (P < 0.05; Figure 1).
Figure 1.
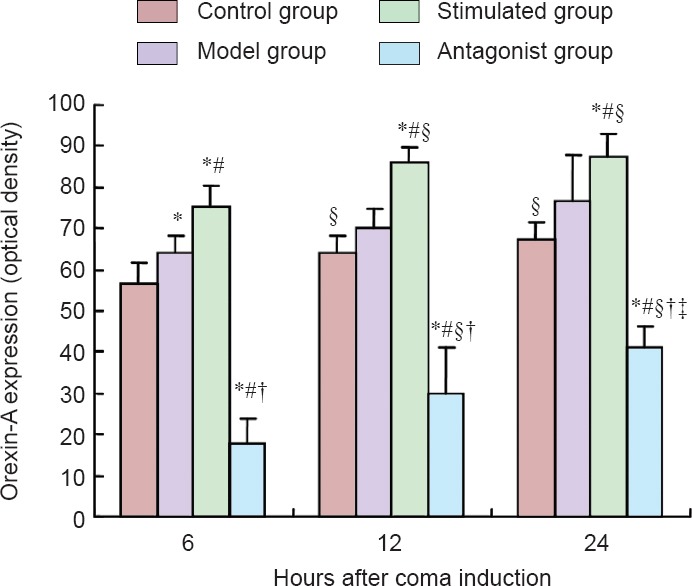
Effect of median nerve electrical stimulation on orexin-A expression in the prefrontal cortex of traumatic brain injury-induced coma rats (enzyme-linked immunosorbent assay analysis).
Data are expressed as the mean ± SD of 10 rats in each group. One-way analysis of variance was performed. *P < 0.05, vs. control group; #P < 0.05, vs. model group; †P < 0.05, vs. stimulated group; §P < 0.05, vs. 6 hours; ‡P < 0.05, vs. 12 hours.
Median nerve electrical stimulation increased OX1R protein expression in the prefrontal cortex of traumatic brain injury-induced coma rats
Results of western blot analysis revealed the following trend for OX1R expression level in the different groups at 6 hours: control group < model group < antagonist group < stimulated group (P < 0.01); at 12 hours, antagonist group < control group < model group < stimulated group (P < 0.01); at 24 hours, control group < antagonist group < model group < stimulated group (P < 0.01). Comparison within groups revealed the following trend for OX1R expression level at three time points as follows: control, model, and stimulated groups: 6 hours < 24 hours < 12 hours (P < 0.01); antagonist group: 24 hours < 6 hours < 12 hours (P < 0.01; Figure 2).
Figure 2.
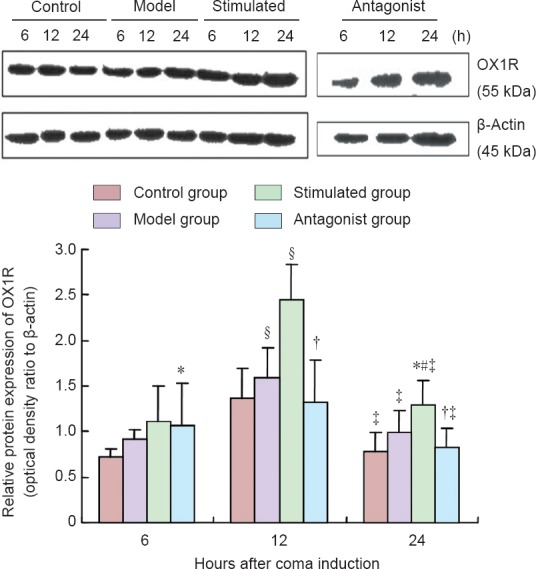
Effect of median nerve electrical stimulation on protein expression of OX1R in the prefrontal cortex of traumatic brain injury-induced coma rats (western blot analysis).
Data are expressed as the mean ± SD of 10 rats in each group. One-way analysis of variance was performed. *P < 0.01, vs. control group; #P < 0.01, vs. model group; †P < 0.01, vs. stimulated group; §P < 0.01, vs. 6 hours; ‡P < 0.01, vs. 12 hours. OX1R: Orexin receptor-1.
Discussion
Traumatic brain injury can result in primary and secondary injuries, such as free radical formation, lipid peroxidation, arachidonic acid decomposition, branched chain amino acids, intracellular Ca2+ overload, blood-brain-barrier damage, and oxidative stress (Ramanjaneya et al., 2009; Garosi and Adamantos, 2011; Feng and Han 2013; Jeter et al., 2013; Khatri and Man, 2013; Wang, 2014). Lesions of the ascending reticular activating system and alterations in the levels of important neurotransmitters including orexin-A, glutamate, 5-hydroxytryptamine, and others are known mechanisms of traumatic brain injury-induced coma. It has been reported that orexin was reduced in cerebrospinal fluid in 95% of patients during 2 months after traumatic brain injury, and led to narcolepsy (Baumann et al., 2009; Ramanjaneya et al., 2009; Jeter et al., 2013). Within 24 hours after traumatic brain injury, OX1R expression level changes can be caused by neural specific nucleoprotein, reduced glial cells, low blood pressure, hypoxia and ischemia, intracranial pressure change, and changes in blood sugar levels (Mihara et al., 2011; Willie et al., 2012). Although there are few studies addressing the relationship between orexin and traumatic brain injury, available evidence suggests that the orexinergic system plays a key role in the pathophysiology of traumatic brain injury. In the present study, we found that orexin-A and OX1R in the model group showed a higher expression level compared with the control group in the prefrontal cortex. In addition, orexin-A and OX1R expression in the prefrontal cortex was upregulated 24 hours after traumatic brain injury. This increase may have been mediated by the physiological stress response, which is critical for neuronal protection in the preliminary stages of traumatic brain injury.
Orexin was initially discovered in 1998, and is a small molecular neural polypeptide synthesized and secreted in the lateral hypothalamic area with feeding-promoting actions. The distribution of orexinergic neuronal projections includes the cerebral cortex, limbic system, thalamus, brain stem, and hypothalamus (Brevig et al., 2010). OX1R is expressed in areas with dense immunoreactive nerve fiber projections and has a wide distribution in central nervous system including the hippocampus, dorsal raphe nucleus, arcuate nucleus, tuberomammillarng nucleus, anterior pretectal nucleus, and raphe nucleus (Kaminski et al., 2010; Dall’Aglio et al., 2011). The expression of OX1R is highest in the prefrontal cortex and locus coeruleus, which play important roles in maintaining wakefulness. Recently, it has been reported that one of the most important functions of the orexinergic system is modulating sleep/wakefulness via exciting hypothalamic-cortical pathways directly. The prefrontal cortex covers one third of the overall cortex area, and plays a very significant role in high-level brain activities, such as consciousness, cognition, and integration. Orexinergic neurons in the hypothalamus project to the prefrontal cortex and excite centrum neurons (Xia et al., 2005). Orexin-A combines with Gq protein and induces phosphatidase activity, second messengers, increases in Ca2+ in the cytoplasm and protein kinase activity (Zawilska et al., 2013; Kukkonen and Leonard, 2014).
Although median nerve electrical stimulation has been widely applied clinically to promote wakefulness in comatose patients, the exact mechanisms of action remain unclear. Median nerve electrical stimulation may exert a wake-promoting action on traumatic brain injury-induced coma via the following mechanisms: (1) Increasing cerebral blood flow. Median nerve electrical stimulation stimulates the dorsal spinal nucleus and contralateral thalamus, which in turn activate the frontal-parietal cortex, leading to a rise in cerebral blood flow (Kukkonen and Leonard, 2014). (2) Related neurotransmitters and pathways. Peripheral electrical signal inputs excite the ascending reticular activating system, including the cholinergic system, causing acetylcholine release, which plays a key role in promoting arousal. Moreover, it has been reported that dopamine metabolites in the midbrain and hypothalamus, such as dihydroxyphenylacetic acid and high aromatic acid levels, are increased after median nerve electrical stimulation, indicating that median nerve electrical stimulation increases dopamine metabolites in the mesencephalon cortical and hypothalamic spinal pathways. (3) Neurotrophic factors such as nerve growth factor and brain-derived neurotrophic factor (Cooper et al., 2005). Nerve growth factor is critical for neural plasticity, which can transform inactive synapses into functional ones. Median nerve electrical stimulation stimulates brain-derived neurotrophic factor secretion in mice after transient ischemic attacks. Thus, our study aimed to explore the relationship between orexin-A and the wake-promoting action of median nerve electrical stimulation, which has not been previously directly studied.
In the present study, we found that only 5 of 30 rats re-awakened in the model group, 22 rats re-awakened in the stimulated group, and 19 rats were restored from a comatose state in the antagonist group. The data support the view that median nerve electrical stimulation could promote wakefulness in traumatic brain injury-induced coma rats. In addition, we found that orexin-A was significantly increased in the prefrontal cortex in the stimulated group compared with the other groups. Data from western blot assay showed that OX1R expression in the stimulated group displayed a significant increasing trend compared with the control and model groups. Our study also revealed that the density of orexin-A and OX1R in the prefrontal cortex was similar to previous studies, but was still lower compared with the stimulated group after median nerve electrical stimulation and OX1R antagonist SB334867 administration, and this difference was statistically significant. The results suggested that median nerve electrical stimulation directly impacted orexin-A and OX1R levels. Additionally, we found time-dependent changes in orexin-A and OX1R levels at 6, 12, and 24 hours. It has been reported that lateral hypothalamic orexin-A neurons are rhythmic and innervated by suprachiasmatic nucleus efferents, which are important components of reproductive and arousal systems (Butler et al., 2012). Orexin neurons are much more active during the circadian night than the circadian day. It has also been reported that both orexinergic innervation and expression of genes encoding orexin receptors (OX1 and OX2) are present in mouse suprachiasmatic nucleus efferents, with OX1 being upregulated at dusk (Belle et al., 2014). Orexin neurons under the influence of circadian rhythm may explain changes in orexin-A and OX1R levels at different time points, because the time of rat euthanization was not constant, and depended on individual differences in recovery time following traumatic brain injury or median nerve electrical stimulation treatment. Previous research revealed that immunoreactivity of the OX1R in the prefrontal cortex can be observed after controlled cortical impact for 6 hours, increases after 12 hours, and peaks at 24 hours. Two days later, it slowly declines (Mihara et al., 2011). Our findings are partially consistent with those of Mihara et al. (2011). However, the results of the present study may be enhanced with a greater sample size and a more refined method of traumatic brain injury. It should be noted that our study examined only orexin-A and OX1R expression in the prefrontal cortex. Whether other brain regions participated in wake-promotion and changes in orexin-A and its receptor level 24 hours following traumatic brain injury-induced coma, how median nerve electrical stimulation elicits orexin-A increases, and the specific mechanisms and pathways involved remain largely unknown and require further exploration. Despite this limitation, our study does suggest that the level of orexin-A plays an important role in the consciousness-promoting process, and the consciousness-promoting action of median nerve electrical stimulation was at least partially due to the up-regulation of orexin-A and OX1R. However, more information could be elicited if other brain regions and neurotransmitters related to orexin-A and/or wakefulness were studied to determine whether orexin-A acts as an arousal “switch”. In addition, the use of orexin-A (−/−) knock-out animals in future studies should also be encouraged to gain further insights into the results found here.
In conclusion, the current study has shown that up-regulation of orexin-A and OX1R in the prefrontal cortex may be one potential mechanism in the wake-promoting effect of median nerve electrical stimulation in a traumatic brain injury-induced coma rat model after OX1R antagonist SB334867 administration. These findings provide an insight into the mechanisms of the consciousness-promoting effect of median nerve electrical stimulation, and show that median nerve electrical stimulation could be a clinically effective method to promote wakefulness from a stuporous state.
Footnotes
Funding: This study was funded by grants from the National Natural Science Foundation of China, No. 81260295, and the Natural Science Foundation of Jiangxi Province of China, No. 20132BAB205063.
Conflicts of interest: None declared.
Copyedited by Barrett R, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Baumann CR, Bassetti CL, Valko PO, Haybaeck J, Keller M, Clark E, Stocker R, Tolnay M, Scammell TE. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle MD, Hughes AT, Bechtold DA, Cunningham P, Pierucci M, Burdakov D, Piggins HD. Acute suppressive and long-term phase modulation actions of orexin on the mammalian circadian clock. J Neurosci. 2014;34:3607–3621. doi: 10.1523/JNEUROSCI.3388-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevig HN, Watson CJ, Lydic R, Baghdoyan HA. Hypocretin and GABA interact in the pontine reticular formation to increase wakefulness. Sleep. 2010;33:1285–1293. doi: 10.1093/sleep/33.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Rainbow MN, Rodriguez E, Lyon SM, Silver R. Twelve-hour days in the brain and behavior of split hamsters. Eur J Neurosci. 2012;36:2556–2566. doi: 10.1111/j.1460-9568.2012.08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EB, Scherder EJ, Cooper JB. Electrical treatment of reduced consciousness: experience with coma and Alzheimer's disease. Neuropsychol Rehabil. 2005;15:389–405. doi: 10.1080/09602010443000317. [DOI] [PubMed] [Google Scholar]
- Cooper JB, Jane JA, Alves WM, Cooper EB. Right median nerve electrical stimulation to hasten awakening from coma. Brain Inj. 1999;13:261–267. doi: 10.1080/026990599121638. [DOI] [PubMed] [Google Scholar]
- Dall’Aglio C, Zannoni A, Mercati F, Forni M, Bacci ML, Boiti C. Differential gene expression and immune localization of the orexin system in the major salivary glands of pigs. Regul Pept. 2011;172:51–57. doi: 10.1016/j.regpep.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- Feng SJ, Han JG. Treatment of traumatic brain injury in rats by RhoA gene silencing combinedwith umbilical cord mesenchymal stem cell transplantation. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:23–30. [Google Scholar]
- Gao XB, Wang AH. Experience-dependent plasticity in hypocretin/orexin neurones: re-setting arousal threshold. Acta Physiol (Oxf) 2010;198:251–262. doi: 10.1111/j.1748-1716.2009.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garosi L, Adamantos S. Head trauma in the cat: 2. assessment and management of traumatic brain injury. J Feline Med Surg. 2011;13:815–823. doi: 10.1016/j.jfms.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey HH. Reducing traumatic brain injuries in youth sports: youth sports traumatic brain injury state laws, January 2009-December 2012. Am J Public Health. 2013;103:1249–1254. doi: 10.2105/AJPH.2012.301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CB, Hergenroeder GW, Ward NH, Moore AN, Dash PK. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30:671–679. doi: 10.1089/neu.2012.2491. [DOI] [PubMed] [Google Scholar]
- Kaminski T, Smolinska N, Nitkiewicz A, Przala J. Expression of orexin receptors 1 (OX1R) and 2 (OX2R) in the porcine pituitary during the oestrous cycle. Anim Reprod Sci. 117:111–118. doi: 10.1016/j.anireprosci.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Khatri N, Man HY. Synaptic activity and bioenergy homeostasis: implications in brain trauma and neurodegenerative diseases. Front Neurol. 2013;4:199. doi: 10.3389/fneur.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signalling cascades. Br J Pharmacol. 2014;171:314–331. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara Y, Dohi K, Yofu S, Nakamachi T, Ohtaki H, Shioda S, Aruga T. Expression and localization of the orexin-1 receptor (OX1R) after traumatic brain injury in mice. J Mol Neurosci. 2011;43:162–168. doi: 10.1007/s12031-010-9438-6. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Morimoto T, Sawai H. Parameters of optic nerve electrical stimulation affecting neuroprotection of axotomized retinal ganglion cells in adult rats. Neurosci Res. 2008;61:129–135. doi: 10.1016/j.neures.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Ramanjaneya M, Conner AC, Chen J, Kumar P, Brown JE, Jöhren O, Lehnert H, Stanfield PR, Randeva HS. Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: diverse roles for orexins A and B. J Endocrinol. 2009;202:249–261. doi: 10.1677/JOE-08-0536. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 2011;32:451–462. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Stephens JR, Levy RH. Effects of valproate and citrulline on ammonium-induced encephalopathy. Epilepsia. 1994;35:164–171. doi: 10.1111/j.1528-1157.1994.tb02928.x. [DOI] [PubMed] [Google Scholar]
- Wang C. Changes in memory function of rats with brain injury after fingolimod administration combined with bone marrow mesenchymal stem cell transplantation. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:4469–4473. [Google Scholar]
- Willie JT, Lim MM, Bennett RE, Azarion AA, Schwetye KE, Brody DL. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J Neurotrauma. 2012;29:1908–1921. doi: 10.1089/neu.2012.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JX, Chen XW, Cheng SY, Hu ZA. Mechanisms of orexin A-evoked changes of intracellular calcium in primary cultured cortical neurons. Neuroreport. 2005;16:783–786. doi: 10.1097/00001756-200505120-00025. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 2012;48:58–81. doi: 10.1016/j.cortex.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilska JB, Urbańska A, Sokołowska P. Orexins/hypocretins stimulate accumulation of inositol phosphate in primary cultures of rat cortical neurons. Pharmacol Rep. 2013;65:513–516. doi: 10.1016/s1734-1140(13)71027-2. [DOI] [PubMed] [Google Scholar]


