Abstract
Previous diffusion tensor imaging (DTI) studies regarding pediatric patients with motor dysfunction have confirmed the correlation between DTI parameters of the injured corticospinal tract and the severity of motor dysfunction. There is also evidence that DTI parameters can help predict the prognosis of motor function of patients with cerebral palsy. But few studies are reported on the DTI parameters that can reflect the motor function outcomes of pediatric patients with hemiplegic cerebral palsy after rehabilitation treatment. In the present study, 36 pediatric patients with hemiplegic cerebral palsy were included. Before and after rehabilitation treatment, DTI was used to measure the fiber number (FN), fractional anisotropy (FA) and apparent diffusion coefficient (ADC) of bilateral corticospinal tracts. Functional Level of Hemiplegia scale (FxL) was used to assess the therapeutic effect of rehabilitative therapy on clinical hemiplegia. Correlation analysis was performed to assess the statistical interrelationship between the change amount of DTI parameters and FxL. DTI findings obtained at the initial and follow-up evaluations demonstrated that more affected corticospinal tract yielded significantly decreased FN and FA values and significantly increased ADC value compared to the less affected corticospinal tract. Correlation analysis results showed that the change amount of FxL was positively correlated to FN and FA values, and the correlation to FN was stronger than the correlation to FA. The results suggest that FN and FA values can be used to evaluate the motor function outcomes of pediatric patients with hemiplegic cerebral palsy after rehabilitation treatment and FN is of more significance for evaluation.
Keywords: nerve regeneration, cerebral palsy, corticospinal tract, diffusion tensor, hemiplegia, motor, rehabilitation, neural regeneration
Introduction
Motor dysfunction is the most common neurodevelopmental problem in pediatric patients with cerebral palsy (Arzoumanian et al., 2003; Wilson-Costello et al., 2005; Drobyshevsky et al., 2007; Ludeman et al., 2008; Trivedi et al., 2010; Williams et al., 2010; Yoshida et al., 2010). Corticospinal tract (CST) is the most important motor pathway involved primarily in voluntary movements of distal extremities (Jang, 2009; Cho et al., 2012). The prognosis of motor outcome is known to be highly related to the state of the CST (Arzoumanian et al., 2003; Drobyshevsky et al., 2007). The extent of CST injury is related to motor dysfunction, and recovery of CST injury is also related to improvement of motor function (deVeber et al., 2000; Sreenan et al., 2000; Trivedi et al., 2008). Therefore, early and precise evaluation for the CST state is essential for establishment of appropriate rehabilitative therapeutic strategy (Rocca et al., 2013). Several modalities, including brain ultrasonography, computed tomography and magnetic resonance imaging (MRI), have been attempted for evaluation of the state of the CST in pediatric patients (Wiklund and Uvebrant, 1991; Seme-Ciglenecki, 2007; Son et al., 2007). However, these techniques have often failed to detect the cause of motor dysfunction and to provide detailed information in pediatric patients (Ancel et al., 2006; Nakayama et al., 2006; Son et al., 2007; Baek et al., 2013).
Diffusion tensor imaging (DTI), a recently developed technique, is a very powerful modality to evaluate the CST state (Arzoumanian et al., 2003; Drobyshevsky et al., 2007; Ludeman et al., 2008; Trivedi et al., 2010; Yoshida et al., 2010). DTI provides information on the microstructural state of neural tracts by virtue of its ability to quantify water diffusion properties (Wakana et al., 2004; Mukherjee and McKinstry, 2006; Kwak et al., 2010). Diffusion tensor tractography (DTT), derived from the DTI technique, provides integrity of the neural tracts and three-dimensional visualization of the architecture (Mori et al., 1999; Mukherjee and McKinstry, 2006; Kwak et al., 2010). DTI and DTT can also provide several reproducible quantitative parameters of water diffusion in brain tissue, including fiber number (FN), fractional anisotropy (FA), and apparent diffusion coefficient (ADC) (Mori et al., 1999, Neil, 2008, Sundaram et al., 2008). The validity of these parameters has been established in several previous studies (Jang et al., 2006; Neil, 2008; Son et al., 2009; Kwak et al., 2010).
Some previous DTI studies in pediatric patients with motor dysfunction have reported significant correlation of DTI parameters of the affected CST with the severity of motor dysfunction (Son et al., 2007; Trivedi et al., 2010; Yoshida et al., 2010; Chang et al., 2012). In addition, it has also been reported that DTI parameters can predict motor dysfunction in pediatric patients (Arzoumanian et al., 2003; Ludeman et al., 2008; Murakami et al., 2008; Son et al., 2009). However, to the best of our knowledge, there has been no study on DTI parameters reflecting the therapeutic effect of rehabilitation treatment in pediatric patients with hemiplegic cerebral palsy. Therefore, we analyzed the correlation of DTI parameters with functional parameters for clinical hemiplegia in pediatric patients with cerebral palsy.
Subjects and Methods
Subjects
Thirty-six subjects were recruited using the following criteria: (1) hemiplegic cerebral palsy patients diagnosed by two pediatric neurologists; (2) regular physical and occupational therapy twice a week by experienced therapists in the same university hospital; (3) absence of diagnosed congenital brain anomaly, chromosomal abnormalities, or genetic syndrome; (4) absence of severe mental retardation or intractable seizure; (5) absence of postnatal brain injury history such as a traumatic event, brain surgery or postnatal neonatal stroke; (6) initial DTI evaluation at an age of ≤ 12 months old; (7) follow-up DTI duration of 6–12 months. Two pediatric neurologists assessed the patients independently, and all patients were evaluated by both neurologists. Participants whose diagnosis was in disagreement between two neurologists were excluded. The study population was originally selected from 124 hemiplegic patients who underwent initial DTI evaluation at an age of ≤ 12 months old. Of the 124 pediatric patients, 89 who underwent follow-up DTI evaluation were selected. Of these 89 pediatric patients, 43 were excluded for their follow-up duration of less than 6 months or over 12 months. Of the remaining subjects, two pediatric patients were excluded for chromosomal abnormalities and four pediatric patients who did not receive regular rehabilitative therapy were excluded. In addition, one patient who showed intractable epilepsy and three other children who were diagnosed as congenital brain anomaly such as pachygyria or schizencephaly were also excluded. Finally, the remaining 36 pediatric patients were recruited for this study. For improvement of hand function, all 36 pediatric patients were intensively provided occupational therapy involving task oriented training that induced reaching, targeting, and grasping of more affected upper extremity. The pediatric patients who reached the goal were then provided additional task involving bimanual activity for more functional improvement. Informed consent was obtained from the parents of all participants, and the study was approved by the institutional review board at the Hospital of School of Medicine, Yeungnam University, Republic of Korea.
Functional level of hemiplegia
Initial and follow-up (mean follow up duration 9.0 ± 2.3 months) functional evaluations were performed by two pediatric neurologists using Functional Level of Hemiplegia scale (FxL) (House et al., 1981; Romain et al., 1999; Fluet et al., 2010). FxL is an observational description of hemiplegic upper extremity use in children with cerebral palsy and it has been widely used in several previous studies on the effect of rehabilitative therapy for motor dysfunction (House et al., 1981; Romain et al., 1999; Fluet et al., 2010). FxL is classified as follows: 0: no use; 1: use as stabilizing weight only; 2: can hold objects placed in the hand; 3: can hold an object and stabilize it for use by the other hand; 4: can actively grasp an object and hold it weakly; 5: can actively grasp an object and stabilize it well; 6: can actively grasp an object and manipulate it against the other hand; 7: can perform bimanual activities easily and occasionally uses the hand spontaneously; 8: uses the hand with complete independence. FxL was evaluated on the same day or at 1 day before DTI scanning.
Diffusion tensor imaging (DTI)
DTI data were acquired using a 1.5-T Philips Gyroscan Intera system (Philips, Ltd, Best, The Netherlands) equipped with a Synergy-L Sensitivity Encoding (SENSE) head coil utilizing a single-shot, spin-echo planar imaging pulse sequence. Sixty-seven contiguous slices were acquired for each of the 32 noncollinear diffusion-sensitizing gradients. Imaging parameters were as follows: matrix = 128 × 128, field of view = 221 × 221 mm2, echo time (TE) = 76 mm, repetition time (TR) = 10,726 ms, parallel imaging reduction factor (SENSE factor) = 2, echo-planar imaging (EPI) factor = 49, b = 1,000 s/mm2, number of excitations (NEX) = 1, and a slice thickness of 2.3 mm. Eddy current-induced image distortions were removed using affine multi-scale two-dimensional registration at the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Fiber connectivity was evaluated using Fiber Assignment by Continuous Tracking (FACT), a three dimensional fiber reconstruction algorithm in Philips PRIDE software (Philips Medical Systems, Best, The Netherlands). The termination criteria used for fiber tracking were fractional anisotropy (FA) threshold of < 0.2 and an angle change of > 45°. A seed region of interest (ROI) was drawn in the CST portion (blue portion) of the anterior mid-pons on a two-dimensional FA color map. On each of the two-dimensional FA color maps, another ROI was drawn in the CST portion (blue portion) of the anterior low-pons (Hong et al, 2010). Fiber tracts passing through both ROIs were designated as final tracts of interest. In order to include only the region of the CST, the typical ROI size was set between 5 and 10 voxels (Son et al., 2007, 2009; Kim et al., 2012). Fiber number (FN), mean value of fractional anisotropy (FA) and apparent diffusion coefficient (ADC) of both sides of the entire CST were measured.
Statistical analysis
Statistical analysis was performed in two steps. In the first step, comparative analysis for DTI parameters such as FN, FA, and ADC between the more affected and less affected sides was performed using paired t-test. Paired t-test was also used for comparison between initial and follow-up data on DTI parameters and FxL. In the second step, Pearson coefficient correlation was used for assessment of statistical significance of correlation between the interval change amounts of DTI parameters and those of FxL. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 (SPSS, Chicago, IL, USA). Statistical significance was accepted for P values of < 0.05.
Results
Demographic data
Among 36 pediatric patients (26 males, 10 females), 20 were born as full-term delivery over 37 weeks of gestational age, and the other 16 were born premature at less than 37 weeks of gestational age. Ages at MRI scanning time and clinical evaluation were adjusted based on corrected age that is by subtracting weeks born prematurely from post-birth age. The mean age at initial evaluation was 5.6 ± 3.2 months and the mean follow up duration was 9.0 ± 2.3 months. Among 36 pediatric patients, 12 had lesions on conventional MRI scan, including right middle cerebral artery infarct in two patients, left fronto-parietal lobe hemorrhage in one patient, and periventricular leukomalacia in nine patients. The other 24 pediatric patients showed no abnormal brain lesion on conventional MRI.
Functional level of hemiplegia scale
At initial evaluation, pediatric patients presented with various hemiplegic symptoms, such as decreased bimanual coordination skill, one hand dominance symptom, and no use of the more affected hand. At follow up evaluation after rehabilitative therapy, all patients showed improvement of their hemiplegic symptoms of upper extremities and increased FxL score. After rehabilitation treatment, clinical function was significantly improved in 35 out of the 36 patients. However, FxL score was not significant in one patient despite improvement in hemiplegic symptoms. All patients were evaluated by two neurologists who were unaware of the other neurologist's evaluation result, and all results were the same for both neurologists. The initial mean score of FxL was 2.4 ± 2.2, and follow up mean score showed a significant increase to 7.1 ± 2.1 (P < 0.05) (Table 1). Regarding baseline data, the 24 patients without brain lesions demonstrated significantly more improvement of FxL (5.04 ± 1.65) compared to the 12 patients with brain lesion (2.58 ± 1.44) (P < 0.05).
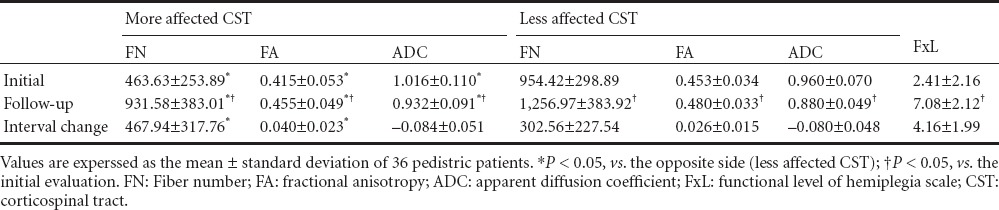
Table 1.
Initial and follow-up data of DTI parameters and FxL in pediatric patients with cerebral palsy
DTI parameters
Results of comparative analysis between the more affected and less affected sides showed a significant decrease in more affected FN and FA, and a significant increase in the more affected ADC at both initial and follow up evaluations (P < 0.05). Comparative analysis between initial and follow up values of FN, FA and ADC showed significant improvement at follow up in both hemispheres of the more affected and less affected sides (P < 0.05). Comparative analysis of interval change amount between more affected and less affected sides showed a significant increase in the amount of FN and FA on the more affected side compared with the less affected side (P < 0.05; Figure 1). However, no significant difference of change amount was observed for ADC (P > 0.05) (Table 1).
Figure 1.
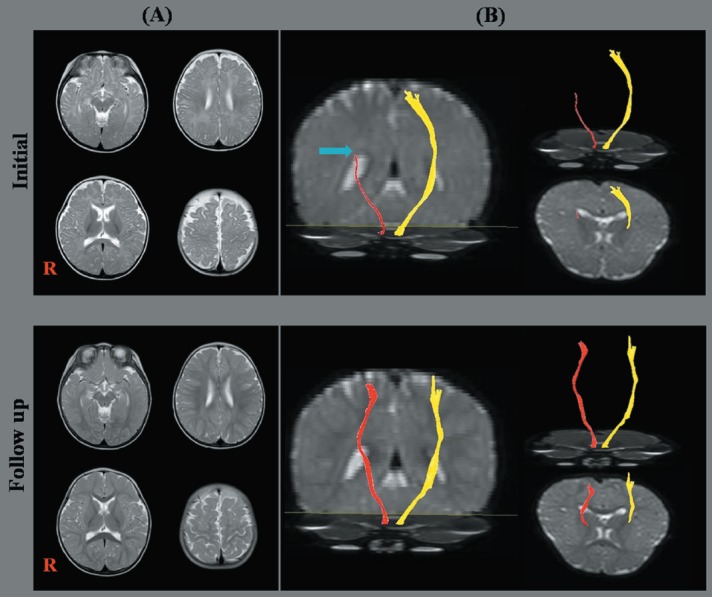
Conventional brain magnetic resonance image (MRI) and diffusion tensor image (DTI) for the corticospinal tract (CST).
(A) T2-weighted brain MRI; (B) DTI for the CST (right CST: red color; left CST: yellow color). Initial DTI for a patient (6-month-old male) shows discontinuity (blue arrow) and decreased fiber density of the right CST compared to the left CST. Follow up DTI for a patient (13-month-old male; the same patient as initial DTI) shows improvements in the disrupted integrity and decreased fiber density of the right CST compared to the initial result.
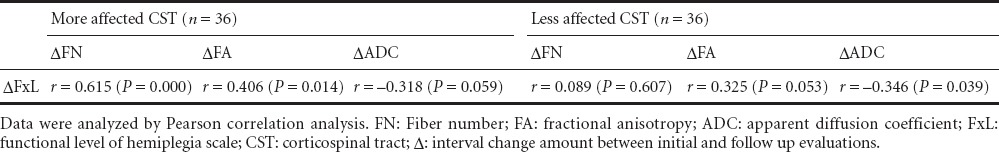
Correlation analysis showed that FN and FA changes were significantly correlated with FxL change, FN change (r = 0.615, P = 0.000) exhibited a stronger positive correlation with FxL change than FA change (r = 0.406, P = 0.014). ADC change (r = –0.318, P = 0.059) showed no significant correlation. The correlations of less affected DTI parameters with FxL change were not significant (Table 2).
Table 2.
Correlation between DTI parameters and FxL in pediatric patients with hemiplegic cerebral palsy
Discussion
In the current study, we investigated the correlation between radiologic parameters of DTI and motor function outcome represented by FxL, demonstrating that FN and FA of the more affected CST are the significant parameters that can reflect the therapeutic effect of rehabilitative therapy.
Several DTI studies have reported on the clinical significance of FN and FA in pediatric patients (Son et al., 2009; Roze et al., 2012; Rickards et al., 2014). Rose et al. (2012) reported significant difference of FA value in CSTs between the affected side and the opposite side. Similarly, Rickards et al. (2014) reported significantly decreased FA value of the affected CST compared with the unaffected CST in patients with hemiparetic cerebral palsy. Another study on the hemiparetic patients with CST disruption reported a significantly decreased FN value of the affected CST compared with the unaffected CST (Son et al., 2009). These results coincided with our results that more affected FN and FA were significantly decreased compared with those of the less affected CST.
Another previous study reported on pediatric patients who showed definite clinical hemiplegia, although they had no definite brain lesion for explainable clinical hemiplegia. In that study, despite normal results of conventional MRI, the patients showed significantly decreased FA value of the affected CST compared with that of the unaffected CST (Son et al., 2007). Similarly, 24 of 36 patients in the current study did not show an abnormal brain lesion on conventional MRI although they showed definite hemiplegic symptoms. By contrast, DTI revealed significant difference of FA, FN and ADC between the more affected and the less affected sides. These findings suggest that DTI is very sensitive to assessment of the CST state in pediatric patients regardless of the conventional MRI lesion.
A few previous DTI studies have reported on recovery of the CST after rehabilitative therapy (Trivedi et al., 2008; Baek et al., 2013). Trivedi et al. (2008) reported significantly increased FA value of the CST in patients with quadriparesis after rehabilitative intervention. Although, this study was coincided with our result which showed improvement of DTI values, it has several differences from our study. First, their patients were quadriplegic cerebral palsy patients. We recruited hemiplegic cerebral palsy patients because we wanted to demonstrate the therapeutic effect more accurately through comparison of the changes between affected and unaffected extremities after treatment. Second, we analyzed whole tract rather than the assessment of ROI. It is well known that there exists some difference of real anatomical location between individual subjects even for the same neural tract, and this difference would be bigger if the subject's age is young. Additionally, several tracts, including spinothalamic, medial lemniscus or corticoreticular pathway run through the similar ROI of the CST. Therefore, we analyzed and assessed real CST. Since the CST is known to be highly related to fine motor movement of the upper extremities (Jang, 2009; Cho et al., 2012), we used FxL, which focused on the use of more affected upper extremity use rather than other evaluation tools such as Bayley Scales of Infant Development (BSID), Erhardt Developmental Prehension Assessment (EDPA) or gross motor function classification system (GMFCS) which could not assess the therapeutic progress of the more affected side in hemiplegic pediatric patients.
Another DTI study on hemiplegic pediatric patients showed significantly increased FA value of entire CSTs after rehabilitative therapy (Baek et al., 2013). In this study, gross motor function measure (GMFM) was used to assess clinical improvement. GMFM score showed significant improvement after treatment, but this change showed no significant correlation with DTI changes. We think this insignificant correlation might be related to the selection of the clinical assessment tool about the gross motor function, not the fine motor function more related to the CST. However, their DTI results coincided with our results, that is, there was significant improvement of all DTI parameters of both CSTs after rehabilitative therapy. In addition, interval change amount of FN and FA values were significantly greater on the more affected side compared with the less affected side. These results would be caused by the rehabilitative treatment, which focuses primarily on the more affected side rather than the less affected side (Bengtsson et al., 2005; Yu et al., 2007; Trivedi et al., 2008; Baek et al., 2013). Recent studies have indicated that rehabilitative therapy is characterized by repetitive practice according to the motor learning theory (Rostami et al., 2012), and leads to treatment-induced neural plasticity, which enhances the synaptogenesis, reorganization, and growth of dendritic arbors within the motor cortex area (Biernaskie and Corbett, 2001; Trivedi et al., 2008; Johnston, 2009). The natural maturation occurred in less affected hemisphere as well as more affected side in immature brain (Morriss et al., 1999; Tanner et al., 2000; Schneider et al., 2004). Our results also showed significant improvement of DTI parameters in both CSTs at follow up evaluation. However, due to the characteristics of rehabilitative therapy, the therapeutic effect after rehabilitation would be greater on the more affected side than the less affected side, and, as a result, radiologic parameters of the more affected side revealed more significant interval change compared with those of the less affected side.
From correlation analysis of radiologic parameters and functional parameters, more affected FN and FA changes revealed significant correlation with FxL changes of functional outcome. In addition, FN change showed stronger correlation with FxL change than FA change. FA value is known to indicate the degree of completion for white matter organization (Assaf and Pasternak, 2008; Neil, 2008; Jang, 2010; Koerte et al., 2011; Rha et al., 2012); increased FA value indicates more unidirectionality of well organization of white matter tracts, and decreased FA value indicates impaired organization of the white matter tracts (Assaf and Pasternak, 2008; Murakami et al., 2008; Neil, 2008; Koerte et al., 2011; Chang et al., 2012; Rha et al., 2012). Compared to FA values, FN values indicate the quantitative information on connectivity within the white matter tracts as determined by the anatomical ROIs (Wilson-Costello et al., 2005; Son et al., 2009; Faria and Hoon, 2010; Jang, 2010; Kwak et al., 2010; Yoshida et al., 2010). Therefore, increased FN values indicate increased numbers of the white matter tracts (Son et al., 2009; Faria and Hoon, 2010; Kwak et al., 2010; Yoshida et al., 2010). Some previous studies have reported these different characteristics of FN and FA values (Jang, 2010; Yoshida et al., 2010; Rha et al., 2012). Yoshida et al. (2010) suggested that the portion of the CST that is lower than the pre-set FA threshold is excluded and that FA of the affected CST can be overestimated. Another report by Jang (2010) suggested that FA value is closely related to integrity of the white matter tracts. Therefore, despite reduced FN of the CST, FA reduction may not be sufficiently reached as low as to reflect the level of damage as long as the integrity of the remaining CST is maintained (Jang, 2010; Yoshida et al., 2010). In addition, Rha et al. (2012) reported a significant high FN value in the high-functioning group compared with the low-functioning group but no significant result for FA value in bilateral spastic cerebral palsy patients. Therefore, because the clinical assessment for this study focused on motor functioning ability, we suggest that FN value, rather than FA value, would show stronger correlation with therapeutic effect. Unlike FN and FA values, ADC showed an insignificant result. This result may be related to specific features of ADC, that is, ADC indicates the magnitude of water diffusion and reflects cellular and organelle density (Drobyshevsky et al., 2007; Assaf and Pasternak, 2008; Neil, 2008; Trivedi et al., 2008).
This is the first study to investigate which DTI parameters can reflect the therapeutic effect of rehabilitative intervention in pediatric patients with hemiplegic cerebral palsy. However, the current study has several limitations. Small number of subjects, due to strict inclusion criteria, is the first limitation of this study. In addition, the young age of subjects who were selected in order to rule out the possibility of secondary musculoskeletal deformity which could influence motor performance can be considered as another limitation. Due to the young age of subjects, we could not perform the full examination for visual acuity, whole electromyography and nerve conduction study in order to rule out the abnormal peripheral nerve system in all subjects. A lack of detailed clinical data such as sensory or cognitive function due to subjects’ age is also a limitation of this study. There exists a limitation from DTI techniques. DTI may underestimate or overestimate the neural fiber tracts because of regions of fiber complexity. Finally, the major potential limitation of this study is the partial volume effect due to the same acquisition parameter for the DTI in all age. The partial volume effect can cause the underestimation of the fiber volume in younger child subjects compared with adult subjects. In future studies, partial volume correction or voxel-based morphometric methods should be used to more closely delineate the change of the CST. Further complementary studies involving larger case number and detailed functional assessments are also needed.
Footnotes
Funding: This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012-013997.
Conflicts of interest: None declared.
Copyedited by Liu C, Li CH, Song LP, Zhao M
References
- Ancel PY, Livinec F, Larroque B, Marret S, Arnaud C, Pierrat V, Dehan M, N’Guyen S, Escande B, Burguet A, Thiriez G, Picaud JC, Andre M, Breart G, Kaminski M. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. 2006;117:828–835. doi: 10.1542/peds.2005-0091. [DOI] [PubMed] [Google Scholar]
- Arzoumanian Y, Mirmiran M, Barnes PD, Woolley K, Ariagno RL, Moseley ME, Fleisher BE, Atlas SW. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Baek SO, Jang SH, Lee E, Kim S, Hah JO, Park YH, Lee JM, Son SM. CST recovery in pediatric hemiplegic patients: Diffusion tensor tractography study. Neurosci Lett. 2013;557:79–83. doi: 10.1016/j.neulet.2013.10.047. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Jang SH, Yoe SS, Lee E, Kim S, Lee DG, Son SM. Diffusion tensor imaging demonstrated radiologic differences between diplegic and quadriplegic cerebral palsy. Neurosci Lett. 2012;512:53–58. doi: 10.1016/j.neulet.2012.01.065. [DOI] [PubMed] [Google Scholar]
- Cho HM, Choi BY, Chang CH, Kim SH, Lee J, Chang MC, Son SM, Jang SH. The clinical characteristics of motor function in chronic hemiparetic stroke patients with complete corticospinal tract injury. NeuroRehabilitation. 2012;31:207–213. doi: 10.3233/NRE-2012-0790. [DOI] [PubMed] [Google Scholar]
- deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Bregman J, Storey P, Meyer J, Prasad PV, Derrick M, MacKendrick W, Tan S. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29:289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- Faria AV, Hoon A. Caveats in diffusion tensor imaging interpretation. Dev Med Child Neurol. 2010;52:887. doi: 10.1111/j.1469-8749.2010.03693.x. [DOI] [PubMed] [Google Scholar]
- Fluet GG, Qiu Q, Kelly D, Parikh HD, Ramirez D, Saleh S, Adamovich SV. Interfacing a haptic robotic system with complex virtual environments to treat impaired upper extremity motor function in children with cerebral palsy. Dev Neurorehabil. 2010;13:335–345. doi: 10.3109/17518423.2010.501362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone JH, Son SM, Jang SH. Somatotopic location of corticospinal tract at pons in human brain: a diffusion tensor tractography study. Neuroimage. 2010;51:952–955. doi: 10.1016/j.neuroimage.2010.02.063. [DOI] [PubMed] [Google Scholar]
- House JH, Gwathmey FW, Fidler MO. A dynamic approach to the thumb-in palm deformity in cerebral palsy. J Bone Joint Surg Am. 1981;63:216–225. [PubMed] [Google Scholar]
- Jang SH. The role of the corticospinal tract in motor recovery in patients with a stroke: a review. NeuroRehabilitation. 2009;24:285–290. doi: 10.3233/NRE-2009-0480. [DOI] [PubMed] [Google Scholar]
- Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabilitation. 2010;27:367–372. doi: 10.3233/NRE-2010-0621. [DOI] [PubMed] [Google Scholar]
- Jang SH, Byun WM, Han BS, Park HJ, Bai D, Ahn YH, Kwon YH, Lee MY. Recovery of a partially damaged corticospinal tract in a patient with intracerebral hemorrhage: a diffusion tensor image study. Restor Neurol Neurosci. 2006;24:25–29. [PubMed] [Google Scholar]
- Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- Koerte I, Pelavin P, Kirmess B, Fuchs T, Berweck S, Laubender RP, Borggraefe I, Schroeder S, Danek A, Rummeny C, Reiser M, Kubicki M, Shenton ME, Ertl-Wagner B, Heinen F. Anisotropy of transcallosal motor fibres indicates functional impairment in children with periventricular leukomalacia. Dev Med Child Neurol. 2011;53:179–186. doi: 10.1111/j.1469-8749.2010.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SY, Yeo SS, Choi BY, Chang CH, Jang SH. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: a diffusion tensor tractography study. Eur Neurol. 2010;63:149–153. doi: 10.1159/000281108. [DOI] [PubMed] [Google Scholar]
- Ludeman NA, Berman JI, Wu YW, Jeremy RJ, Kornak J, Bartha AI, Barkovich AJ, Ferriero DM, Henry RG, Glenn OA. Diffusion tensor imaging of the pyramidal tracts in infants with motor dysfunction. Neurology. 2008;71:1676–1682. doi: 10.1212/01.wnl.0000304084.59964.e2. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC. Changes in brain water diffusion during childhood. Neuroradiology. 1999;41:929–934. doi: 10.1007/s002340050869. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006;16:19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Murakami A, Morimoto M, Yamada K, Kizu O, Nishimura A, Nishimura T, Sugimoto T. Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics. 2008;122:500–506. doi: 10.1542/peds.2007-2816. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Yasokawa YT, Miwa K, Yoshimura SI, Iwama T. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry. 2006;77:850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27:1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- Rha DW, Chang WH, Kim J, Sim EG, Park ES. Comparing quantitative tractography metrics of motor and sensory pathways in children with periventricular leukomalacia and different levels of gross motor function. Neuroradiology. 2012;54:615–621. doi: 10.1007/s00234-011-0996-2. [DOI] [PubMed] [Google Scholar]
- Rickards T, Sterling C, Taub E, Perkins-Hu C, Gauthier L, Graham M, Griffin A, Davis D, Mark VW, Uswatte G. Diffusion tensor imaging study of the response to constraint-induced movement therapy of children with hemiparetic cerebral palsy and adults with chronic stroke. Arch Phys Med Rehabil. 2014;95:506–514. doi: 10.1016/j.apmr.2013.08.245. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Turconi AC, Strazzer S, Absinta M, Valsasina P, Beretta E, Copetti M, Cazzagon M, Falini A, Filippi M. MRI predicts efficacy of constraint-induced movement therapy in children with brain injury. Neurotherapeutics. 2013;10:511–519. doi: 10.1007/s13311-013-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain M, Benaim C, Allieu Y, Pelissier J, Chammas M. Assessment of hand after brain damage with the aim of functional surgery. Ann Chir Main Memb Super. 1999;18:28–37. doi: 10.1016/s0753-9053(99)80054-4. [DOI] [PubMed] [Google Scholar]
- Rostami HR, Arastoo AA, Nejad SJ, Mahany MK, Malamiri RA, Goharpey S. Effects of modified constraint-induced movement therapy in virtual environment on upper-limb function in children with spastic hemiparetic cerebral palsy: a randomised controlled trial. NeuroRehabilitation. 2012;31:357–365. doi: 10.3233/NRE-2012-00804. [DOI] [PubMed] [Google Scholar]
- Roze E, Harris PA, Ball G, Elorza LZ, Braga RM, Allsop JM, Merchant N, Porter E, Arichi T, Edwards AD, Rutherford MA, Cowan FM, Counsell SJ. Tractography of the corticospinal tracts in infants with focal perinatal injury: comparison with normal controls and to motor development. Neuroradiology. 2012;54:507–516. doi: 10.1007/s00234-011-0969-5. [DOI] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Seme-Ciglenecki P. Predictive values of cranial ultrasound and assessment of general movements for neurological development of preterm infants in the Maribor region of Slovenia. Wien Klin Wochenschr. 2007;119:490–496. doi: 10.1007/s00508-007-0839-7. [DOI] [PubMed] [Google Scholar]
- Son SM, Ahn YH, Sakong J, Moon HK, Ahn SH, Lee H, Yu IK, Shin YJ, Jang SH. Diffusion tensor imaging demonstrates focal lesions of the corticospinal tract in hemiparetic patients with cerebral palsy. Neurosci Lett. 2007;420:34–38. doi: 10.1016/j.neulet.2007.04.054. [DOI] [PubMed] [Google Scholar]
- Son SM, Park SH, Moon HK, Lee E, Ahn SH, Cho YW, Byun WM, Jang SH. Diffusion tensor tractography can predict hemiparesis in infants with high risk factors. Neurosci Lett. 2009;451:94–97. doi: 10.1016/j.neulet.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Sreenan C, Bhargava R, Robertson CM. Cerebral infarction in the term newborn: clinical presentation and long-term outcome. J Pediatr. 2000;137:351–355. doi: 10.1067/mpd.2000.107845. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner SF, Ramenghi LA, Ridgway JP, Berry E, Saysell MA, Martinez D, Arthur RJ, Smith MA, Levene MI. Quantitative comparison of intrabrain diffusion in adults and preterm and term neonates and infants. AJR Am J Roentgenol. 2000;174:1643–1649. doi: 10.2214/ajr.174.6.1741643. [DOI] [PubMed] [Google Scholar]
- Trivedi R, Gupta RK, Shah V, Tripathi M, Rathore RK, Kumar M, Pandey CM, Narayana PA. Treatment-induced plasticity in cerebral palsy: a diffusion tensor imaging study. Pediatr Neurol. 2008;39:341–349. doi: 10.1016/j.pediatrneurol.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Shah V, Goyel P, Paliwal VK, Rathore RK, Gupta RK. Correlation of quantitative sensorimotor tractography with clinical grade of cerebral palsy. Neuroradiology. 2010;52:759–765. doi: 10.1007/s00234-010-0703-8. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wiklund LM, Uvebrant P. Hemiplegic cerebral palsy: correlation between CT morphology and clinical findings. Dev Med Child Neurol. 1991;33:512–523. doi: 10.1111/j.1469-8749.1991.tb14916.x. [DOI] [PubMed] [Google Scholar]
- Williams J, Lee KJ, Anderson PJ. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52:232–237. doi: 10.1111/j.1469-8749.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Hayakawa K, Yamamoto A, Okano S, Kanda T, Yamori Y, Yoshida N, Hirota H. Quantitative diffusion tensor tractography of the motor and sensory tract in children with cerebral palsy. Dev Med Child Neurol. 2010;52:935–940. doi: 10.1111/j.1469-8749.2010.03669.x. [DOI] [PubMed] [Google Scholar]
- Yu C, Shu N, Li J, Qin W, Jiang T, Li K. Plasticity of the corticospinal tract in early blindness revealed by quantitative analysis of fractional anisotropy based on diffusion tensor tractography. Neuroimage. 2007;36:411–417. doi: 10.1016/j.neuroimage.2007.03.003. [DOI] [PubMed] [Google Scholar]


