Abstract
Mismatch negativity is generated automatically, and is an early monitoring indicator of neuronal integrity impairment and functional abnormality in patients with brain injury, leading to decline of cognitive function. Antipsychotic medication cannot affect mismatch negativity. The present study aimed to explore the relationships of mismatch negativity with neurocognition, daily life and social functional outcomes in patients after brain injury. Twelve patients with traumatic brain injury and 12 healthy controls were recruited in this study. We examined neurocognition with the Wechsler Adult Intelligence Scale-Revised China, and daily and social functional outcomes with the Activity of Daily Living Scale and Social Disability Screening Schedule, respectively. Mismatch negativity was analyzed from electroencephalogram recording. The results showed that mismatch negativity amplitudes decreased in patients with traumatic brain injury compared with healthy controls. Mismatch negativity amplitude was negatively correlated with measurements of neurocognition and positively correlated with functional outcomes in patients after traumatic brain injury. Further, the most significant positive correlations were found between mismatch negativity in the fronto-central region and measures of functional outcomes. The most significant positive correlations were also found between mismatch negativity at the FCz electrode and daily living function. Mismatch negativity amplitudes were extremely positively associated with Social Disability Screening Schedule scores at the Fz electrode in brain injury patients. These experimental findings suggest that mismatch negativity might efficiently reflect functional outcomes in patients after traumatic brain injury.
Keywords: nerve regeneration, brain injury, cognition disorders, diagnostic techniques, Wechsler Intelligence Scale, event-related potential, neuronal plasticity, electrophysiology, neuropsychology, activity of daily living, work capacity evaluation, electroencephalogram, neural regeneration, NSFC grant
Introduction
At least 10 million cases of severe traumatic brain injury (TBI) occur annually worldwide, so TBI is a major cause of morbidity (Manor et al., 2008; Kumaria et al., 2009). In the military, thousands of new brain injuries occur each year, further heightening public awareness of TBI (Duncan, 2011; Scheibel, 2012). Peoples’ working memory capacity, regulative and evaluative components of cognitive function are damaged in mild and severe TBI (Perlstein et al., 2006; Larson et al., 2009, 2011; Chen et al., 2010; Yu et al., 2010). TBI is a difficult-to-understand neurological disease, so the sequelae and treatment of TBI are the key foci of public concern and have attracted great scientific interest (Feng and Han, 2013; Wang, 2014). Mismatch negativity (MMN) is an event-related potential component that measures preattentional sensory processing (Campanella et al., 2014) and is generated automatically. Näätänen et al. (2007) found that MMN reflects an automatic measurement of perceptual detection, and is also an indicator of the sensory prerequisites of cognition. MMN deficits are generally accompanied by cognitive decline, no matter what disorders cause them (Näätänen et al., 2012), and MMN has been widely applied in the treatment of a variety of diseases, including dyslexia, schizophrenia, memory disorders, Huntington's disease, Fibromyalgia, TBI and coma (Kujala et al., 2007; Näätänen et al., 2007; Zarza-Lucianez, 2007; Duncan et al., 2009; Kaipio, 2013; Beste et al., 2014; Choi et al., 2014; Baldeweg et al., 2015). Both the progression of disorders and premorbid neurocognitive impairment and functional abnormality are reflected by MMN abnormality in TBI cases without macroscopic lesions on conventional MRI (Umbricht et al., 2006; Kaipio et al., 2013). MMN can also be used to check for vegetative and minimally conscientious state during the subacute phase of severe TBI (Zarza-Lucianez et al., 2007; Pachalska et al., 2011), and cannot be affected by antipsychotic medication (Catts et al., 1995; Umbricht et al., 1998, 1999). MMN deficiency has been observed in TBI patients (Jones et al., 2000); Kaipio et al. (2001, 2013) have reported that MMN relates to global social functioning in patients after TBI. In healthy participants, MMN and Global Assessment of Functioning scores also have a significant correlation (Light et al., 2007; Calcus et al., 2014). The correlation of MMN with social cognitive ability and functional outcomes has also been reported in American and Han Chinese schizophrenia patients (Wynn et al., 2010; Lin et al., 2012). However, little evidence has focused on the application of MMN to TBI.
In this study, we aimed to demonstrate the correlation of MMN with neurocognition and functional outcomes in patients after TBI and in healthy controls, and provide evidence for a better understanding of MMN differences. We speculated that MMN amplitude, neurocognition and functional outcomes would be higher in healthy controls than in TBI patients. MMN is particularly associated with functional outcomes in TBI patients, and could be used to predict neuronal regeneration.
Subjects and Methods
Participants
The age of all participants ranged from 16 years to 59 years, with a mean of 34.9 ± 8.5 years. Participants’ education ranged from 5 years to 14 years, with a mean of 8.7 ± 3.2 years. Twelve patients suffering TBI for over 6 months (8 males and 4 females) and 12 healthy controls (8 males and 4 females) were recruited from the Affiliated Hospital of Chifeng University, China.
All participants’ hearing ability was confirmed by the 512-Hz tuning fork test (Burkey et al., 1998). All participants had normal or corrected-to-normal visual acuity, and all were identified as right-handed.
TBI patients were diagnosed according to the criteria in the Structured Clinical Interview for Diagnostics. All patients were in stable condition and taking no medicine. None of the patients had a history of central nervous system disease, alcohol or drug abuse, or electroconvulsive therapy.
Healthy controls were recruited through posters displayed in the hospital. They were healthy people on physical examination, and their mean age was 34.5 ± 8.8 years. An initial screening interview was conducted by a board-certified psychiatrist, to exclude participants with identifiable psychiatric disorders, histories of head injury, or neurological disorders. All Structured Clinical Interviews and cognition impairment administrations were conducted by the same neurologists.
All participants signed a written informed consent form prior to experimentation. This study was approved by the Institutional Review Board of the Affiliated Hospital of Chifeng University in China.
Neurocognition
Neurocognition was evaluated according to the Wechsler Adult Intelligence Scale-Revised China (WAIS-RC) in patients at 6 months after TBI (Yao et al., 2007). The measurement consisted of performance intelligence quotient (IQ), verbal IQ and full IQ. There are 13 subtests for the measures. There is an obvious functional obstacle when the total score is < 70 points; a lower score indicates worse performance.
Functional outcome measures
Activity of Daily Living (ADL) scale
The ADL scale was developed by Lawton and Brody in the United States in 1969. This scale evaluates participants’ functioning in everyday life. It includes the Physical Self-maintenance Scale and the Instrumental Activities of Daily Living Scale. ADL is mainly used for the evaluation of daily life ability and includes 14 items, six items on the Physical Self-maintenance Scale and eight items on the Instrumental Activities of Daily Living Scale. Total scores on this scale range from 14 points to 56 points. There is an obvious functional obstacle when the total score is ≥ 22 points; a higher score indicates worse performance. ADL evaluation was performed 6 months after TBI and every day after neurocognition detection.
China version of Social Disability Screening Schedule (C-SSDS)
The C-SSDS test was originally developed by the WHO Disability Assessment Schedule in 1988. The validity and reliability of the C-SSDS were confirmed by the China Mental Illness Epidemiological Work Group from twelve regions. This scale subjectively measures adults’ social, occupational, and psychological functioning. It is suitable for cases aged from 15 years to 59 years. C-SSDS includes 10 items. Each score on this scale ranges from 0 to 2; 0 point refers to healthy or very minor defects, 1 point refers to a functional defect, and 2 points refers to a serious functional defect. There is an obvious social function obstacle when the total score is ≥ 2 points; a higher score indicates worse performance. The C-SSDS test was performed 6 months after TBI and every day following the ADL test.
Electroencephalogram (EEG) recording
After neurocognition and function assessment, EEG was recorded the next day. The participants were seated in a comfortable chair in a sound-attenuated room. Stimulus presentation and data synchronization with the EEG were accomplished using E-Prime (Psychology Software Tools, Pittsburgh, PA, USA). The EEG was continuously recorded (band pass 0.1–200 Hz, sampling rate 1,000 Hz) with Neuroscan Synamp Amplifier (Compumedics USA, El Paso, TX, USA), using an electrode cap with 32 Ag/AgCl electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FT8, T3, C3, Cz, C4, T4, TP7, CP3, CP4, TP8, T5, P3, Pz, P4, T6, O1, Oz, O2 and four electro-oculogram (EOG) electrodes) mounted according to the extended international 10-20 system. Vertical electric orbital upper and vertical electric orbital lower were recorded above and below the left eyes. Horizontal EOG was recorded at the outer canthus of each eye. The ground electrode was placed on the forehead, and the reference electrodes were located at the root of the nose. Electrode impedance was maintained below 5 kΩ throughout the experiment.
The auditory stimuli consisted of sounds at 85 dB SPL and 1,000 Hz. The standard and deviant tones lasted 200 ms (Deviat and standard probabilities: 20% and 80%, respectively). The interstimulus interval was 600 ms. The participants were asked to watch a hexapod movie without paying attention to sound. The experimental procedure was 15 minutes long. Breaks were permitted only when the participants asked to rest.
EOG artifacts were corrected using the method proposed by Semlitsch et al. (1986). The EEG was segmented in epochs of 600 ms, time-locked to face onset and included a 200-ms pre-stimulus baseline. Averaging of the event-related potential waves and related procedures was performed using the NeuroScan version 4.5 software (Compumedics U.S.A., Ltd., El Paso, TX, USA). Eye blinks were removed using the established mathematical procedures (Semlitsch et al., 1986). Trials contaminated by amplifier clipping, bursts of electromyographic activity, or peak-to-peak deflection exceeding ±100 μV were excluded from averaging. The averaged event-related potentials were digitally filtered with a low-pass filter at 30 Hz (24 dB/Octave) and then divided into 1,000-ms epochs from –100-ms prestimulus to 400-ms poststimulus. The MMN wave was generated by subtracting the standard event-related potential wave from the deviant ones. The mean voltage of the MMN amplitude was measured between 100 ms and 300 ms. Larger MMN peaks have generally been seen at the frontal electrodes (Umbricht et al., 2006; Wynn et al., 2010; El et al., 2014). Based on the observed scalp topography of the MMN in the control group and on electrode choice in previous MMN studies, the following fronto-central electrodes were chosen for analysis: F3, F4, Fz, C3, C4, Cz, FC3, FC4, FCz (Giard et al., 1990; Lin et al., 2012; Neuhoff et al., 2012; Xiying et al., 2012), and was performed using the NeuroScan version 4.5 software package (Compumedics, U.S.A., Ltd.). This fronto-central region is known to be of interest for auditory stimulus perception and processing (Giard et al., 1990) and these electrodes have also been used for group comparisons between TBI and controls in former cognition studies (Sarno et al., 2006; Doi et al., 2007; Lew et al., 2007a, b; Kodama et al., 2010).
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 software manufactured by SPSS Inc. (IBM Corporation, Armonk, NY, USA). A chi-squared test was used to examine differences in demographic variables. The measurement data were not normally distributed as determined by the Kolmogorov-Smirnov test (Ks < 0.05). Comparisons between groups were made using the Mann-Whitney U test (Pereira et al., 2015). A P value of less than 0.05 was considered statistically significant. A Mann-Whitney U test with 9 electrodes as within-participants variables and the two groups as the between-participants variable was applied to assess patterns of MMN activity (Lin et al., 2012). There was no significant difference in neurocognition, social cognitive ability, and functional outcome scores in the assumption of homogeneity of variance; thus, Spearman's partial correlation analysis with age, sex and education as covariates was performed to analyze the relationships between MMN amplitudes and WAIS-RC, ADL, and C-SSDS scores (Seung et al., 2014).
Results
MMN amplitudes between two groups
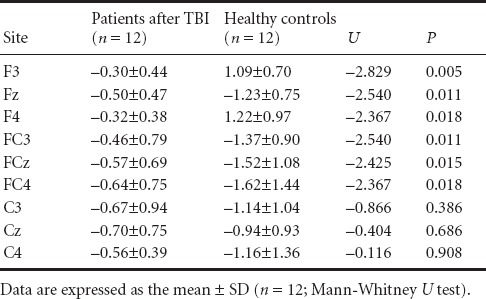
There were no significant differences between groups in age distribution (F = 6.53, P > 0.05) or education (F = 0.34, P > 0.05). Comparisons in MMN amplitudes between groups showed a significant difference (U = –2.425, P < 0.05). Figure 1 shows the grand-averaged MMN waveforms and topographical maps for the two groups. The TBI patients displayed obviously reduced MMN amplitudes compared with normal controls at the FCz electrode. The fronto-central electrodes (F3, Fz, F4, FC3, FCz, FC4) with significantly higher MMN amplitudes in healthy controls than in TBI patients are shown in Table 1 (P < 0.05).
Figure 1.
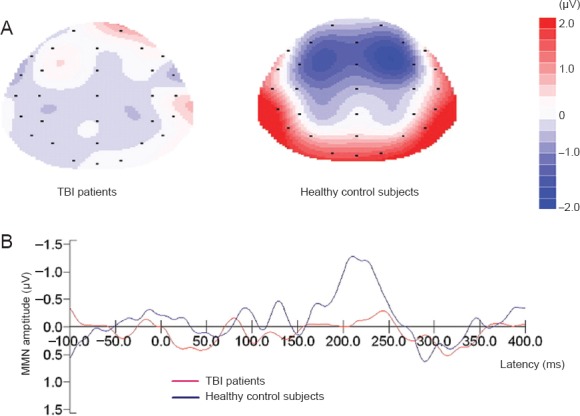
Topographic maps of MMN (A) and MMN waves (B) at FCz in traumatic brain injury (TBI) patients and healthy control participants.
The MMN wave was generated by subtracting the standard event-related potential wave from the deviant ones. MMN amplitude was measured as the mean voltage between 100 ms and 300 ms. Blue region indicates low voltage and red region indicates high voltage. MMN: Mismatch negativity.
Table 1.
Comparison of mismatch negativity (MMN) amplitude (μV) between traumatic brain injury (TBI) patients and healthy controls
The relationship of MMN with neurocognition and functional outcome
There were significant positive correlations between MMN amplitudes, neurocognition, and functional outcome scores in F3, Fz, F4, FC3, FCz, FC4, C3, Cz, C4 (Table 2). The strongest positive correlations were found between MMN at the FCz electrode and ADL (r = 0.671, P < 0.001). The scatter plots between MMN and ADL scores, and between MMN and C-SSDS scores in TBI patients, are revealed in Figure 2. MMN amplitude was extremely positively associated with ADL in FCz and was also extremely positively associated with C-SSDS in Fz in TBI patients (r = 0.502, P < 0.01).
Table 2.
Correlation of MMN amplitudes with neurocognition and functional outcomes through Spearman's partial correlations analysis, taking the age, sex and education as the covariates
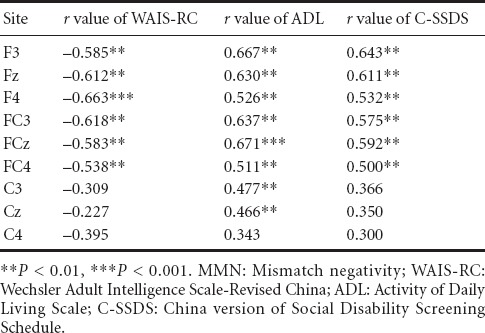
Figure 2.
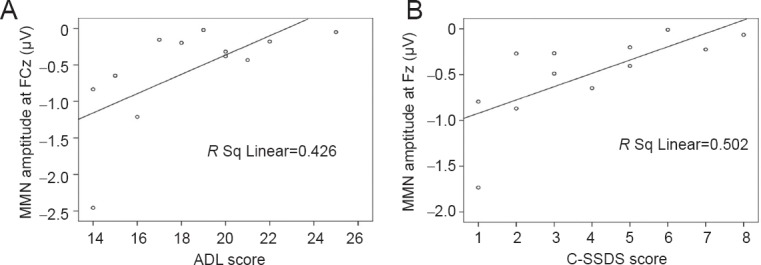
Scatter plots between MMN at the FCz electrode and ADL scores (A) and between MMN at the Fz electrode and C-SSDS scores (B) in patients after traumatic brain injury.
MMN amplitude was extremely positively associated with ADL in FCz. MMN amplitudes were extremely positive associated with C-SSDS in Fz in TBI patients. MMN: Mismatch negativity; ADL: Activity of Daily Living Scale; C-SSDS: China version of Social Disability Screening Schedule.
Discussion
Event-related potentials recorded in a healthy young group and a healthy elderly group have been documented for the early treatment of cognitive alterations and dementia, and can be used as an early discrimination between normal and pathological brain aging (Freigang et al., 2014). Schmidt et al. (2013) present a model-based investigation of mechanisms underlying the reduction of MMN amplitudes under ketamine, and demonstrated the potential of modeling pharmacological modulation from EEG data. MMN can be used to confirm plastic neural damage, and can also predict the recovery of patients in a coma and in infants learning a language (Kujala and Naatanen, 2010). Kaipio et al. (2001) reported fast vigilance attenuation in closed head injury patients, and indicated that MMN amplitude for the pitch deviant declined in healthy controls less than in patients. MMN may have clinical utility in detecting deviations from normal cognitive processing (Lew et al., 2007; Paukkunen et al., 2011). The relationships of MMN to social cognitive ability or functional outcome have not been studied, so we compared the relevance of MMN, neurocognition, and daily and social functioning in patients after TBI and in healthy controls. In this study, we concluded that MMN amplitudes were lower in patients after TBI than in healthy controls.
In terms of daily and social functioning in the study, MMN in healthy controls and patients after TBI was significantly associated with the ADL and C-SSDS scores. MMN and neuropsychological test (WAIS-RC) scores in controls and TBI patients also have an obvious relationship. So, the main finding of this study is that MMN in the fronto-central region was significantly negatively correlated with neurocognition and positively correlated with functional outcomes between two groups. The significant positive relevancy of MMN and functional outcomes was emphasized. MMN can be significantly predicted in patients after TBI.
Cognitive function is closely related to age (Lampit et al., 2014; Roy et al., 2014; Shafto et al., 2014), and MMN amplitude is in a downward trend in the elderly (Kiang et al., 2009). The C-SSDS asks for a restriction on age. So in this study, the age of participants is considered. Gender can also influence cognitive dysfunction after TBI. Gender, therefore, may be a factor affecting the degree of brain injury recovery (Garcia et al., 1999; Roof et al., 2000; Yune et al., 2004). We put the gender factor in the reference system for research. Cognitive rehabilitation after TBI is associated with the number of years of education: the higher the education level, the better the cognitive function recovery (Schneider et al., 2014). In the present study, there were no significant differences between groups in aspects of age distribution or education.
As far as we know, this study is the first demonstration to explore the relationships of MMN with neurocognition, social cognitive ability, and daily and social functional outcomes. In brief, MMN has significant relationships to neurocognition, and daily and social functioning in TBI patients, to generalize the results. MMN is best predicted by functional outcome in Han Chinese TBI patients, and can predict neuronal regeneration. The present study has three limitations. First, although the participants were asked to watch a hexapod movie without paying attention to sound, we could not completely rule out the possibility that they occasionally paid attention to the sound. Second, the experimental procedure was 15 minutes long. Breaks were permitted only when the participants asked to rest. The rest times may have affected the EEG recording and may influence the results. Third, we performed our study with a limited number of participants, so further studies should be conducted involving a larger number of participants with regular breaks in the experimental procedure. In future, electrophysiological and neuropsychological studies combining biomarkers from different modalities should be investigated to predict the functional recovery of patients after traumatic brain injury earlier and more accurately.
Footnotes
Funding: This study was supported by grants from the National Natural Science Foundation of China, No. 81172911, 81373251; the National High Technology Research and Development Program of China (863 Program), No. 2015AA020503; the Science and Technology Development Project of Suzhou of China, No. SZP201304; and Priority Academic Program Development of Jiangsu Higher Education Institutes of China.
Conflicts of interest: None declared.
Copyedited by Jackson C, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Baldeweg T, Hirsch SR. Mismatch negativity indexes illness-specific impairments of cortical plasticity in schizophrenia: a comparison with bipolar disorder and Alzheimer's disease. Int J Psychophysiol. 2015;95:145–155. doi: 10.1016/j.ijpsycho.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Beste C, Humphries M, Saft C. Striatal disorders dissociate mechanisms of enhanced and impaired response selection-Evidence from cognitive neurophysiology and computational modelling. Neuroimage Clin. 2014;4:623–634. doi: 10.1016/j.nicl.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV, Hardiman MJ. Measurement of mismatch negativity in individuals: a study using single-trial analysis. Psychophysiology. 2010;47:697–705. doi: 10.1111/j.1469-8986.2009.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey JM, Lippy WH, Schuring AG, Rizer FM. Clinical utility of the 512-Hz Rinne tuning fork test. Am J Otol. 1998;19:59–62. [PubMed] [Google Scholar]
- Calcus A, Deltenre P, Hoonhorst I, Collet G, Markessis E, Colin C. MMN and P300 are both modulated by the featured/featureless nature of deviant stimuli. Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.11.020. doi: 10.1016/j.clinph.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Campanella S, Colin C. Event-related potentials and biomarkers of psychiatric diseases: the necessity to adopt and develop multi-site guidelines. Front Behav Neurosci. 2014;8:428. doi: 10.3389/fnbeh.2014.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts SV, Shelley AM, Ward PB, Liebert B, McConaghy N, Andrews S, Michie PT. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- Chen AJ, D’Esposito M. Traumatic brain injury: from bench to bedside [corrected] to society. Neuron. 2010;66:11–14. doi: 10.1016/j.neuron.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Choi W, Lim M, Kim JS, Kim DJ, Chung CK. Impaired pre-attentive auditory processing in fibromyalgia: a mismatch negativity (MMN) study. Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.10.012. doi: 10.1016/j.clinph.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Doi R, Morita K, Shigemori M, Tokutomi T, Maeda H. Characteristics of cognitive function in patients after traumatic brain injury assessed by visual and auditory event-related potentials. Am J Phys Med Rehabil. 2007;86:641–649. doi: 10.1097/PHM.0b013e318115aca9. [DOI] [PubMed] [Google Scholar]
- Duncan CC, Summers AC, Perla EJ, Coburn KL, Mirsky AF. Evaluation of traumatic brain injury: brain potentials in diagnosis, function, and prognosis. Int J Psychophysiol. 2011;82:24–40. doi: 10.1016/j.ijpsycho.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, Polich J, Reinvang I, Van Petten C. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- El Karoui I, King JR, Sitt J, Meyniel F, Van Gaal S, Hasboun D, Adam C, Navarro V, Baulac M, Dehaene S, Cohen L, Naccache L. Event-related potential, time-frequency, and functional connectivity facets of local and global auditory novelty processing: an intracranial study in humans. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu143. doi: 10.1093/cercor/bhu143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng SJ, Han JG. Treatment of traumatic brain injury in rats by RhoA gene silencing combined with umbilical cord mesenchymal stem cell transplantation. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:23–30. [Google Scholar]
- Freigang C, Rubsamen R, Richter N. Pre-attentive cortical processing of behaviorally perceptible spatial changes in older adults-a mismatch negativity study. Front Neurosci. 2014;8:146. doi: 10.3389/fnins.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Estrada J, Luquin S, Fernandez AM, Garcia-Segura LM. Dehydroepiandrosterone, pregnenolone and sex steroids down-regulate reactive astroglia in the male rat brain after a penetrating brain injury. Int J Dev Neurosci. 1999;17:145–151. doi: 10.1016/s0736-5748(98)00065-3. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, van der Elst W, Jolles J, Verhey FR, McGlinchey RE, Milberg WP, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SJ, Vaz Pato M, Sprague L, Stokes M, Munday R, Haque N. Auditory evoked potentials to spectro-temporal modulation of complex tones in normal subjects and patients with severe brain injury. Brain. 2000;123:1007–1016. doi: 10.1093/brain/123.5.1007. [DOI] [PubMed] [Google Scholar]
- Kaipio ML, Cheour M, Ohman J, Salonen O, Naatanen R. Mismatch negativity abnormality in traumatic brain injury without macroscopic lesions on conventional MRI. Neuroreport. 2013;24:440–444. doi: 10.1097/WNR.0b013e32836164b4. [DOI] [PubMed] [Google Scholar]
- Kaipio ML, Novitski N, Tervaniemi M, Alho K, Ohman J, Salonen O, Naatanen R. Fast vigilance decrement in closed head injury patients as reflected by the mismatch negativity (MMN) Neuroreport. 2001;12:1517–1522. doi: 10.1097/00001756-200105250-00043. [DOI] [PubMed] [Google Scholar]
- Kodama T, Morita K, Doi R, Shoji Y, Shigemori M. Neurophysiological analyses in different color environments of cognitive function in patients with traumatic brain injury. J Neurotrauma. 2010;27:1577–1584. doi: 10.1089/neu.2009.1119. [DOI] [PubMed] [Google Scholar]
- Kujala T, Näätänen R. The adaptive brain: a neurophysiological perspective. Prog Neurobiol. 2010;91:55–67. doi: 10.1016/j.pneurobio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Kujala T, Tervaniemi M, Schroger E. The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol Psychol. 2007;74:1–19. doi: 10.1016/j.biopsycho.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kumaria A, Tolias CM. Normobaric hyperoxia therapy for traumatic brain injury and stroke: a review. Br J Neurosurg. 2009;23:576–584. doi: 10.3109/02688690903050352. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Farrer TJ, Clayson PE. Cognitive control in mild traumatic brain injury: conflict monitoring and conflict adaptation. Int J Psychophysiol. 2011;82:69–78. doi: 10.1016/j.ijpsycho.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DA, Kellison IL, Schmalfuss IM, Perlstein WM. Double jeopardy! The additive consequences of negative affect on performance-monitoring decrements following traumatic brain injury. Neuropsychology. 2009;23:433–444. doi: 10.1037/a0015723. [DOI] [PubMed] [Google Scholar]
- Lee SH, Sung K, Lee KS, Moon E, Kim CG. Mismatch negativity is a stronger indicator of functional outcomes than neurocognition or theory of mind in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:213–219. doi: 10.1016/j.pnpbp.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Lew HL, Gray M, Poole JH. Temporal stability of auditory event-related potentials in healthy individuals and patients with traumatic brain injury. J Clin Neurophysiol. 2007a;24:392–397. doi: 10.1097/WNP.0b013e31814a56e3. [DOI] [PubMed] [Google Scholar]
- Lew HL, Thomander D, Gray M, Poole JH. The effects of increasing stimulus complexity in event-related potentials and reaction time testing: clinical applications in evaluating patients with traumatic brain injury. J Clin Neurophysiol. 2007b;24:398–404. doi: 10.1097/WNP.0b013e318150694b. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Y, Sun G, Gao L, Zhao L. Visual mismatch negativity elicited by facial expressions: new evidence from the equiprobable paradigm. Behav Brain Funct. 2012;8:7. doi: 10.1186/1744-9081-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19:1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Liu CM, Chiu MJ, Liu CC, Chien YL, Hwang TJ, Jaw FS, Shan JC, Hsieh MH, Hwu HG. Differentiation of schizophrenia patients from healthy subjects by mismatch negativity and neuropsychological tests. PLoS one. 2012;7:e34454. doi: 10.1371/journal.pone.0034454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor T, Barbiro-Michaely E, Rogatsky G, Mayevsky A. Real-time multi-site multi-parametric monitoring of rat brain subjected to traumatic brain injury. Neurol Res. 2008;30:1075–1083. doi: 10.1179/174313208X346107. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118:2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S, Ponton C. The mismatch negativity (MMN)--a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin Neurophysiol. 2012;123:424–458. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Neuhoff N, Bruder J, Bartling J, Warnke A, Remschmidt H, Muller-Myhsok B, Schulte-Korne G. Evidence for the late MMN as a neurophysiological endophenotype for dyslexia. PLoS One. 2012;7:e34909. doi: 10.1371/journal.pone.0034909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachalska M, Lukowicz M, Kropotov JD, Herman-Sucharska I, Talar J. Evaluation of differentiated neurotherapy programs for a patient after severe TBI and long term coma using event-related potentials. Med Sci Monit. 2011;17:CS120–128. doi: 10.12659/MSM.881970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukkunen AK, Leminen M, Sepponen R. The effect of measurement error on the test-retest reliability of repeated mismatch negativity measurements. Clin Neurophysiol. 2011;122:2195–2202. doi: 10.1016/j.clinph.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Pereira J, Hastie P, Araújo R, Farias C, Rolim R, Mesquita I. A comparative study of students’ track and field technical performance in sport education and in a direct instruction approach. J Sports Sci Med. 2015;14:118–27. [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Larson MJ, Dotson VM, Kelly KG. Temporal dissociation of components of cognitive control dysfunction in severe TBI: ERPs and the cued-Stroop task. Neuropsychologia. 2006;44:260–274. doi: 10.1016/j.neuropsychologia.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 2000;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Roy M, Retzer A, Sikabofori T. Personality development and intellectual disability. Curr Opin Psychiatry. 2015;28:35–39. doi: 10.1097/YCO.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Sarno S, Erasmus LP, Frey M, Lippert G, Lipp B. Electrophysiological correlates of active and passive attentional states after severe traumatic brain injury. Funct Neurol. 2006;21:21–29. [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, Levin HS. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J Int Neuropsychol Soc. 2012;18:89–100. doi: 10.1017/S1355617711001433. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Diaconescu AO, Kometer M, Friston KJ, Stephan KE, Vollenweider FX. Modeling ketamine effects on synaptic plasticity during the mismatch negativity. Cereb Cortex. 2013;23:2394–2406. doi: 10.1093/cercor/bhs238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider EB, Sur S, Raymont V, Duckworth J, Kowalski RG, Efron DT, Hui X, Selvarajah S, Hambridge HL, Stevens RD. Functional recovery after moderate/severe traumatic brain injury: a role for cognitive reserve? Neurology. 2014;82:1636–1642. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Korne G, Deimel W, Bartling J, Remschmidt H. Auditory processing and dyslexia: evidence for a specific speech processing deficit. Neuroreport. 1998;9:337–340. doi: 10.1097/00001756-199801260-00029. [DOI] [PubMed] [Google Scholar]
- Schulte-Korne G, Deimel W, Bartling J, Remschmidt H. Speech perception deficit in dyslexic adults as measured by mismatch negativity (MMN) Int J Psychophysiol. 2001;40:77–87. doi: 10.1016/s0167-8760(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK, Dixon M, Taylor JR, Rowe JB, Cusack R, Calder AJ, Marslen-Wilson WD, Duncan J, Dalgleish T, Henson RN, Brayne C, Matthews FE, Cam CAN. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014;14:204. doi: 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, Kane J. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–725. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, Kane J. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- Wang C. Changes in memory function of rats with brain injury after fingolimod administration combined with bone marrow mesenchymal stem cell transplantation. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:4469–4473. [Google Scholar]
- Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry. 2010;67:940–947. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Chen H, Jiang L, Tam WC. Replication of factor structure of Wechsler Adult Intelligence Scale-III Chinese version in Chinese mainland non-clinical and schizophrenia samples. Psychiatry Clin Neurosci. 2007;61:379–384. doi: 10.1111/j.1440-1819.2007.01672.x. [DOI] [PubMed] [Google Scholar]
- Yu Z, Morrison B., 3rd Experimental mild traumatic brain injury induces functional alteration of the developing hippocampus. J Neurophysiol. 2010;103:499–510. doi: 10.1152/jn.00775.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarza-Lucianez D, Arce-Arce S, Bhathal H, Sanjuan-Martin F. Mismatch negativity and conscience level in severe traumatic brain injury. Rev Neurol. 2007;44:465–468. [PubMed] [Google Scholar]


