Abstract
Interleukin-6 has been shown to be involved in nerve injury and nerve regeneration, but the effects of long-term administration of high concentrations of interleukin-6 on neurons in the central nervous system is poorly understood. This study investigated the effects of 24 hour exposure of interleukin-6 on cortical neurons at various concentrations (0.1, 1, 5 and 10 ng/mL) and the effects of 10 ng/mL interleukin-6 exposure to cortical neurons for various durations (2, 4, 8, 24 and 48 hours) by studying voltage-gated Na+ channels using a patch-clamp technique. Voltage-clamp recording results demonstrated that interleukin-6 suppressed Na+ currents through its receptor in a time- and dose-dependent manner, but did not alter voltage-dependent activation and inactivation. Current-clamp recording results were consistent with voltage-clamp recording results. Interleukin-6 reduced the action potential amplitude of cortical neurons, but did not change the action potential threshold. The regulation of voltage-gated Na+ channels in rat cortical neurons by interleukin-6 is time- and dose-dependent.
Keywords: nerve regeneration, brain injury, inflammatory reaction, interleukin-6, voltage-gated Na+ channel, cortical neurons, cerebrospinal fluid, neuroimmunomodulation, neuroprotection, action potential, patch clamp, neurophysiology, NSFC grants, neural regeneration
Introduction
Interleukin-6 (IL-6) is a pleiotropic pro-inflammatory cytokine, involved in the induction, growth and differentiation of cells in the immune and hematopoietic systems, and initiates and coordinates inflammatory reaction (Akira et al., 1990; Heinrich et al., 1990; Hirano, 1992; Qian et al., 2013; Sun et al., 2014). IL-6 is an essential regulatory factor for neuroimmunomodulation in the central nervous system, and protects neurons and promotes nerve regeneration (Penkowa et al., 2000; Swartz et al., 2001; Herrmann et al., 2003).
IL-6 in the central nervous system is mainly derived from neurons, astrocytes and microglia (Schobitz et al., 1993; Nakanishi et al., 2007; Zhong et al., 2014) and under normal physiological conditions its production in the central nervous system is strictly regulated (Hopkins and Rothwell, 1995; Van Wagoner and Benveniste, 1999). Nevertheless, the changes in IL-6 levels are strongly associated with neuropathological and physiological changes caused by inflammation or infection (Benveniste, 1992; Merrill, 1992; Hedrich et al., 2012). In clinical and experimental neuropathology, the damaging and protective functions of IL-6 have been reported. The damaging effects of IL-6 are mainly mediated by IL-6, tumor necrosis factor (TNF)-α and IL-1 β that regulate inflammatory reactions in the central nervous system (Gadient and Otten, 1997; Benveniste, 1998; Erta et al., 2012). IL-6 is correlated with glial cell activation and the production of inflammatory regulatory factors such as reactive oxygen molecules, cytokines, chemokines and prostaglandins (Erta et al., 2012, Almolda et al., 2014). The secretion of these inflammatory factors induces blood-brain barrier damage, lymphocyte infiltration and neurodegeneration (Almolda et al., 2014). However, some studies have suggested that IL-6 inhibits inflammation, protects neurons, and promotes nerve regeneration. In a rat model of spinal cord injury, intrathecal injection of IL-6 enhanced axonal regeneration (Cao et al., 2006). Intraventricular injection of IL-6 significantly reduced brain damage in rats with cerebral ischemia (Loddick et al., 1998). In addition, studies using IL-6 deficient rats also demonstrated the neuroprotective and promoting effects of IL-6 on nerve regeneration (Murphy et al., 1999; Swartz et al., 2001). These results suggest that IL-6 functions in the central nervous system are complex and multi-directional.
Voltage-gated Na+ channels are transmembrane proteins composed of an α subunit with the bore and auxiliary β subunits, and regulate the Na+ influx of excitable cells, and are a key factor for regulating the generation and propagation of action potentials (Hodgkin and Huxley, 1952; Hille, 1986; Chen et al., 2014). A previous study demonstrated that the electrophysiological characteristics of voltage-gated Na+ channels were altered in fibroblast gene and its homologous gene knockout rats. In these rats, the cerebellar granule neurons lost the ability to generate repeated action potentials (Hans et al., 1999). Moreover, voltage-gated Na+ channel affected the resting potential of neurons, and have an important role in the regulation of action potential threshold (Rush and Cummins, 2007). Thus, changes in Na+ channel function and expression have key effects on the excitability of normal nerve cells.
Numerous studies have verified that IL-6 is involved in nerve damage (Gadient and Otten, 1997; Benveniste, 1998; Erta et al., 2012; Almolda et al., 2014), and is associated with nerve regeneration (Loddick et al., 1998; Murphy et al., 1999; Swartz et al., 2001; Cao et al., 2006). The present study investigated the effects of IL-6 on the characteristics and expression of voltage-gated Na+ channels in rat cortical neurons cultured in vitro and neuronal excitability using a patch-clamp technique. In addition, fluorescent quantitative PCR was used to explore electrophysiologically the effects of IL-6 in the central nervous system under pathological conditions.
Materials and Methods
Experimental animals
Healthy female pregnant Sprague-Dawley rats weighing 180–220 g at gestational day 18 were purchased from Guangdong Provincial Medical Experimental Animal Center of China. The use of animals throughout the study was in accordance with the Experimental Animal Breeding and Guide of Ethics Committee of Medical College of Shantou University in China.
Culture of cortical neurons
Rat cortical neurons were cultured as previously described (Chen et al., 2014). Healthy pregnant Sprague-Dawley rats at gestational day 18 were anesthetized with ether. Fetal rats were obtained by caesarean section. After removal of the hippocampus and meninges, the cortex was obtained and placed in precooled Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Grand Island, NY, USA). Tissues were triturated, digested with 0.25% ethylenediamine tetra-acetic acid-Trypsin (Invitrogen) in a CO2 incubator at 37°C for 5–7 minutes. The digestion was terminated by adding 0.5 mL fetal bovine serum (HyClone, Logan, UT, USA). Cortical tissue was agitated by Pasteur pipette and centrifuged at 900 × g for 10 minutes. The supernatant was discarded. Cells were resuspended with DMEM/F12 (Invitrogen), stirred uniformly and filtered through a 200-mesh filter. After an appropriate dilution, cell counts were conducted. Overall, 2–5 × 106 cells in each 6-well plate were seeded on a glass plate coated with 12.5 μg/mL polylysine (Sigma, St. Louis, MO, USA). After 4 days of culture, cells were incubated with Neurobasal + 1% B-27 (Gibco, Grand Island, NY, USA). At 24 hours later, 0.01 mM cytarabine (Sigma) was added and after 24 hours of incubation, the medium was replaced to suppress glial cell growth. Thereafter, half of the medium was replaced every 4 days. After 6–12 days of culture, experiments were conducted.
Identification of the purity of neurons and the expression of voltage-gated Na+ channel in neurons as measured by immunofluorescence staining
Primary antibodies: Hoechst stain was used to label cell nuclei. Specific neuronal nuclear antigen antibody rat anti-neuN (1:1,000, Millipore, Darmstadt, Germany) was utilized to label neurons. Reactive rabbit anti-Nav antibody (1:200; Alomone, Jerusalem, Israel) was used to label voltage-gated Na+ channels. Secondary antibodies were Alexa488 goat anti-rat IgG (1:600; Invitrogen), and Alexa546-Cy3 goat anti-rabbit IgG (1:1,000; Invitrogen). An indirect immunofluorescence technique was used to detect and calculate the purity of neurons and voltage-gated Na+ channel expression in neurons.
Drug treatment groups
Dose-dependent experiments
In the experimental groups, neurons were separately treated with 0.1 ng/mL (n = 27, n represents cell number), 1 ng/mL (n = 22), 5 ng/mL (n = 17), or 10 ng/mL (n = 34) IL-6 for 24 hours. Simultaneously, to detect whether IL-6 receptor inhibitor or anti-gp130 antibody interfered with IL-6 effects on voltage-gated Na+ channel in neurons, IL-6 receptor inhibitor IL-6ra (1 μg/mL; Sigma; n = 12) in the IL-6 + IL-6ra group (n = 12) and monoclonal anti-mouse-gp130 antibody (1 μM; Sigma; n = 12) in the IL-6 + anti-mouse-gp130 group (n = 12) were separately added for 24 hours at 30 minutes before treatment with IL-6. In the control group (n = 17), PBS prepared by our laboratory was used to treat cortical neurons for 24 hours.
Time-dependent experiments
In the experimental groups, 10 ng/mL IL-6 was used to treat rat cortical neurons for 2 (n = 17), 4 (n = 17), 8 (n = 22), 24 (n = 25), or 48 hours (n = 33). In the control group (n = 17), neurons were treated with PBS for 24 hours.
IL-6 effects on Na+ channel activation in cortical neurons
In the experimental groups: neurons were treated with 0.1 ng/mL IL-6 (n = 11), 1 ng/mL (n = 11), or 5 ng/mL (n = 11) for 24 hours, and 10 ng/mL for 2 (n = 11), 4 (n = 11), 8 (n = 11), 24 (n = 34), or 48 hours (n = 11). Neurons in the control group (n = 26) were treated with PBS for 24 hours.
IL-6 effects on Na+ channel inactivation in cortical neurons
In the experimental groups, neurons were treated with 0.1 ng/mL IL-6 (n = 11), 1 ng/mL (n = 11), or 5 ng/mL (n = 11) for 24 hours, and 10 ng/mL for 2 (n = 11), 4 (n = 11), 8 (n = 11), 24 (n = 22), or 48 hours (n = 11). Neurons in the control group (n = 16) were treated with PBS for 24 hours.
IL-6 effects on action potential amplitude and threshold in neurons
In the experimental groups, neurons were treated with 0.1 ng/mL (n = 15), 1 ng/mL (n = 17), or 5 ng/mL (n = 11), and 10 ng/mL (n = 21) IL-6 for 24 hours. Neurons in the control group (n = 23) were treated with PBS for 24 hours.
Whole-cell voltage-clamp recording
Electrophysiological recordings of rat cortical neurons were performed as previously described (Chen et al., 2014). Bath solution for whole-cell voltage-gated Na+ current recording consisted of 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM hydroxyethyl piperazine ethanesulfonic acid, and 10 mM glucose, at pH 7.4 adjusted by NaOH. The solution inside the electrode was composed of 145 mM CsCl, 1 mM MgCl2, 10 mM ethylene-bis (oxy-ethylene-nitrilo) tetra-acetic acid, 4 mM TEA-Cl,10 mM hydroxyethyl piperazine ethanesulfonic acid, and 5 mM ATP-Na2, at pH 7.3 adjusted by CsOH. Patch pipettes were pulled to a tip resistance of 5−10 MΩ from a hard glass capillary tube. Voltage-clamp recording was obtained using Axon-200B amplifier (Axon, New York, NJ, USA). Series resistance compensation was 50–70%. Collection frequency was 200 kHz.
To study activation properties, neurons were clamped at −80 mV and depolarized to potentials ranging from −80 to 65 mV for 20 ms with 5 mV stepping and 0.5 Hz frequency. To examine the inactivation properties, Na+ currents were recorded at −25 mV after pre-pulse from −100 to 0 mV for 40 ms with 5 mV stepping.
Action potential amplitude and threshold in neurons recorded by current clamp
The pipette solution contained 130 mM KCl, 10 mM NaCl, 1 mM MgCl2, 10 mM ethylene-bis (oxy-ethylene-nitrilo) tetra-acetic acid, 10 mM hydroxyethyl piperazine ethanesulfonic acid, 10 mM glucose, 5 mM ATP-Na2, at a pH value of 7.2 regulated with KOH. Neurons were held around −80 mV by injecting a depolarization current and the spike was elicited by a 500 pA depolarizing ramp current for 100 ms.
Fluorescent quantitative PCR
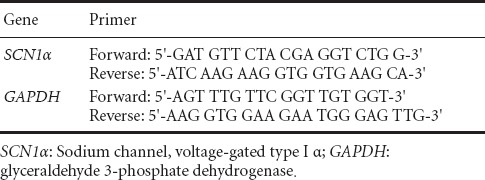
To explore IL-6 effects on Na+ channel protein expression, α1A subunit mRNA expression was measured using real-time quantitative PCR. RNA was extracted using TRIZOL (Invitrogen) according to the manufacturer's instructions. The purified RNA was reverse transcribed to cDNA with oligo dT-15 primers. Fluorescent quantitative PCR was performed using Applied Biosystems 7300 QPCR system (Applied Biosystems Corporation, Foster City, USA) with a SYBR green fluorophore. A 20 μL of total reaction volume included 10 μL SYBR Green PCR Master Mix, 1 μL cDNA template and 0.8 μL forward/reverse primers (Table 1). The templates were amplified using the following PCR protocol: 95°C for 2 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Experimental results were detected using the built-in software of Applied Biosystems 7300 QPCR system.
Table 1.
SCN1α primer sequences
Statistical analysis
Measurement data were expressed as the mean ± SEM. Electrophysiological data were analyzed using Clampfit 8.0 software (Axon, Silicon Valley, USA). Statistical data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The differences between different concentrations of IL-6 and the control group and the differences between IL-6 treatment for durations and the control group were compared using one-way analysis of variance and the least significant difference test. Action potential threshold and excitation frequency in the IL-6 treatment group and control group were analyzed by the Student's t-test. A value of P < 0.05 was considered statistically significant.
Results
Identification of the purity of neurons
The purity of neurons was > 95%. Voltage-gated Na+ channel expression was stably detected in >99% of neurons (Figure 1A–D).
Figure 1.
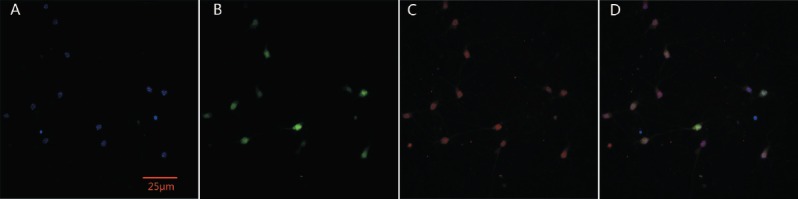
Purity of neurons and voltage-gated Na+ channel expression in primary cultured rat cortical neurons.
(A) Hoechst stained cells (blue); (B) NeuN-labeled neurons (green); (C) Nav antibody-labeled voltage-gated Na+ channel (red); (D) superimposed image of A, B and C. Scale bar: 25 μm.
IL-6 suppressed Na+ currents in neurons in a dose-dependent manner
Before the whole-cell voltage-gated Na+ channel current was recorded, primary cultured cortical neurons were treated with 0.1, 5, 1, or 10 ng/mL IL-6 for 24 hours. Neurons were clamped at −80 mV and depolarized at a collection frequency of 200 kHz to elicit an inward current, which has the properties of rapid activation and inactivation (Figure 2A).
Figure 2.
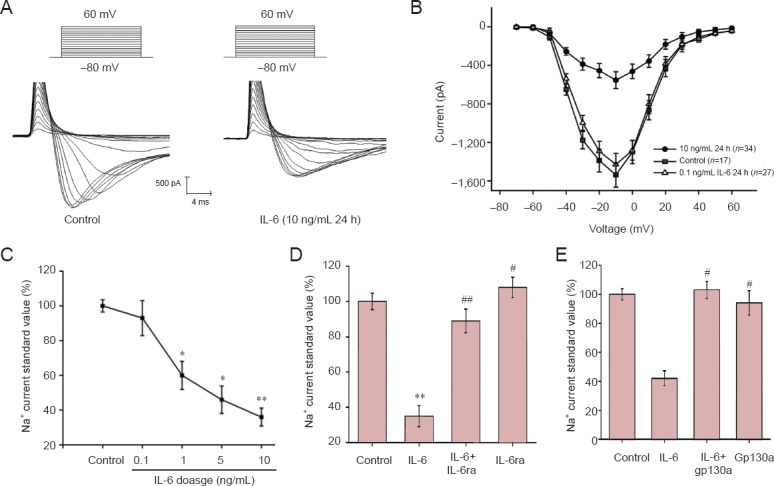
Inhibitory effect of interleukin-6 (IL-6) and interleukin-6 receptor inhibitor (IL-6ra) on voltage-gated Na+ channel currents in neurons is dose-dependent and correlates with gp130.
(A) At −80 to 60 mV depolarization, typical whole cell Na+ current curves in neurons of the control group (n = 17) and IL-6 group (10 ng/mL, n = 34, 24 hours). (B) Current-voltage curves of control group, 0.1 (n = 27) and 10 ng/mL IL-6 group. *P < 0.05, **P < 0.01, vs. the control group (mean ± SEM; Student's t-test). (C) Dose-dependent effect of IL-6 (from 0.1 to 10 ng/mL) on Na+ currents. Each current value was normalized according to the control group and expressed as a percentage of the control group. (D) At −10 mV, standard Na+ current values of control group, IL-6 group (1 μg/mL; n = 12), IL-6 + IL-6ra group (gp130a, 1 μg/mL; n = 12) and IL-6ra group (1 μg/mL; n = 12). (E) At −10 mV, standard Na+ current peak values of the control group, IL-6 group, IL-6 + gp130 antibody (1 μM) group (n = 12) and gp130 antibody group (n = 12). (D, E) *P < 0.05, **P < 0.01, vs. control group; #P < 0.05, ##P < 0.01, vs. IL-6 group (mean ± SEM; one-way analysis of variance, least significant difference test). n represents cell number. h: Hours.
To eliminate the effects of different neuronal volumes on Na+ current recording, the peak current normalized by the whole-cell capacitance was applied to calculate the density. Membrane capacitance was obtained with an Axon-200B single-probe low-noise patch-clamp amplifier. The activation potential of voltage-gated Na+ channel current was markedly inhibited by 1, 5, and 10 ng/mL IL-6, but not by 0.1 ng/mL IL-6 (Figure 2B).
The peak currents were different at −10 mV and various doses of IL-6, which indicated that the inhibition was dose-dependent. The Na+ currents were suppressed by about 7%, 40% (P < 0.05), 54% (P < 0.05), and 64% (P < 0.01) by treatment with 0.1, 1, 5 and 10 ng/mL of IL-6, respectively (Figure 2C). To explore whether the inhibition was dependent on the IL-6 receptor, IL-6 and IL-6ra (1 μg/mL; Sigma; n = 12) were simultaneously added to cultures. IL-6ra abolished the inhibitory effects of IL-6 on Na+ currents, but did not alter the currents when used alone (Figure 2D). Anti-gp130 antibody (1 μg/mL; Sigma, USA; n = 12) was used to investigate whether IL-6 inhibited Na+ current via activation of the JAK/STAT signaling pathway. Combined actions of IL-6 and anti-gp130 exhibited an inhibitory effect on Na+ currents indicating these effects might be mediated by the JAK/STAT signaling pathway.
IL-6 suppressed Na+ currents in neurons in a time-dependent manner
To explore whether IL-6 affected Na+ currents in a time-dependent manner, 10 ng/mL IL-6 was used. Neurons were treated with IL-6 for 2 (n = 17), 4 (n = 17), 8 (n = 22), 24 (n = 25), or 48 hours (n = 33). IL-6 suppressed Na+ currents at 2, 4, 8 and 24 hours (P < 0.05; Figure 3A). As shown in Figure 3B, the standard peak current at −10 mV in the different groups suggested that the inhibitory effect of IL-6 was time-dependent. The reduction of the currents induced by IL-6 treatment at 2, 4, 8, 24 and 48 hours were 49% (P < 0.05), 55% (P < 0.05), 59% (P < 0.01), 65% (P < 0.01) and 40% (P < 0.05), respectively.
Figure 3.
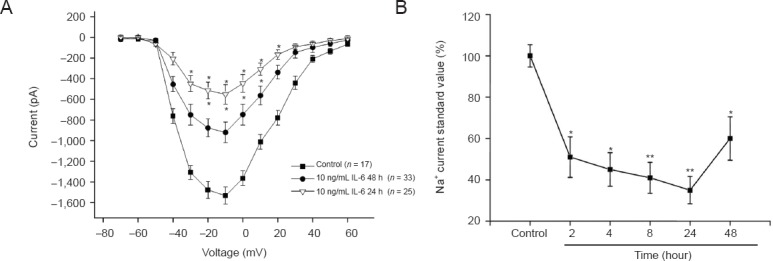
Interleukin-6 (IL-6) effects on voltage-gated Na+ channel currents in rat cortical neurons is time-dependent.
(A) Current-voltage relationship after treatment with 10 ng/mL IL-6 for 24 hours (n = 25) or 48 hours (n = 33). *P < 0.05, vs. the control group (n = 17) (mean ± SEM; Student's t-test). (B) Treatment (10 ng/mL) with IL-6 for 2–48 hours affected Na+ current in a time-dependent manner. Each current value was normalized according to the control group and expressed as a percentage of the control group. *P < 0.05, **P < 0.01, vs. the control group (mean ± SEM; one-way analysis of variance followed by the least significant difference test). h: Hours.
IL-6 effects on Na+ channel activation and inactivation in neurons
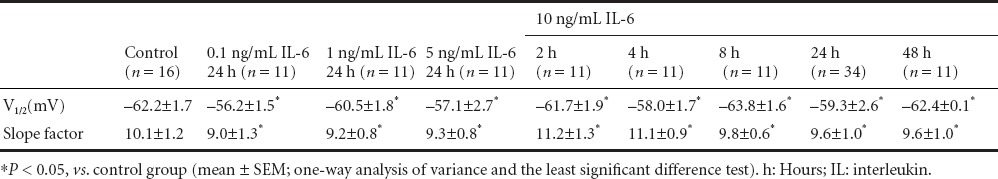
To explore the effects of IL-6 on other aspects of voltage-gated Na+ channels, we analyzed and compared voltage-dependent Na+ channel activation and inactivation at different doses and various time points. For the analysis of activation, the conductance (g) value at each voltage was calculated and was then normalized by the maximal conductance (g/gmax). The conductance-voltage curves were fitted using the Boltzmann equation (Figure 4A). For the analysis of inactivation, the peak currents at −25 mV of the test voltage were normalized by the maximum current (I/Imax) (Figure 4B, insert). The current-voltage curves were also fitted using the Boltzmann equation (Figure 4B). Results indicated that IL-6 had no effect on activation and inactivation at any dose or for any duration of incubation. These treatments significantly reduced the whole-cell Na+ currents (P < 0.05) (10 ng/mL for 24 hours and 10 ng/mL for 4 hours; Figure 4A, B). All parameters (V1/2 and slope factor) of voltage-dependent activation and inactivation are summarized in Tables 2 and 3. These results suggested that IL-6 did not modulate Na+ currents by regulating the essential characteristics of the voltage-gated Na+ channels in neurons. The regulatory effect of IL-6 on Na+ currents may result from a biochemical change, such reducing the number of Na+ channels in the plasma membrane. Using real-time fluorescent quantitative PCR, the content of α1A (SCN1α) subunit mRNA in neurons was measured before and after IL-6 application. Expression of the α1A subunit was also inhibited by 10 ng/mL of IL-6 in a time-dependent manner (P < 0.05). Two hours after IL-6 treatment, α1A subunit expression was significantly reduced (P < 0.05), only slightly decreased at 4 hours (P < 0.05), and was not reduced at time points after 4 hours compared with the control group (Figure 4C).
Figure 4.
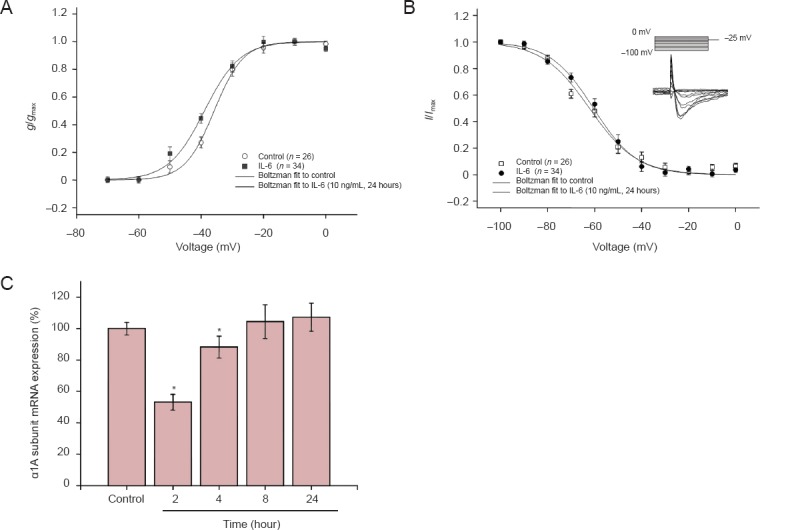
Interleukin-6 (IL-6) effects on electrophysiological properties and expression of Na+ channels in cortical neurons.
(A) Voltage-dependent curve (Boltzmann equation and ignition parameters) in the 10 ng/mL IL-6 24-hour group (n = 11). (B) Inactivation characteristic curve and voltage-dependent inactivation curve (Boltzmann equation and ignition parameters) in the 10 ng/mL IL-6 24-hour group. Vertical coordinates of A and B: Standardized conductance value (g/gmax), and standardized current value (I/Imax). (C) α1A subunit mRNA expression: *P < 0.05, vs. the control group (mean ± SEM; one-way analysis of variance followed by the least significant difference test).
Table 2.
Voltage-dependent activation parameters
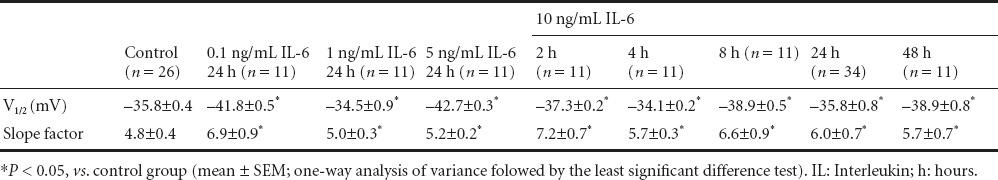
Table 3.
Voltage-dependent inactivation parameters
IL-6 effects on action potential amplitude and threshold in neurons
Voltage-gated Na+ channels are essential for producing action potential upstroke and overshoot. To investigate the effects of IL-6 on action potentials, a current clamp was utilized to record action potentials (Figure 5A). IL-6 treatment did not affect action potential threshold (about −40 mV; Figure 5B). The peak of the spike was lowered significantly after 1, 5 and 10 ng/mL IL-6 treatment for 24 hours (P < 0.05, P < 0.01). However, 0.1 ng/mL IL-6 did not alter the neuronal action potential. These results were consistent with the data recorded by the current clamp, suggesting that IL-6 diminished the amplitude of action potentials by inhibiting voltage-gated Na+ channels in cortical neurons.
Figure 5.
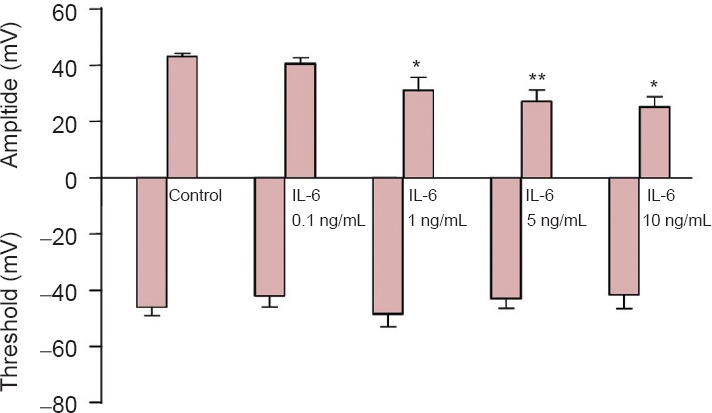
Interleukin-6 (IL-6) effects on action potentials.
The amplitude and threshold of action potentials in the control group (n = 23) and 0.1 (n = 15), 1 (n = 17), 5 (n=11) and 10 ng/mL (n = 21) IL-6 groups. *P < 0.05, **P < 0.01, vs. control group (mean ± SEM; one-way analysis of variance followed by the least significant difference test).
Discussion
IL-6 plays an important role in the pathophysiological processes of the central nervous system through IL-6 receptor-induced intracellular signaling cascades (Nelson et al., 2002). Results from this study demonstrated that IL-6 suppressed voltage-gated Na+ channel currents and diminished the peak of action potential in neurons (using receptor IL-6ra and gp130) in time- and dose-dependent manners, but did not alter voltage-gated Na+ channel activation and inactivation. The present study investigated the effects of IL-6 on voltage-gated Na+ channels and provided a possible explanation for the effects of high IL-6 concentrations on neuronal protection and regeneration in the central nervous system under pathological conditions.
Previous studies reported that IL-6 levels in the cerebrospinal fluid of patients with nervous system disease were present between pg/mL and ng/mL (Pleines et al., 2001; Woiciechowsky et al., 2002; Minambres et al., 2003; Nagafuchi et al., 2006; Helmy et al., 2012). In these studies, different doses of IL-6 (0.1–10 ng/mL) from the cerebrospinal fluid of patients with nervous system diseases were used to treat cortical neurons. Thus, IL-6 doses reached a high level under pathological conditions.
This study investigated the effects of pathological levels of IL-6 on voltage-gated Na+ channels of rat normal cortical neurons cultured in vitro. Results verified that IL-6 suppressed the Na+ current of voltage-gated Na+ channels in a dose- and time-dependent manner and inhibited the amplitude of neuronal action potentials. This suggested that IL-6 reduces neuronal excitability by inhibiting Na+ currents of voltage-gated Na+ channels. Taken together, under pathological conditions, high levels of IL-6 can reduce the energy consumption of neurons to maintain transmembrane Na+ concentration differences by decreasing Na+ currents (Wang et al., 2003). However, IL-6 diminished steady-state maladjustment-induced neuronal excitotoxicity in the central nervous system by reducing the amplitude of action potentials (Jabaudon et al., 2000; Nishizawa, 2001). The death rate of neurons was higher after cryolesion (Swartz et al., 2001) or sciatic nerve transection (Murphy et al., 1999) in IL-6 deficient rats compared with normal rats. This study provided a partial explanation for the neuroprotection and repair properties of IL-6 from the aspects of neuronal excitability.
Experimental results revealed that IL-6 levels were noticeably reduced at 2 hours, which indicated that IL-6 diminished Na+ channel expression on cell membranes possibly by down-regulating voltage-gated Na+ channel mRNA transcriptional levels. mRNA levels returned to normal at 8 hours, but the current was restored only 48 hours later. These data confirmed that the reduction in protein expression might occur at 2–48 hours. This sequential change indicated that the effects of IL-6 may be compensated for by other signaling pathways after long-term treatment, such as the increased tolerance of neurons to IL-6 and the induction of negative regulatory pathways that lead to neuronal sensitivity to prolonged IL-6 exposure (Nelson et al., 2002).
In the presence of IL-6 receptor antibody or gp130 antibody, IL-6 did not alter Na+ currents of voltage-gated Na+ channels, indicating the inhibitory effect of IL-6 on Na+ currents might be controlled by its receptor and gp130 downstream pathways. JAK/STAT signaling pathway is a downstream pathway of IL-6 and gp130 regulates neurogenesis or neuroprotection in the nervous system under pathological conditions (Hirano et al., 1997). A previous study showed that dorsal root ganglion neurons cultured in vitro blocked neurite outgrowth after treatment with a Jak2 kinase inhibitor (Liu and Snidwer, 2001). Another study verified that microglia-derived IL-6 effectively inhibited neuronal cell death after neural precursor cells were infected by HSV-1, and demonstrated that the IL-6/STAT3 axis plays a key role in neuroprotection (Chucair-Elliott et al., 2014). Time-dependent results revealed that the inhibitory effect of IL-6 on Na+ currents at 48 hours was weaker than that at 24 hours, and that this inhibitory effect was reduced with prolonged time. These results indicated that a high-dose of IL-6 over a long treatment period probably activated a negative feedback mechanism, which then decreased the inhibitory effects on Na+ currents, a key characteristic of the JAK/STAT signaling pathway (Nelson et al., 1999; Oberbach et al., 2012; Sansone and Bromberg, 2012). This mechanism also alters the production of cytokines in the late stages of inflammatory reactions.
The effects of IL-6 in the central nervous system are pleiotropic. The precise mechanisms of the effects observed in this study require further investigation because cytokines do not act in isolation in vivo. Rather they are part of an intricate regulatory pathway of molecules with numerous feedback loops. However, this study partially explained the effects of IL-6 on neuronal protection or regeneration from the perspective of ion channels and provided a new concept and target for developing drugs to treat central nervous system diseases in the clinic.
Footnotes
Funding: This work was supported by a grant from the National Natural Science Foundation of China, No. 30972766, 31170852, 81001322, 81172795, 81173048; and the Specialized Research Fund for the Doctoral Program of Colleges and Universities, No. 20094402110004.
Conflicts of interest: None declared.
Copyedited by Croxford L, Frenchman B, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- Almolda B, Villacampa N, Manders P, Hidalgo J, Campbell IL, Gonzalez B, Castellano B. Effects of astrocyte-targeted production of interleukin-6 in the mouse on the host response to nerve injury. Glia. 2014;62:1142–1161. doi: 10.1002/glia.22668. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu F, Guo J, Sheng J, Li W, Zhao X, Wang G, Li K. Chronic haloperidol increases voltage-gated Na + currents in mouse cortical neurons. Biochem Biophys Res Commun. 2014;450:55–60. doi: 10.1016/j.bbrc.2014.05.081. [DOI] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Conrady C, Zheng M, Kroll CM, Lane TE, Carr DJ. Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia. 2014;62:1418–1434. doi: 10.1002/glia.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)-a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Hans VH, Kossmann T, Lenzlinger PM, Probstmeier R, Imhof HG, Trentz O, Morganti-Kossmann MC. Experimental axonal injury triggers interleukin-6 mRNA, protein synthesis and release into cerebrospinal fluid. J Cereb Blood Flow Metab. 1999;19:184–194. doi: 10.1097/00004647-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Hedrich CM, Bruck N, Paul D, Hahn G, Gahr M, Rosen-Wolff A. “Mutation negative” familial cold autoinflammatory syndrome (FCAS) in an 8-year-old boy: clinical course and functional studies. Rheumatol Int. 2012;32:2629–2636. doi: 10.1007/s00296-011-2019-3. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy AA, Naseer MM, Shafie SE, Nada MA. Role of interleukin 6 and alpha-globulins in differentiating Alzheimer and vascular dementias. Neurodegener Dis. 2012;9:81–86. doi: 10.1159/000329568. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, Vogel J, Prinz S, Schwab S, Monyer H, Brombacher F, Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic channels: molecular pores of excitable membranes. Harvey Lect. 1986;82:47–69. [PubMed] [Google Scholar]
- Hirano T. The biology of interleukin-6. Chem Immunol. 1992;51:153–180. [PubMed] [Google Scholar]
- Hirano T, Nakajima K, Hiki M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Jabaudon D, Scanziani M, Gahwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc Natl Acad Sci U S A. 2000;97:5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Snider WD. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Merrill JE. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Dev Neurosci. 1992;14:1–10. doi: 10.1159/000111642. [DOI] [PubMed] [Google Scholar]
- Minambres E, Cemborain A, Sanchez-Velasco P, Gandarillas M, Diaz-Reganon G, Sanchez-Gonzalez U, Leyva-Cobian F. Correlation between transcranial interleukin-6 gradient and outcome in patients with acute brain injury. Crit Care Med. 2003;31:933–938. doi: 10.1097/01.CCM.0000055370.66389.59. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Borthwick LS, Johnston RS, Kuchel G, Richardson PM. Nature of the retrograde signal from injured nerves that induces interleukin-6 mRNA in neurons. J Neurosci. 1999;19:3791–3800. doi: 10.1523/JNEUROSCI.19-10-03791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi M, Nagafuchi Y, Sato R, Imaizumi T, Ayabe M, Shoji H, Ichiyama T. Adult meningism and viral meningitis, 1997-2004: clinical data and cerebrospinal fluid cytokines. Int Med. 2006;45:1209–1212. doi: 10.2169/internalmedicine.45.1769. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Campbell IL, Gruol DL. Altered physiology of Purkinje neurons in cerebellar slices from transgenic mice with chronic central nervous system expression of interleukin-6. Neuroscience. 1999;89:127–136. doi: 10.1016/s0306-4522(98)00316-9. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic interleukin-6 exposure alters electrophysiological properties and calcium signaling in developing cerebellar purkinje neurons in culture. J Neurophysiol. 2002;88:475–486. doi: 10.1152/jn.2002.88.1.475. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- Oberbach A, Schlichting N, Heinrich M, Till H, Stolzenburg JU, Neuhaus J. Free fatty acid palmitate impairs the vitality and function of cultured human bladder smooth muscle cells. PLoS One. 2012;7:e41026. doi: 10.1371/journal.pone.0041026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Giralt M, Carrasco J, Hadberg H, Hidalgo J. Impaired inflammatory response and increased oxidative stress and neurodegeneration after brain injury in interleukin-6-deficient mice. Glia. 2000;32:271–285. doi: 10.1002/1098-1136(200012)32:3<271::aid-glia70>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Pleines UE, Morganti-Kossmann MC, Rancan M, Joller H, Trentz O, Kossmann T. S-100 beta reflects the extent of injury and outcome, whereas neuronal specific enolase is a better indicator of neuroinflammation in patients with severe traumatic brain injury. J Neurotrauma. 2001;18:491–498. doi: 10.1089/089771501300227297. [DOI] [PubMed] [Google Scholar]
- Qian YJ, Nie J, Pan X, Hu MH, Mi CB. Orthodontic force effects on the expression of periodontal interleukin-6 mRNA in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:8672–8677. [Google Scholar]
- Rush AM, Cummins TR. Painful research: Identification of a small-molecule inhibitor that selectively targets Na(v)1.8 sodium channels. Mol Interv. 2007;7:192–195. doi: 10.1124/mi.7.4.4. [DOI] [PubMed] [Google Scholar]
- Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur J Neurosci. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Chen R, Dong JH, Jiang Q, Sun ZK, Guo ZH. Effect of lipopolysaccharide stimulation combined with heat stress on release of interleukin-6 and tumor necrosis factor-alpha from human intestinal epithelial cells cultured in vitro. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:8238–8242. [Google Scholar]
- Swartz KR, Liu F, Sewell D, Schochet T, Campbell I, Sandor M, Fabry Z. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001;896:86–95. doi: 10.1016/s0006-8993(01)02013-3. [DOI] [PubMed] [Google Scholar]
- Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Xiao AY, Sheline C, Hyrc K, Yang AZ, Goldberg MP, Choi DW, Yu SP. Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. J Cell Sci. 2003;116:2099–2110. doi: 10.1242/jcs.00420. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Schoning B, Cobanov J, Lanksch WR, Volk HD, Docke WD. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma. 2002;52:339–345. doi: 10.1097/00005373-200202000-00021. [DOI] [PubMed] [Google Scholar]
- Zhong SH, Fang Y, Peng JZ, Gao TH. The expression of inflammatory factors in different periods after acute spinal cord injury in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:2806–2811. [Google Scholar]


