Abstract
Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) represent a promising young-state stem cell source for cell-based therapy. hUCMSC transplantation into the transected sciatic nerve promotes axonal regeneration and functional recovery. To further clarify the paracrine effects of hUCMSCs on nerve regeneration, we performed human cytokine antibody array analysis, which revealed that hUCMSCs express 14 important neurotrophic factors. Enzyme-linked immunosorbent assay and immunohistochemistry showed that brain-derived neurotrophic factor, glial-derived neurotrophic factor, hepatocyte growth factor, neurotrophin-3, basic fibroblast growth factor, type I collagen, fibronectin and laminin were highly expressed. Treatment with hUCMSC-conditioned medium enhanced Schwann cell viability and proliferation, increased nerve growth factor and brain-derived neurotrophic factor expression in Schwann cells, and enhanced neurite growth from dorsal root ganglion explants. These findings suggest that paracrine action may be a key mechanism underlying the effects of hUCMSCs in peripheral nerve repair.
Keywords: nerve regeneration, human umbilical cord-derived mesenchymal stem cells, conditioned medium, Schwann cells, dorsal root ganglion, axons, peripheral nerve regeneration, neurotrophic factors, neural regeneration
Introduction
Cell-based therapy is a promising strategy for improving peripheral nerve regeneration. Schwann cells and mesenchymal stem cells (MSCs) are commonly used in cell-based therapies for neural injuries and disorders (Alsanie et al., 2013; Hsu et al., 2013). Although Schwann cells are the most important support cells in tissue grafting, their clinical use is limited because autologous Schwann cells are difficult to obtain and amplify, and they rapidly lose their phenotypic characteristics (Kingham et al., 2007). MSCs are multipotent, tissue-specific stem cells that are easily isolated (Zhu et al., 2014). MSCs exert trophic, anti-inflammatory and immunomodulatory effects. These characteristics make MSCs the most attractive support cell in cell-based therapy for neurological diseases (Caplan, 2009).
Bone marrow is the main autologous source of MSCs used to treat nerve injury (Huang et al., 2014; Ma, 2014). Human bone marrow mesenchymal stem cell transplantation was used for treating neurological diseases in several clinical trials with encouraging early or long-term results (Kumar et al., 2009; Dai et al., 2013; Jiang et al., 2013). However, some patients may not be able to use their own cells because of age or underlying diseases (Romanov et al., 2003). Human umbilical cord-derived MSCs (hUCMSCs) represent a “younger” stem cell type and have higher proliferation rates and greater expansion capacity than bone marrow mesenchymal stem cells in vitro. hUCMSCs are regarded as an alternative source of bone marrow mesenchymal stem cells because of favorable intrinsic properties, such as high self-renewal capacity (Li et al., 2014; Zhang et al., 2014). Additionally, their use is ethically less problematic, and they are abundantly available, hypo-immunogenic and non-tumorigenic (Fong et al., 2014). A number of experimental and clinical trials have examined the efficacy and safety of treating neurological diseases with hUCMSCs (Bongso and Fong, 2013; Cui et al., 2014). However, the mechanisms underlying the ability of hUCMSCs to enhance nerve regeneration remain to be elucidated.
Wang et al. (2009) analyzed the beneficial effects of MSCs on peripheral nerve regeneration. Their findings suggested that cell replacement, growth factor production, extracellular matrix molecule synthesis, immune modulation, and other factors may be involved. Thus, MSCs may contribute to nerve regeneration by secreting growth factors and depositing basal lamina components, thereby establishing a favorable microenvironment for nerve regeneration (Caplan and Dennis, 2006). In the present study, we investigated the paracrine effects of hUCMSCs by analyzing neurotrophic factor expression and extracellular matrix production by these cells. Furthermore, we examined the effects of hUCMSC-conditioned medium on Schwann cell properties and neurite outgrowth from dorsal root ganglia.
Materials and Methods
hUCMSC culture and identification
A total of 18 healthy human umbilical cords were obtained from healthy mothers at the General Hospital of Chinese PLA following their informed consent. All experimental procedures were approved by the Institutional Ethics Committee, Chinese PLA General Hospital in China. hUCMSCs were isolated and cultured as previously described (Peng et al., 2011). The hUCMSCs were cultured for expansion in Dulbecco's modified Eagle's medium (DMEM)/F12, containing 10% fetal bovine serum and 100 U/mL penicillin/streptomycin, at 37°C in a 5% CO2 incubator. When the cultures reached 80% confluence, cells were resuspended with 0.25% ethylenediamine tetraacetic acid and reseeded in new culture flasks. Single-cell suspensions were washed three times in PBS, counted, and adjusted to the appropriate concentrations. 106 cells per sample were stained with anti-CD29, CD44, CD90, CD105, CD71, CD73, CD34 and CD45 antibodies (1:1,000; BD Pharmingen, San Diego, CA, USA). A FACScan machine (Gilson, Middleton, WI, USA) was used to analyze antibody binding. To evaluate the multipotent differentiation capacity of stem cells, second passage hUCMSCs were treated with osteogenic induction medium, adipogenic induction medium or chondrogenic medium for 21 days as described previously (Liu et al., 2012). After the differentiation process was completed, cells were stained with Alizarin red for osteoblasts, Oil-red O for adipocytes, and Safranin O for chondrocytes.
Extracellular matrix components deposited by hUCMSCs
To identify the extracellular matrix components deposited by hUCMSCs, we reconstituted the 3D cell-free extracellular matrix microenvironment as previously described (Lai et al., 2010). hUCMSC-derived extracellular matrix on coverslips was fixed with 4% formaldehyde and incubated with mouse anti-human collagen type I monoclonal antibody, mouse anti-human laminin monoclonal antibody or mouse anti-human fibronectin monoclonal antibody (1:200; Sigma, St. Louis, MO, USA) overnight at 4°C in a humidified chamber. After washing three times with PBS, the samples were conjugated with FITC-labeled goat anti-mouse IgG (1:250; Beijing Zhongshan Biological Reagent Company, Beijing, China) for 2 hours at room temperature. The stained samples were examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Human cytokine antibody array analysis
Two hUCMSC cell lines were expanded to 107 cells and harvested at passage 2. Proteins were isolated and assayed with a RayBioHuman Cytokine Antibody ArrayKit (R&D Systems, Minneapolis, MN, USA). The membrane was blocked with blocking buffer, incubated with the protein sample for 1–2 hours, and then incubated with diluted biotin-conjugated antibodies (ready to use) and horseradish peroxidase-conjugated streptavidin for 2 hours. After incubation with detection buffer, the membrane was exposed to X-ray film, and signals were detected using a chemiluminescence imaging system, Fluorchem 9000 (Alpha Innotech Corp., San Leandro, CA, USA). Relative cytokine expression levels were quantified by densitometry.
hUCMSC conditioned medium preparation and enzyme-linked immunosorbent assay (ELISA)
Second passage hUCMSCs were seeded in DMEM/F12 containing 10% fetal bovine serum. After the cells reached 90% confluence, the medium was replaced with DMEM/F12 medium containing 1% fetal bovine serum, followed by culture for an additional 48 hours at 37°C in a humidified 5% CO2 incubator. The hUCMSC-conditioned medium was collected, filtered through a 0.22-μm filter, and immediately frozen at −80°C until use. DMEM/F12 medium containing 1% fetal bovine serum was used as a control. The conditioned media were analyzed using human brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), hepatocyte growth factor (HGF), neurotrophin-3 (NT-3), basic fibroblast growth factor (bFGF), nerve growth factor (NGF)-β and vascular endothelial growth factor (VEGF) ELISA kits (Boster, Wuhan, China) according to the manufacturer's protocols. All samples were analyzed in triplicate, and the optical density was measured at 450 nm (Wellscan MK3, Labsystems Dragon, Helsinki, Finland).
Schwann cell harvest and culture with hUCMSC-conditioned medium
Schwann cells were harvested from the sciatic nerves of specific-pathogen-free 3-day-old Sprague-Dawley rats (provided by Animal Center of Military Academy of Medical Sciences of Chinese PLA; license No. SCXK (Jun) 2012-0004) as previously described (Gu et al., 2012). To determine Schwann cell viability in hUCMSC-conditioned medium, MTT assay was performed as previously described (Yang et al., 2008). Briefly, Schwann cells (1 × 104 cells/well) were seeded into 96-well plates and incubated with hUCMSC-conditioned medium or control medium (DMEM/F12 containing 1% fetal bovine serum) for 12, 24 or 48 hours. Optical density was measured using a microplate reader (Beckman, Brea, CA, USA) at 570 nm. Five replicates from each sample were measured.
EdU/Hoechst 33342 double staining
According to the EdU Labeling/Detection Kit manual (Ribobio, Guangzhou, China), Schwann cells were cultured in 24-well plates at 5 × 104 cells per well and incubated in 10 μM EdU labeling medium for 48 hours at 37°C in a humidified 5% CO2 incubator. At the appropriate time, cultured cells were fixed with 4% paraformaldehyde for 30 minutes. After three PBS washes, staining was performed with 200 μL of 1 × Apollo® reaction cocktail at 37°C for 30 minutes. Following permeabilization with 0.2% Triton X-100 in PBS, the cells were stained with 5 g/mL Hoechst 33342 dye for 30 minutes, and observed under a fluorescence microscope (Olympus). The percentage of EdU-positive cells was calculated.
Western blot assay
Schwann cells were cultured in hUCMSC-conditioned medium or control medium for 48 hours. Total protein concentration was then determined. Protein samples were separated at 120 V on 10% gels by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then the separated proteins were transferred onto polyvinylidene difluoride membranes and incubated with rabbit anti-human NGF-β monoclonal antibody (1:500; Chemicon, Temecula, CA, USA), rabbit anti-human BDNF monoclonal antibody (1:500; Chemicon) or mouse anti-β-actin antibody (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000, Santa Cruz Biotechnology) or goat anti-mouse IgG (1:1,000, Santa Cruz Biotechnology) for 1 hour, treated with an enhanced chemiluminescence substrate for 1 minute, and developed by exposure to Kodak X-OMAT light-sensitive film (Kodak, Rochester, NY, USA). Protein expression was expressed as the ratio of the optical density of the target protein to that of β-actin.
Dorsal root ganglion explant culture and immunofluorescence staining
Dorsal root ganglia were dissected from Sprague-Dawley rat pups on postnatal day 1 as previously described (Yang et al., 2007). Dorsal root ganglion explants were treated with hUCMSC-conditioned medium, negative control medium or 50 ng/mL NGF-β (positive control) for 72 hours. Neurite outgrowth from the explants was observed and analyzed using phase contrast microscopy (Olympus). Immunofluorescence staining was also performed. Dorsal root ganglia were fixed for 10 minutes in 4% paraformaldehyde at 4°C and washed three times with PBS. Dorsal root ganglia were immunostained overnight at 4°C with mouse anti-rat neurofilament 200 monoclonal antibody (1:500; Sigma) to identify axons. The ganglia were then incubated with Alexa 488-conjugated goat anti-mouse IgG (1:200; Beijing Zhongshan Biological Reagent Company) at room temperature for 2 hours. Immunostained axons were visualized under a fluorescence microscope (Olympus), and images were captured using a digital camera (Olympus).
Statistical analysis
All data are expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance followed by Tukey's test using SPSS 17.0 software package (SPSS, Chicago, IL, USA). A P-value < 0.05 was considered statistically significant.
Results
Biological characterization of hUCMSCs
Figure 1A shows a phase contrast image of passage 2 hUCMSCs, which are adherent and display a fibroblast-like shape. Passage 2 hUCMSCs cultured with different media were characterized by the presence of different cell types due to their multi-differentiation potential; osteogenic (Figure 1B), adipogenic (Figure 1C) and chondrogenic (Figure 1D) cells were revealed by Alizarin red staining, Oil-red O staining and Safranin O staining, respectively. Figure 1E illustrates that isolated hUCMSCs were positive for the mesenchymal markers CD29, CD44, CD90, CD105, CD71 and CD73, but negative for CD34 (hematopoietic/endothelial marker) and CD45 (hematopoietic marker).
Figure 1.
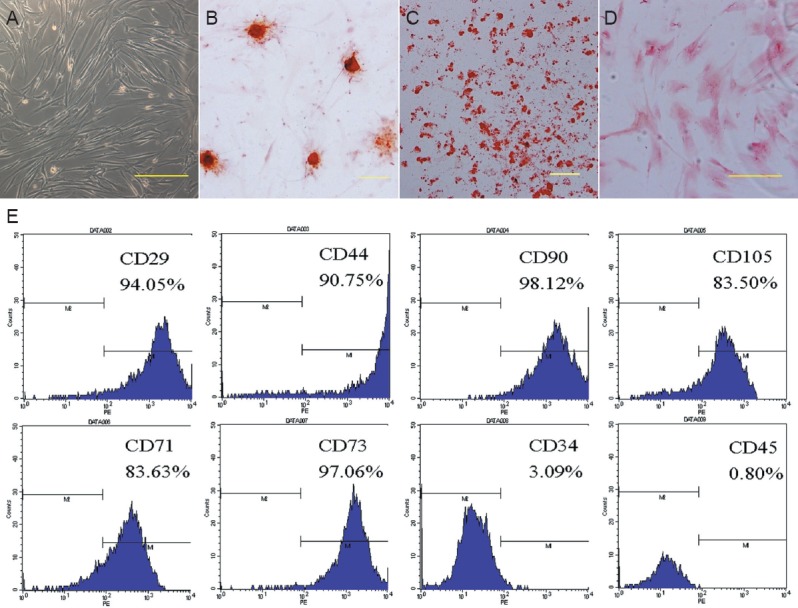
Biological characteristics of human umbilical cord mesenchymal stem cells (hUCMSCs).
(A) Phase contrast images of passage 2 hUCMSCs. hUCMSCs can be differentiated into osteogenic (B; Alizarin red staining), adipogenic (C; Oil-red O staining) and chondrogenic lineages (D; Safranin O staining) following treatment with different culture media. Scale bars in A–D: 200 μm. (E) Flow cytometry showing that hUCMSCs are positive for CD29, CD44, CD90, CD105, CD71 and CD73, and negative for CD34 and CD45.
hUCMSCs expressed extracellular matrix and neurotrophic proteins related to neurogenesis
We analyzed the composition of extracellular matrix deposited by hUCMSCs using immunostaining. As shown in Figure 2A, immunostaining revealed the presence of collagen type I, laminin and fibronectin, which are reportedly involved in peripheral nerve regeneration (Gao et al., 2013). Figure 2B summarizes the results of human cytokine antibody array analysis for approximately 79 proteins. As shown, 14 growth factors were expressed by hUCMSCs at highly significant levels. Of particular interest were BDNF, NT-3, neurotrophin-4/5 (NT-4/5), GDNF, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), leukemia inhibitory factor (LIF), insulin-like growth factor-1 (IGF-1), transforming growth factor-β (TGF-β), interleukin-6 (IL-6), neutrophil activating protein-2 (NAP-2), VEGF, HGF and stem cell factor (SCF). To quantify the levels of these secreted proteins more precisely, hUCMSC-conditioned medium was assayed using ELISA. As shown in Figure 2C, D, all samples of hUCMSC-conditioned medium assayed contained high levels of BDNF, GDNF, HGF, NT-3 and bFGF. NGF-β and VEGF were secreted at low levels.
Figure 2.
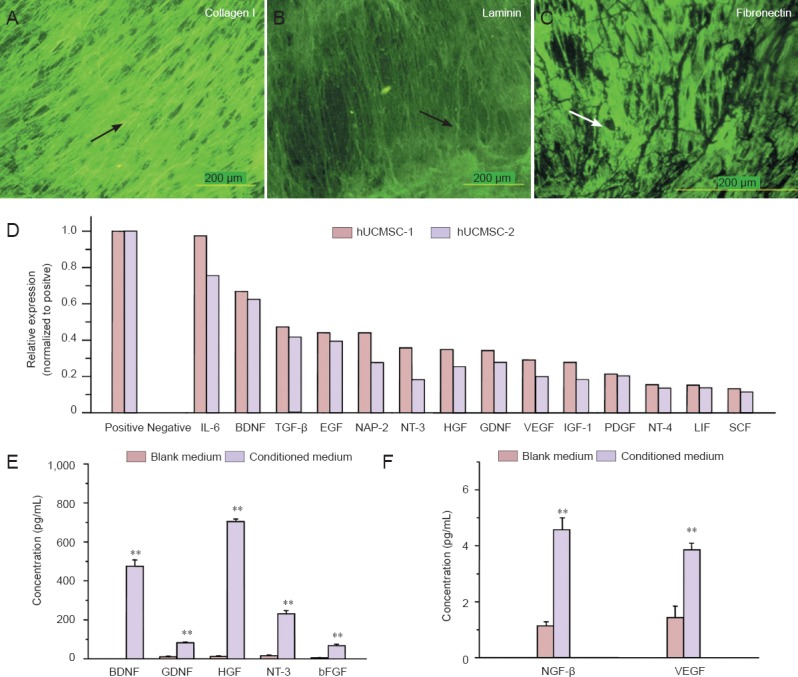
hUCMSCs express and secrete extracellular matrix and neurotrophic factors that enhance nerve regeneration.
(A–C) Extracellular matrix components deposited by hUCMSCs were visualized by immunofluorescence staining. Arrows indicate positive expression. FITC was the dye. Scale bars: 200 μm. (D) Cytokine antibody array assay revealed neurotrophic factor expression. hUCMSC-1 and hUCMSC- 2 represent cultures derived from different umbilical cords. (E, F) BDNF, GDNF, HGF, NT-3, bFGF, NGF-β and VEGF protein levels were detected in hUCMSC-conditioned medium and control medium by ELISA. All data are expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance followed by Tukey's test. **P < 0.01, vs. control (blank) medium. hUCMSCs: Human umbilical cord-derived mesenchymal stem cells; IL-6: interleukin-6; BDNF: brain-derived neurotrophic factor; TGF-β: tumor growth factor-β; EGF: epidermal growth factor; NAP-2: neutrophil activating protein-2; NT-3: neurotrophin-3; HGF: hepatocyte growth factor; GDNF: glial-derived neurotrophic factor; VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor-1; PDGF: platelet-derived growth factor; NT-4: neurotrophin-4; LIF: leukemia inhibitory factor; SCF: stem cell factor; bFGF: basic fibroblast growth factor; NGF-β: nerve growth factor-β; ELISA: enzyme linked immunosorbent assay.
Effect of hUCMSC-conditioned medium on rat Schwann cell culture
As shown by the MTT assay, 12 hours of treatment with hUCMSC-conditioned medium did not significantly change Schwann cell viability compared with control medium treatment. However, 24 and 48 hours of treatment with hUCMSC-conditioned medium significantly increased Schwann cell viability compared with control medium (Figure 3A). EdU/Hoechst immunostaining showed that 48 hours of treatment with hUCMSC-conditioned medium increased the proliferation rate of Schwann cells compared with control medium (Figure 3B, C). Western blot analysis revealed that NGF-β and BDNF protein levels were significantly higher in Schwann cells cultured with hUCMSC-conditioned medium for 48 hours compared with control medium (Figure 3D–F).
Figure 3.
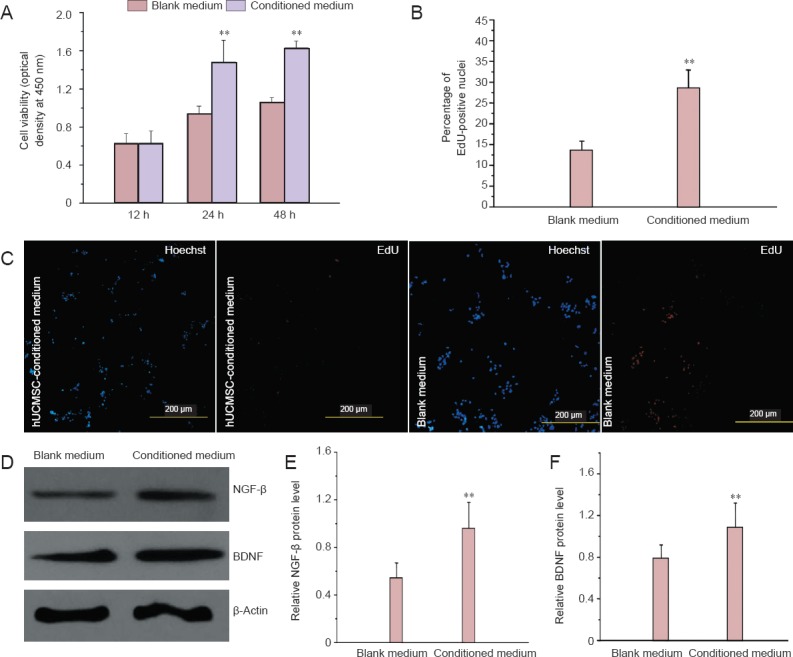
Effects of hUCMSC-conditioned medium on Schwann cells.
(A) Viability of Schwann cells cultured in hUCMSC-conditioned medium or control medium (MTT assay). (B) The percentage of proliferating Schwann cells cultured in hUCMSC-conditioned medium and control medium for 48 hours (EdU/Hoechst immunostaining). (C) EdU/Hoechst double staining of Schwann cells treated with control medium and hUCMSC-conditioned medium for 48 hours. Scale bars: 200 μm. (D–F) NGF-β and BDNF protein levels in Schwann cells cultured in hUCMSC-conditioned medium or control medium for 48 hours. All data are expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance followed by Tukey's test. **P < 0.01, vs. blank medium. hUCMSCs: Human umbilical cord-derived mesenchymal stem cells; MTT: 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; h: hours.
Effect of hUCMSC-conditioned medium on rat dorsal root ganglion explants
Rat dorsal root ganglion explants were cultured in control medium, hUCMSC-conditioned medium or 50 ng/mL NGF-containing medium for 3 days. Phase contrast imaging and neurofilament 200 immunostaining revealed the extent of neurite outgrowth from dorsal root ganglion explants in the three different groups (Figure 4A). A comparison of cumulative neurite lengths from cultured dorsal root ganglion explants indicated that neurites grew faster in both hUCMSC-conditioned medium and 50 ng/mL NGF-containing medium than in control medium (P < 0.01; Figure 4B). hUCMSC-conditioned medium treatment of dorsal root ganglion explants for 72 hours significantly increased the mean axon area ratio (axon area/DRG area) compared with control medium treatment. The mean axon area ratio of hUCMSC-conditioned medium-treated explants was similar to that of 50 ng/mL NGF-treated explants (P < 0.01; Figure 4C).
Figure 4.
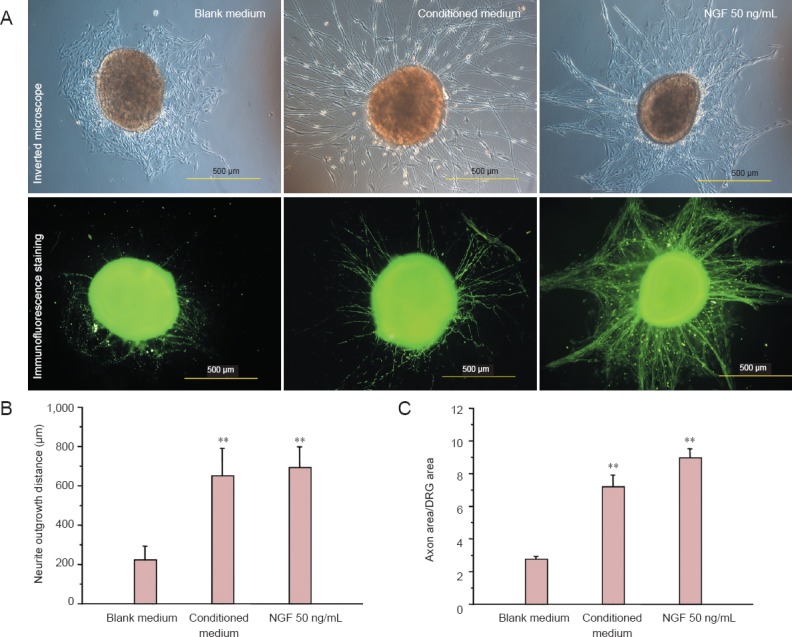
Effect of hUCMSC-conditioned medium on neurite outgrowth from DRG explants.
(A) Morphology of DRG explants and staining for NF200 after 72-hour treatment with control medium, hUCMSC-conditioned medium or 50 ng/mL NGF. Scale bars: 500 μm. The neurite outgrowth distance (B) and percentage of axon area/DRG area (C) of the DRG explants were measured and calculated under a fluorescence microscope. All data are expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance followed by Tukey's test. **P < 0.01, vs. blank medium. hUCMSCs: Human umbilical cord-derived mesenchymal stem cells; DRG: dorsal root ganglion; NF200: neurofilament 200; NGF: nerve growth factor.
Discussion
Numerous studies have shown that MSCs have beneficial effects on peripheral nerve reconstruction (Ribeiro et al., 2013). However, the underlying mechanisms remain unclear. Several studies have suggested that the transdifferentiation of MSCs plays a crucial role in peripheral nerve regeneration, because the implanted MSCs can differentiate into Schwann-like cells at the site of injury (Chen et al., 2006; Yang et al., 2009). However, some studies have questioned the transdifferentiation capability of MSCs, because the expression of Schwann cell phenotypic markers does not prove that these cells function as Schwann cells (Peng et al., 2011). Matsuse et al. (2010) demonstrated that after transplanting MSCs into the sciatic nerve stump, only a few MSCs could spontaneously differentiate into Schwann cells in vivo. The current consensus is that MSCs promote peripheral nerve through the release of cytokines, growth factors and neuroregulatory molecules.
To better understand the role of MSCs in peripheral nerve regeneration, we examined the paracrine actions of hUCMSCs on neural tissue and Schwann cells. We first performed a human cytokine antibody array assay to identify the proteins expressed by hUCMSCs. Approximately 79 proteins were identified, and we chose 14 proteins that were expressed at highly significant levels and that had previously reported neurotrophic properties, including BDNF, NT-3, NT-4/5, GDNF, PDGF, EGF, LIF, IGF-1, TGF-β, IL-6, NAP-2, VEGF, HGF and SCF. These proteins play important roles in enhancing angiogenesis and neurogenesis during the development and regeneration of peripheral nerves. NGF, BDNF, NT-3 and NT-4/5 play crucial roles in neuronal survival, differentiation and maintenance. NGF promotes the survival and differentiation of sensory and sympathetic neurons and is the prototypical neurotrophin (Truzzi et al., 2008). BDNF supports motor neuron survival and promotes axonal growth in motor and sensory neurons (Zhao et al., 2013). NT-3 supports the survival, growth and differentiation of neurons, and encourages neuronal synapse formation. NT-4/5 is a recently identified neurotrophin with potential neurotrophic effects on various neuronal subpopulations, and it promotes the survival of motor and sensory neurons (Shakhbazau et al., 2013).
In addition to the four members of the neurotrophin family, other growth factors with neurotrophic actions include GDNF and FGF. GDNF is a potent survival factor for midbrain dopaminergic neurons and many other types of neuronal populations (Dubový et al., 2011). FGF is a potent mitogen that may promote not only glial and Schwann cell proliferation but also angiogenesis to affect the development of both the central and peripheral nervous systems (Wang et al., 2008). Some other bioactive molecules with neurotrophin-like actions have also been tested to determine the possibility of their serving as additives in neural scaffolds. These factors include IGF-1, VEGF, LIF and PDGF (Verheyen et al., 2013).
Angiogenesis and the growth of new blood vessels also play important roles in nerve regeneration. VEGF stimulates axonal outgrowth, enhances the survival and proliferation of Schwann cells, and improves intraneural angiogenesis by promoting endothelial sprouting during peripheral nerve regeneration (Verheyen et al., 2013). HGF is a neurotrophic factor for motor, sensory and parasympathetic neurons in vitro. Li et al. (2008) reported that HGF has potent angiogenic and neuroregenerative effects in allogeneic grafting of peripheral nerves. All of these findings indicate that hUCMSCs secrete a variety of cytokines. ELISA was performed to analyze proteins secreted by hUCMSCs. The results revealed that neurotrophic factors, including BDNF, HGF, GDNF, NT-3 and bFGF, are expressed at significantly high levels; some other factors (NGF-β and VEGF) were secreted at low concentrations.
Neurotrophic factors and extracellular matrix proteins are two important growth-inducing factors that promote Schwann cell proliferation and stimulate neuronal neurite outgrowth. In addition to neurotrophic factors, we also examined the expression of extracellular matrix proteins secreted by hUCMSCs. Immunostaining results revealed that hUCMSCs deposited extracellular matrix containing collagen I, laminin and fibronectin. Collagen, laminin and fibronectin have been shown to play important roles in axonal development and growth. Our findings indicate that hUCMSC-derived extracellular matrix may contribute to the ability of these cells to promote peripheral nerve regeneration.
Bone marrow mesenchymal stem cells promote the proliferation of Schwann cells and enhance neuronal survival in co-culture systems in vitro, likely by secreting soluble factors and extracellular matrix proteins (Wang et al., 2009). Different MSC sources have different secretory factor profiles. In this study, we observed that hUCMSC-conditioned medium increased Schwann cell viability and proliferation, enhanced NGF and BDNF protein expression in Schwann cells, and promoted neurite outgrowth from dorsal root ganglia. Schwann cells are the primary structural and functional cells in the peripheral nervous system and play a crucial role in peripheral nerve regeneration, and can secrete various neurotrophic factors to promote axon growth. Our results demonstrate that the paracrine effects of hUCMSCs maintain cell viability, and also promote growth factor secretion by Schwann cells. These effects synergistically enhance nerve regeneration.
In conclusion, hUCMSCs secrete various neurotrophic factors and deposit extracellular matrix proteins to modulate Schwann cell behavior and promote neurite outgrowth. Our findings suggest that paracrine mechanisms possibly underlie the effectiveness of hUCMSC-based cell therapy in the treatment of peripheral nerve injuries.
Footnotes
Funding: This research was supported by the National Natural Science Foundation of China, No. 31100696, 31170946; a grant from the National High Technology Research and Development Program of China (863 Program), No. 2012AA020502; a grant from the National Program on Key Basic Research Project of China (973 Program), No. 2014CB542201; and a grant from Beijing Metropolis Beijing Nova Program, No. 2011115.
Conflicts of interest: None declared.
Copyedited by Patel B, Norman C, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Alsanie WF, Niclis JC, Petratos S. Human embryonic stem cell- derived oligodendrocytes: protocols and perspective. Stem Cells Dev. 2013;22:2459–2476. doi: 10.1089/scd.2012.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's jelly of the human umbilical cord. Stem Cell Rev. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang XD, Chen G, Lin WW, Yao J, Gu XS. Study of in vivo differentiation of rat bone marrow stromal cells into schwann cell-like cells. Microsurgery. 2006;26:111–115. doi: 10.1002/micr.20184. [DOI] [PubMed] [Google Scholar]
- Cui B, Li E, Yang B, Wang B. Human umbilical cord blood-derived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp Ther Med. 2014;7:1233–1236. doi: 10.3892/etm.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Dubový P, Raška O, Klusαkovα I, Stejskal L, Celakovský P, Haninec P. Ciliary neurotrophic factor promotes motor reinnervation of the musculocutaneous nerve in an experimental model of end-to-side neurorrhaphy. BMC Neurosci. 2011;12:58. doi: 10.1186/1471-2202-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong CY, Tam K, Cheyyatraivendran S, Gan SU, Gauthaman K, Armugam A, Jeyaseelan K, Choolani M, Biswas A, Bongso A. Human Wharton's jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J Cell Biochem. 2014;115:290–302. doi: 10.1002/jcb.24661. [DOI] [PubMed] [Google Scholar]
- Gao X, Wang Y, Chen J, Peng J. The role of peripheral nerve ECM components in the tissue engineering nerve construction. Rev Neurosci. 2013;24:443–453. doi: 10.1515/revneuro-2013-0022. [DOI] [PubMed] [Google Scholar]
- Gu Y, Ji Y, Zhao Y, Liu Y, Ding F, Gu X, Yang Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials. 2012;33:6672–6681. doi: 10.1016/j.biomaterials.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Chen SL, Wang DY, Chiu IM. Stem cell-based therapy in neural repair. Biomed J. 2013;36:98–105. doi: 10.4103/2319-4170.113226. [DOI] [PubMed] [Google Scholar]
- Huang JF, Huang JF, Zhang WC. Bone marrow mesenchymal stem cells differentiate into neuron-like cells induced by combination of two cytokines. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:829–834. [Google Scholar]
- Jiang PC, Xiong WP, Wang G, Ma C, Yao WQ, Kendell SF, Mehling BM, Yuan XH, Wu DC. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp Ther Med. 2013;6:140–146. doi: 10.3892/etm.2013.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7:241–248. [PubMed] [Google Scholar]
- Lai Y, Sun Y, Skinner CM, Son EL, Lu Z, Tuan RS, Jilka RL, Ling J, Chen XD. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010;19:1095–1107. doi: 10.1089/scd.2009.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Yao XY, Yang LM, Zhao PW, Zhang GH. Induced differentiation of human umbilical cord mesenchymal stem cells into neural stem cells in vitro. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:75–80. [Google Scholar]
- Li Z, Peng J, Wang G, Yang Q, Yu H, Guo Q, Wang A, Zhao B, Lu S. Effects of local release of hepatocyte growth factor on peripheral nerve regeneration in acellular nerve grafts. Exp Neurol. 2008;214:47–54. doi: 10.1016/j.expneurol.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Liu S, Yuan M, Hou K, Zhang L, Zheng X, Zhao B, Sui X, Xu W, Lu S, Guo Q. Immune characterization of mesenchymal stem cells in human umbilical cord Wharton's jelly and derived cartilage cells. Cell Immunol. 2012;278:35–44. doi: 10.1016/j.cellimm.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Ma HY. Bone marrow mesenchymal stem cell transplantation combined with edaravone inhibits neuronal apoptosis after cerebral infarction. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:1615–1620. [Google Scholar]
- Matsuse D, Kitada M, Kohama M, Nishikawa K, Makinoshima H, Wakao S, Fujiyoshi Y, Heike T, Nakahata T, Akutsu H, Umezawa A, Harigae H, Kira J, Dezawa M. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J Neuropathol Exp Neurol. 2010;69:973–985. doi: 10.1097/NEN.0b013e3181eff6dc. [DOI] [PubMed] [Google Scholar]
- Peng J, Wang Y, Zhang L, Zhao B, Zhao Z, Chen J, Guo Q, Liu S, Sui X, Xu W, Lu S. Human umbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84:235–243. doi: 10.1016/j.brainresbull.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Ribeiro J, Gartner A, Pereira T, Gomes R, Lopes MA, Gonçalves C, Varejão A, Luís AL, Maurício AC. Perspectives of employing mesenchymal stem cells from the Wharton's jelly of the umbilical cord for peripheral nerve repair. Int Rev Neurobiol. 2013;108:79–120. doi: 10.1016/B978-0-12-410499-0.00004-6. [DOI] [PubMed] [Google Scholar]
- Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- Shakhbazau A, Mohanty C, Shcharbin D, Bryszewska M, Caminade AM, Majoral JP, Alant J, Midha R. Doxycycline-regulated GDNF expression promotes axonal regeneration and functional recovery in transected peripheral nerve. J Control Release. 2013;172:841–851. doi: 10.1016/j.jconrel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol. 2008;128:2031–2040. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- Verheyen A, Peeraer E, Lambrechts D, Poesen K, Carmeliet P, Shibuya M, Pintelon I, Timmermans JP, Nuydens R, Meert T. Therapeutic potential of VEGF and VEGF-derived peptide in peripheral neuropathies. Neuroscience. 2013;244:77–89. doi: 10.1016/j.neuroscience.2013.03.050. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding F, Gu Y, Liu J, Gu X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009;1262:7–15. doi: 10.1016/j.brainres.2009.01.056. [DOI] [PubMed] [Google Scholar]
- Wang ZL, Cheng SM, Ma MM, Ma YP, Yang JP, Xu GL, Liu XF. Intranasally delivered bFGF enhances neurogenesis in adult rats following cerebral ischemia. Neurosci Lett. 2008;446:30–35. doi: 10.1016/j.neulet.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wu H, Hu N, Gu X, Ding F. Effects of bone marrow stromal cell-conditioned medium on primary cultures of peripheral nerve tissues and cells. Neurochem Res. 2009;34:1685–1694. doi: 10.1007/s11064-009-9963-2. [DOI] [PubMed] [Google Scholar]
- Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, Yang F, Wang S, Xu W, Wang A, Lu S. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–2387. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen X, Ding F, Zhang P, Liu J, Gu X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials. 2007;28:1643–1652. doi: 10.1016/j.biomaterials.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Xiao Q, Lv G. Chitosan conduits with human umbilical cord mesenchymal stem cells induce differentiation and growth of the nerve lateral bud. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:6985–6989. [Google Scholar]
- Zhao T, Yan W, Xu K, Qi Y, Dai X, Shi Z. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy. 2013;15:792–804. doi: 10.1016/j.jcyt.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Tao HR, Shen ZL. Rat's Schwann cells induce neural differentiation of human umbilical cord mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7250–7254. [Google Scholar]


