Abstract
Connexin subunits are proteins that form gap junction channels, and play an important role in communication between adjacent cells. This review article discusses the function of connexins/hemichannels/gap junctions under physiological conditions, and summarizes the findings regarding the role of connexins/hemichannels/gap junctions in the physiological and pathological mechanisms underlying central nervous system diseases such as brain ischemia, traumatic brain and spinal cord injury, epilepsy, brain and spinal cord tumor, migraine, neuroautoimmune disease, Alzheimer's disease, Parkinson's disease, X-linked Charcot-Marie-Tooth disease, Pelizaeus-Merzbacher-like disease, spastic paraplegia and maxillofacial dysplasia. Connexins are considered to be a potential novel target for protecting the central nervous system.
Keywords: connexin, gap junction, biosynthetic pathways, biodegradation, brain, central nervous system diseases
Introduction
The majority of cell types that compose the neurovascular unit, including neurons, astrocytes and endothelial cells, have been found to express connexins (Cx), which play vital functions in the human central nervous system (CNS). Since Paul (1986) cloned and sequenced the first Cx in 1986, the Cx gene family has been found to constitute 19 members in mouse and 20 in human, 19 of which can be grouped as sequence-orthologous pairs (Willecke et al., 2002). Cxs are commonly named according to their molecular weight (e.g., Cx43 has a molecular weight of 43 kDa) predicted from their primary amino acid sequence (Beyer et al., 1987). All Cxs have a topology composed of four membrane spanning regions, two extracellular loops, a cytoplasmic loop and cytoplasmic termini (Kumar and Gilula, 1996). Their primary function is to form jap junctions (GJs) and directly exchange ions and small molecules between adjacent cells (Leithe et al., 2012). Cxs have a short half-life of only a few hours, which is responsible for responding to physiological requirements to either up- or down-regulate the extent of GJ channel expression (Laird, 2006). This function appears to be essential for Cx-mediated gap junctional intercellular communication (GJIC) (Guo et al., 2003), which implies that modulation of Cx synthesis and degradation rate may be important for control of GJ levels under physiological or pathophysiological conditions (Laird, 2010). The most well established function of Cxs is to form GJs between adjacent cells. This network determines how easily small molecules diffuse by GJIC and how far locally initiated signals can be spread (Anders et al., 2014). Cxs can also form hemichannels (HCs) to exchange ions and signaling molecules between the intra- and extra-cellular environments. HCs can serve as autocrine/paracrine cellular communication pathways (Vega et al., 2013). Furthermore, Cxs have critical effects on cell adhesion, motility and migration that do not involve intercellular channels (Matsuuchi and Naus, 2013). Thus, Cxs/HCs/GJs have complex functions depending on the differing environments. Despite the fact that these functions remain to be fully elucidated, emerging evidence suggests a critical role of their dysfunction in the pathogenesis of CNS diseases (Seifert et al., 2006). In this review, we will discuss the function of Cxs/HCs/GJs under physiological conditions. Additionally, we will describe recent findings of diseases associated with Cxs/HCs/GJs in the CNS. An improved understanding of the function of Cxs/HCs/GJs in CNS pathogenesis offers the potential for development of novel strategies to treat neurological disorders.
Biosynthesis and Degradation of Cxs
Cx biosynthesis
Similar to other plasma membrane proteins, Cxs are delivered to the cell surface through the secretory pathway (Berthoud et al., 2004). Cxs are thought to be co-translationally thread into the rough endoplasmic reticulum (ER) via the translocon and encoded start and stop transfer sequences (Laird, 2006). The majority of Cxs are subsequently delivered through the Golgi compartment. On their transit from the ER through the trans-Golgi network, Cxs are oligomerized into hexamers (or connexons). Proper folding and oligomerization of Cxs appear to be important steps for quality control systems. If newly synthesized Cxs are not correctly folded, they may be expelled in a proteasome-dependent manner via a process termed ER-associated degradation (Berthoud et al., 2004). At least 40% of newly synthesized wild-type Cx43 is estimated to undergo this process, which has been hypothesized to be a mechanism for regulating GJIC under physiological and pathological conditions (VanSlyke and Musil, 2002; Kelly et al., 2007). Connexon-containing vesicles are transported from the trans-Golgi network to the plasma membrane. Once they reach and fuse with the plasma membrane, some connexons dock with each other in the adjacent cells and become parts of GJ plaques (Berthoud et al., 2004; Laird, 2006). Cxs can also form HCs that communicate with the extracellular space and maintain the integrity of the lumens of these intracellular compartments (Froger et al., 2010). There is strong evidence to suggest that microtubules facilitate Cx trafficking to improve the efficiency of the delivery process, although they do not appear to be essential (Johnson et al., 2002; Lauf et al., 2002).
Cx degradation
The half-life of Cxs is very short, which indicates a rapid rate of metabolism. Gaietta et al. (2002) first reported that newly synthesized Cxs were transported to the plasma membrane and incorporated at the periphery of existing GJs, whereas old Cxs were removed to the central core. These finding suggest that the older Cxs in the center of GJs were likely to be destined for internalization and degradation in the cytoplasm. Approximately two decades earlier, researchers discovered that the entire GJs or fragments may form double-membrane circular structures. These structures named “annular junctions” were bound to be degraded (Severs et al., 1989). A later study revealed that annular junctions originated from pre-existing GJ plaques between contacting cells (Jordan et al., 2001), and they were thus renamed ‘connexosomes’ to reflect their Cx-rich status (Laird, 2006). The connexosomes internalize into one of two opposing cells to form double-membrane GJ vesicles, and are quite large and structurally different from typical endocytic organelles. Therefore, the internalization mechanism is likely to be distinct from conventional endocytic processes such as classical endosomes orphagosomes (Hesketh et al., 2010). It is now known that Cxs are internalized by at least two distinct pathways. Piehl et al. (2007) reported that complete or large portions of GJ plaques were subdivided into large cytoplasmic vesicles (0.5–5 μm in diameter) that were slowly degraded by endo/lysosomal pathways, which occurred over a period of 20–60 minutes. Another continuous and fast (few seconds) internalization mechanism was reported for small GJ vesicles (0.18–0.27 μm in diameter), which bud from the central regions of plaques and translocate much more rapidly (within minutes) into cells for degradation (Falk et al., 2009). After entering the early endosome, endocytosed proteins can be transported further downstream in the degradation pathway to the lysosome, to undergo recycling to the plasma membrane, or are transported to the trans-Golgi network (Scita and Di Fiore, 2010).
The Function of Cxs/HCs/GJs under Physiological Conditions
Channel-dependent function
The most well established function of Cxs is the formation of GJs (Figure 1). The term ‘GJ’ comes from histological studies, where they were first described by electron microscopy using heavy metal incubation (Revel and Karnovsky, 1967). GJ channels are large, poorly selective, aqueous pores that are assembled by the head-to-head docking of two half GJ channels or HCs, each delivered by one of the partner cells (De Bock et al., 2014a). Freeze-fracture analysis revealed that GJs can form GJ plaques that contain less than a dozen to up to 200,000 units, and which extend from several nanometers to a few micrometers in diameter (Bruzzone et al., 1997; Falk, 2000). GJ plaques composed of multiple GJ channels spanning the two plasma membranes are found in almost all tissues (Sosinsky and Nicholson, 2005). Hydrophilic molecules smaller than 1 kDa are able to travel through the GJ channels, which is critical for direct exchange of ions and small metabolites between adjacent cells (Alexander and Goldberg, 2003). It is widely believed that connexons also play critical functions in the exchange of ions and signaling molecules between the intra- and extracellular environment, which are often termed HCs (Stout et al., 2004; Spray et al., 2013). HCs are not incorporated into GJs, and they mainly allow direct contact between the cytosol and extracellular space. Under normal conditions, most HCs are primarily in a closed state, while they can open following stimuli such as mechanical stress, ischemia, inflammation and high extracellular calcium (Goodenough and Paul, 2003; Burra et al., 2010; Eugenin et al., 2012; Fiori et al., 2014). HC opening allows the free exchange of ions and small metabolic or signaling molecules with a molecular weight < 1–2 kDa in a bidirectional manner, which may contribute to paracrine signaling (Wang et al., 2013a).
Figure 1.
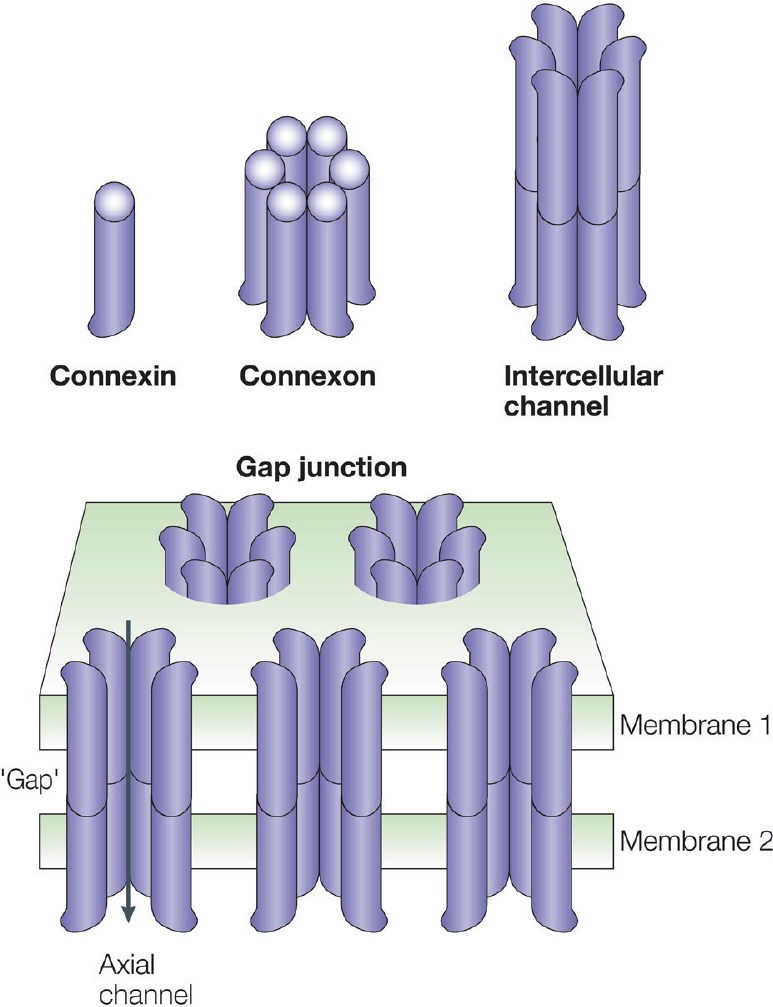
Connexin (Cx), connexon/hemichannel (HC) and intercellular channel and gap junction (GJ).
Schematic showing the relationships between the Cx monomer, the hexameric assembly of Cxs into an HC and the two HCs forming an intercellular channel. Clusters of intercellular channels are known as GJs, which provide an axial channel (arrow) that allows exchange of ions and small metabolites between adjacent cells. This figure is reproduced from Nature Reviews Molecular Cell Biology (Goodenough and Paul, 2003) with permission.
Channel-independent function
In addition to the established role of GJs and HCs, there are many channel-independent roles in cytoskeletal changes. These processes include alterations in cell morphology, the regulation of cell polarity and an influence on cell motility (Kameritsch et al., 2012; Matsuuchi and Naus, 2013). The underlying mechanism involved in these processes includes the initial breakdown of the existing actin cytoskeletal network matrix. Although the exact mechanistic details remain unclear, the C-terminal region of Cxs is thought to contain the majority of the regulatory and protein-protein interaction domains (Cina et al., 2009; Solan and Lampe, 2009). Extensive evidence also suggests that Cxs regulate cell growth via a variety of processes, which plays an important role in the control of gene expression and tumor formation (Kardami et al., 2007; Cronier et al., 2009). Although some of these effects may be linked to GJIC, increasing evidence indicates that at least some of these changes are the results of the interaction between Cxs and other scaffold or signaling molecules (Olbina and Eckhart, 2003; Fu et al., 2004).
Both Cx47 and Cx32 knock-out mice were found to develop profound CNS demyelination associated with gross tremors and tonic seizures that cause death by postnatal weeks 5–6 (Menichella et al., 2003). This study provided the first evidence that GJIC was crucial for normal CNS myelination. In addition, a recent study supported that Cx was a rather unexplored but promising target for influencing CNS barrier function (De Bock et al., 2014a). Nevertheless, the mechanisms underlying non-channel activities remain largely unknown, but likely reflect cell-type, tissue-type and Cx-type specificity of responses, as well as multiple protein-protein interactions.
The Function of Cxs/HCs/GJs in the Human CNS Disorders
Brain ischemia
Brain ischemia remains a leading cause of morbidity and mortality in the developed world. Neuronal cell death can occur in the core region within seconds to minutes after ischemia. If blood flow is not restored quickly, the progression of cell injury and death will last for several hours to days, thereby expanding the infarct region to the surrounding area, termed the penumbra (Turner et al., 2013). Cxs have been suggested to contribute to the wave of delayed secondary injury that spreads from the core region to the penumbra (De Bock et al., 2014b). Ischemia-related injury is mostly associated with changes of astrocyte GJIC and an aberrant opening of HCs (Retamal et al., 2007; Wang et al., 2013b). The opening HCs leads to cell dysfunction via the collapse of membrane potential and entry of Ca2+, and also promotes release of glutamate and ATP that may further cell death (Decrock et al., 2011). Additionally, GJs may contribute to the propagation of cortical spreading depression (CSD), a wave of tissue depolarization followed by neuronal inactivation associated with increased infarct volume after cerebral ischemia (Theis et al., 2003).
Traumatic injury
Traumatic brain and spinal cord injury may cause immediate neuronal and glial cell death, followed by a cascade of secondary events leading to on-going spread of tissue damage. This secondary injury includes the inflammatory response, free radical formation and excitotoxicity, which lead to demyelination, axonal degeneration, glial scarring and neovascularization surrounding the area of initial damage (Hausmann, 2003; Norenberg et al., 2004). In the rodent, the level of Cx43 protein in astrocytic GJs is up-regulated after traumatic injury of the CNS (Cronin et al., 2008). Treatment with a Cx43 mimetic peptide also reduces secondary tissue damage after spinal cord injury by reducing gliosis and cytokine release (O’Carroll et al., 2013). Cx36 plays a detrimental role in injury-mediated neuronal death, and elimination of Cx36 and/or inactivation of the mechanisms for increased Cx36 expression is neuroprotective (Wang et al., 2012). In addition, the expression of Cxs (Cx29 and Cx32) on oligodendrocytes is increased in the narrow band border between the penumbra and the core regions of injury (Moon et al., 2010).
Epilepsy
Epilepsy is a CNS condition characterized by the periodic and unpredictable occurrence of seizures. Enhanced GJIC between neurons is considered a major factor involved in direct intercellular cytoplasmic connections and the promotion of hypersynchronous neuronal activity associated with seizures (Seifert et al., 2010). Interestingly, neurosurgical specimens from patients with temporal lobe epilepsy typically demonstrate marked reactive gliosis (Seifert et al., 2010). The expression of astrocytic Cx mRNAs (Cx30 and Cx43) is several fold higher than that of neuronal Cx mRNAs (Cx36 and Cx45), and glial cells outnumber neuronal cells in mammalian hippocampal and cortical tissues (Mylvaganam et al., 2014). GJs in astrocytes appear to play a dual role: on one hand they counteract the generation of hyperactivity by facilitating clearance of elevated extracellular K+ levels, while on the other hand they constitute a pathway for energetic substrate delivery to fuel neuronal hyper-activity (Steinhäuser et al., 2012). Although there is no doubt that Cx-based GJs and HCs are related to epilepsy, the specific details of their involvement remain to be elucidated.
Tumors
Tumors of the CNS are relatively rare compared with tumors of other tissues, accounting for less than 2% of all malignancies (Parkin et al., 2001). However, brain tumors are often lethal and the average survival rate of patients is low after diagnosis. Glioma is one of the most frequent primary brain tumors in adults, which is a space-occupying mass in the brain that causes a high intracranial pressure, vessel occlusion and brain edema (Behin et al., 2003). Glioma has been traditionally classified by the World Health Organization into four grades, of which grade IV is the most malignant (Sin et al., 2012). In the last decade, Cx has been shown to participate in diverse cellular processes including development, differentiation, homeostasis and survival. Interestingly, Cx43 was identified at areas of cell-to-cell contact between co-cultured glioma cells and astrocytes, and this cellular coupling had profound effects on the phenotypic transformation of astrocytes and the susceptibility of surrounding tissue to glioma invasion (Zhang et al., 1999). However, the potential role of Cxs in tumor growth and expansion remains controversial (Schalper et al., 2014). For example, although it is widely recognized that Cxs exhibit tumor suppressive properties, recent evidence suggests a role in promoting tumor growth and metastasis under some circumstances (Naus and Laird, 2010). In general, decreasing Cx43 expression is associated with increasing proliferation and higher tumor grades. In a screen of 18 human samples in which Cx43 expression was examined in different glioma stages, a reduction of Cx43 was found to be associated with glioma progression (Huang et al., 1999). A similar result was also obtained by other groups (Soroceanu et al., 2001; Pu et al., 2004). However, a recent study of 32 human samples reported elevated levels of Cx43 mRNA, but reduced levels of Cx43 protein, in high-grade glioma, suggesting an alteration of post-transcriptional mechanisms (Caltabiano et al., 2010). Therefore, it is clear that Cx43 expression is highly heterogeneous, and it may perform different functions depending on local microenvironment of the tumor (Naus and Laird, 2010).
Migraine
Migraine is a complex familial disorder of the brain characterized by recurrent unilateral headache, usually accompanied by nausea, vomiting, photophobia and/or phonophobia (Edvinsson and Uddman, 2005). Although several hypotheses have been proposed for the initiation of migraine, its mechanism remains unclear (Goadsby et al., 2009). A mutation in a calcium gene channel has been suggested to sensitize individual neurons to environmental factors, resulting in a wave of CSD when the attack occurs (Edvinsson and Uddman, 2005). Neuronal activity is thought to be the main cause of migraine initiation. However, it has also been shown that neuronal-glial communication via GJs and paracrine signaling was involved in CSD activity and migraine pathology (Thalakoti et al., 2007). Tonabersat was first identified as an anti-epileptic drug and showed significant efficacy as a novel GJ modulator for the treatment of migraine (Silberstein, 2009). Tonabersat can inhibit Cx26 GJIC between glial cells and neurons in the sensory part of the trigeminal nerve and prevent CSD, resulting in reduced migraine attacks in animal models and in humans (Damodaram et al., 2009). Nevertheless, further studies are required to determine the exact roles of Cxs in migraine.
Neuroautoimmune disease
Multiple sclerosis is a chronic inflammatory demyelinating disease of the CNS, characterized by degeneration of oligodendrocytes, demyelination of neurons and consequently axonal loss and neurological deficits (Compston and Coles, 2002). Similarly, demyelination occurs in neuromyelitis optica, a variant of multiple sclerosis, although with a different pathophysiology and localization. In an experimental autoimmune encephalomyelitis model of multiple sclerosis, demyelinating lesions were found to exhibit significantly decreased Cx43 expression, while recovering lesions had markedly increased Cx43 expression (Roscoe et al., 2007). Masaki et al. (2013) also reported that oligodendrocyte Cx32/Cx47 expression was decreased in most active and chronic lesions from multiple sclerosis and neuromyelitis optica patients. Interestingly, Cx43-specific antibodies were absent in all samples in that study. Further, Cx43 loss was significantly associated with a rapidly progressive disease course of multiple sclerosis and neuromyelitis optica. Therefore, the differential expression of Cx43 in active and chronic lesions implies differing roles at different stages in multiple sclerosis and neuromyelitis optica (Moinfar et al., 2014). However, the underlying mechanisms of Cx43 signaling in these diseases remains poorly understood (Kielian, 2008).
Alzheimer's disease (AD)
AD is one of the most prevalent forms of dementia in humans. The major clinical manifestations are cognitive deficits such as memory and learning impairment. AD is histologically characterized by substantial neuronal and synaptic loss associated with extracellular β-amyloid (Aβ) accumulation in the form of senile plaques (Selkoe, 2001). Several studies have shown that Aβ can stimulate astrogliosis, which is also observed in the human AD brain (Nagele et al., 2004; Olabarria et al., 2010). The expression of Cx43 and Cx30, the two main Cxs in astrocytes, was reported to increase in the immediate vicinity of the majority of Aβ plaques in AD mouse models (Mei et al., 2010). Further, brain samples of AD patients displayed an increase in Cx43 expression in senile plaques (Nagy et al., 1996). Moreover, Aβ peptide has been shown to promote astrocyte activation and the release of glutamate and ATP through Cx43 HCs, resulting in neuronal death by opening of Cx36 HCs in neurons (Orellana et al., 2011).
Parkinson's disease (PD)
PD is an adult-onset neurodegenerative disease characterized by the loss of dopaminergic neurons in the substantia nigra-striatum. The clinical symptoms of PD include progressive tremor, muscle rigidity and gait disturbance. Rufer et al. (1996) reported that immunoreactive Cx43 protein was increased in the striatum of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, although no evidence of increased functional coupling was observed. Kawasaki et al. (2009) also reported that Cx43 expression was enhanced in a rat PD model induced by rotenone and in vitro in cultured astrocytes stimulated with rotenome. These data suggest that modulation of Cx43 in astrocytes with a participation of GJIC may play an important role in PD pathology (Kawasaki et al., 2009). However, the mechanism linking enhanced astrocyte GJIC and dopaminergic neuron death requires urgent clarification. Tremors are also commonly observed in PD patients. Although the causes of these tremors remains unclear, the inferior olive is thought to play a key role (Loewenstein, 2002). Interestingly, there is some evidence for a role of GJIC in inferior olive neurons (Loewenstein, 2002), although no difference in the severity of harmaline-induced tremors was observed between Cx36 knockout mice and wild-type mice (Long et al., 2002). Further experiments are required to clarify the mechanism of tremors in PD patients.
X-linked Charcot-Marie-Tooth disease (CMTX)
CMTX is an inherited motor and sensory neurological disorder that causes progressive distal muscle weakness and atrophy, sensory loss and decreased or absent tendon reflexes (Scherer and Kleopa, 2012); cognitive impairment can also occur (Stancanelli et al., 2012). Over the last two decades, there have been rapid advances in understanding the molecular basis for CMTX, with more than 400 different mutations in GJB1 described (Scherer and Kleopa, 2012). GJB1 can encode Cx32, a GJ protein expressed by many cell types, including oligodendrocytes and Schwann cells. Cx32 forms reflexive channels that allow the passage of ions and signaling molecules across the myelin sheath in Schwann cells (Bicego et al., 2006). Nevertheless, despite this broad expression pattern of Cx32, peripheral neuropathy is usually the sole clinical manifestation of CMTX. It is possible that co-expression of other Cxs may offset some damage caused by the loss of Cx32 (Scherer and Kleopa, 2012). A GJB1 gene replacement strategy may be useful treatment of patients with CMTX (Shy et al., 2007).
Pelizaeus-Merzbacher-like disease (PMLD)
PMLD is an autosomal recessive inherited severe leukoencephalopathy in humans caused by mutations in GJA12 (or GJC2) gene encoding for Cx47, and is characterized by nystagmus, progressive spasticity, ataxia and hypomyelination on MRI (Uhlenberg et al., 2004). Cx47 is primarily expressed in the cell bodies of oligodendrocytes, and mutant proteins appear to accumulate partially in the endoplasmic reticulum. Mutations associated with PMLD disrupt Cx47/Cx47 and Cx47/Cx43 GJIC between oligodendrocytes and astrocytes, impede the passage of molecules between cells and results in loss of function (Orthmann-Murphy et al., 2007; Mi et al., 2013). These changes result in a decreased number of cells coupled within glial networks, rather than detrimental function of the mutated Cx47 protein (Tress et al., 2011).
Spastic paraplegia
Spastic paraplegia is a syndrome describing inherited disorders in which lower extremity weakness and spasticity are the predominant symptoms. There are more than 50 genetic types of spastic paraplegia (Fink, 2013). Orthmann-Murphy et al. (2009) described three cases of SPG44 from one family with a homozygous 133M mutation of GJA12/GJC2. This phenotype caused a milder symptom: late onset (first and second decades), cognitive impairment, slowly progressive, spastic paraplegia, dysarthria and upper extremity involvement. The mutant proteins form GJ plaques at cell borders similar to Cx47, but fail to form functional homotypic channels. This is particularly interesting because the spastic paraplegia phenotype is thought to represent a length-dependent axonopathy, and suggests that mutant forms of Cx47 may directly interfere with interactions between oligodendrocytes and their associated axons (Abrams and Scherer, 2012).
Maxillofacial dysplasia
Oculodentodigital dysplasia is a clinically variable genetic disorder caused by mutations of the GJA1 gene at chromosome 6q22–23, predominantly inherited in an autosomal dominant fashion (Paznekas et al., 2003). The genetic mutations affect highly conserved amino acid residues located in different portions of the Cx43 protein (Jamsheer et al., 2014). The phenotype of oculodentodigital dysplasia comprises craniofacial, dental and digital abnormalities (Paznekas et al., 2003). Hallermann-Streiff syndrome is a rare disorder, mostly reported in case studies. Hallermann-Streiff syndrome is an autosomal recessive or sporadic syndrome that shows substantial overlap with oculodentodigital dysplasia, and is characterized by dyscephalia, and facial and dental abnormalities (Thomas et al., 2013). However, while oculodentodigital dysplasia is a dominantly inherited disorder due to mutations in GJA1, the inheritance pattern of Hallermann-Streiff syndrome remains controversial (Pizzuti et al., 2004). As noted, some patients with a ‘full blown’ Hallermann-Streiff syndrome phenotype had no mutations in the GJA1 coding region (Abrams and Scherer, 2012).
Conclusion/Perspective
It is now clear that Cxs can form GJs and syncytial networks, and play a key role in the direct exchange of ions and small molecules between adjacent cells. Cxs can also form HCs that provide large, relatively nonselective conductances in single plasma membranes. In addition, the function of Cxs is independent from HCs in the regulation of growth, development and differentiation of the CNS. Although the molecular basis of Cxs/HCs/GJs function remains to be fully determined, novel physiological functions are continuing to be found. The combination of specific Cxs/HCs/GJs blockers with gene ablation approaches would provide the most compelling evidence for their function. The discovery of Cx-linked human diseases has provided further interest in the biology of HCs/GJs. The roles of Cxs in human CNS diseases appear to be dependent on the particular role of the Cxs and the interaction with other proteins in a given tissue. Clearly, the establishment of additional animal models of Cx-linked diseases will help to elucidate the roles of Cxs in normal and pathological conditions in the human CNS, although the methods for separating the intercellular channel, hemichannel and non-channel activities of Cxs need to be developed. A complete understanding of these communication systems will provide essential information for the development of novel therapeutic approaches.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China (General Program), No. 81271293 and the National Science Foundation for Young Scientists of China, No. 81000490.
Conflicts of interest: None declared.
Copyedited by Dean J, Robens J, Li CH, Song LP, Zhao M
References
- Abrams CK, Scherer SS. Gap junctions in inherited human disorders of the central nervous system. Biochim Biophys Acta. 2012;1818:2030–2047. doi: 10.1016/j.bbamem.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- Anders S, Minge D, Griemsmann S, Herde MK, Steinhäuser C, Henneberger C. Spatial properties of astrocyte gap junction coupling in the rat hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130600. doi: 10.1098/rstb.2013.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Minogue PJ, Laing JG, Beyer EC. Pathways for degradation of connexins and gap junctions. Cardiovasc Res. 2004;62:256–267. doi: 10.1016/j.cardiores.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicego M, Morassutto S, Hernandez VH, Morgutti M, Mammano F, D’Andrea P, Bruzzone R. Selective defects in channel permeability associated with Cx32 mutations causing X-linked Charcot-Marie-Tooth disease. Neurobiol Dis. 2006;21:607–617. doi: 10.1016/j.nbd.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. In: Ejb Reviews. Springer; 1996. (1997) Connections with connexins: the molecular basis of direct intercellular signaling; pp. 135–161. [DOI] [PubMed] [Google Scholar]
- Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107:13648–13653. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltabiano R, Torrisi A, Condorelli D, Albanese V, Lanzafame S. High levels of connexin 43 mRNA in high grade astrocytomas. Study of 32 cases with in situ hybridization. Acta Histochem. 2010;112:529–535. doi: 10.1016/j.acthis.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Cina C, Maass K, Theis M, Willecke K, Bechberger JF, Naus CC. Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. J Neurosci. 2009;29:2009–2021. doi: 10.1523/JNEUROSCI.5025-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci. 2008;39:152–160. doi: 10.1016/j.mcn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Damodaram S, Thalakoti S, Freeman SE, Garrett FG, Durham PL. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache. 2009;49:5–20. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock M, Vandenbroucke RE, Decrock E, Culot M, Cecchelli R, Leybaert L. A new angle on blood-CNS interfaces: A role for connexins? FEBS Lett. 2014a;588:1259–1270. doi: 10.1016/j.febslet.2014.02.060. [DOI] [PubMed] [Google Scholar]
- De Bock M, Decrock E, Wang N, Bol M, Vinken M, Bultynck G, Leybaert L. The dual face of connexin-based astroglial Ca(2+) communication: a key player in brain physiology and a prime target in pathology. Biochim Biophys Acta. 2014b;1843:2211–2232. doi: 10.1016/j.bbamcr.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Decrock E, Vinken M, Bol M, D’Herde K, Rogiers V, Vandenabeele P, Krysko DV, Bultynck G, Leybaert L. Calcium and connexin-based intercellular communication, a deadly catch? Cell Calcium. 2011;50:310–321. doi: 10.1016/j.ceca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Uddman R. Neurobiology in primary headaches. Brain Res Rev. 2005;48:438–456. doi: 10.1016/j.brainresrev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Basilio D, Sαez JC, Orellana JA, Raine CS, Bukauskas F, Bennett MV, Berman JW. The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system. J Neuroimmune Pharmacol. 2012;7:499–518. doi: 10.1007/s11481-012-9352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM. Connexin-specific distribution within gap junctions revealed in living cells. J Cell Sci. 2000;113:4109–4120. doi: 10.1242/jcs.113.22.4109. [DOI] [PubMed] [Google Scholar]
- Falk MM, Baker SM, Gumpert AM, Segretain D, Buckheit RW. Gap junction turnover is achieved by the internalization of small endocytic double-membrane vesicles. Mol Biol Cell. 2009;20:3342–3352. doi: 10.1091/mbc.E09-04-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JK. Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms. Acta Neuropathol. 2013;126:307–328. doi: 10.1007/s00401-013-1115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori MC, Reuss L, Cuello LG, Altenberg GA. Functional analysis and regulation of purified connexin hemichannels. Front Physiol. 2014;5:71. doi: 10.3389/fphys.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger N, Orellana JA, Calvo CF, Amigou E, Kozoriz MG, Naus CC, Sαez JC, Giaume C. Inhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotective. Mol Cell Neurosci. 2010;45:37–46. doi: 10.1016/j.mcn.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC. CCN3 (NOV) interacts with connexin43 in C6 glioma cells: possible mechanism of connexin-mediated growth suppression. J Biol Chem. 2004;279:36943–36950. doi: 10.1074/jbc.M403952200. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Goadsby P, Charbit A, Andreou A, Akerman S, Holland P. Neurobiology of migraine. Neuroscience. 2009;161:327–341. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–295. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Guo Y, Martinez-Williams C, Rannels DE. Gap junction-microtubule associations in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1213–1221. doi: 10.1152/ajplung.00066.2003. [DOI] [PubMed] [Google Scholar]
- Hausmann O. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, Kass DA, Machamer CE, Van Eyk JE, Tomaselli GF. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RP, Hossain MZ, Sehgal A, Boynton AL. Reduced connexin43 expression in high-grade human brain glioma cells. J Surg Oncol. 1999;70:21–24. doi: 10.1002/(sici)1096-9098(199901)70:1<21::aid-jso4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jamsheer A, Sowinska-Seidler A, Socha M, Stembalska A, Kiraly-Borri C, Latos-Bielenska A. Three novel GJA1 missense substitutions resulting in oculo-dento-digital dysplasia (ODDD) - further extension of the mutational spectrum. Gene. 2014;539:157–161. doi: 10.1016/j.gene.2014.01.066. [DOI] [PubMed] [Google Scholar]
- Johnson RG, Meyer RA, Li XR, Preus DM, Tan L, Grunenwald H, Paulson AF, Laird DW, Sheridan JD. Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules. Exp Cell Res. 2002;275:67–80. doi: 10.1006/excr.2002.5480. [DOI] [PubMed] [Google Scholar]
- Jordan K, Chodock R, Hand AR, Laird DW. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci. 2001;114:763–773. doi: 10.1242/jcs.114.4.763. [DOI] [PubMed] [Google Scholar]
- Kameritsch P, Pogoda K, Pohl U. Channel-independent influence of connexin 43 on cell migration. Biochim Biophys Acta. 2012;1818:1993–2001. doi: 10.1016/j.bbamem.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol. 2007;94:245–264. doi: 10.1016/j.pbiomolbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Hayashi T, Nakachi K, Trosko J, Sugihara K, Kotake Y, Ohta S. Modulation of connexin 43 in rotenone-induced model of Parkinson's disease. Neuroscience. 2009;160:61–68. doi: 10.1016/j.neuroscience.2009.01.080. [DOI] [PubMed] [Google Scholar]
- Kelly SM, Vanslyke JK, Musil LS. Regulation of ubiquitin-proteasome system mediated degradation by cytosolic stress. Mol Biol Cell. 2007;18:4279–4291. doi: 10.1091/mbc.E07-05-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem. 2008;106:1000–1016. doi: 10.1111/j.1471-4159.2008.05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Laird D. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithe E, Sirnes S, Fykerud T, Kjenseth A, Rivedal E. Endocytosis and post-endocytic sorting of connexins. Biochim Biophys Acta. 2012;1818:1870–1879. doi: 10.1016/j.bbamem.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Loewenstein Y. A possible role of olivary gap-junctions in the generation of physiological and pathological tremors. Mol Psychiatry. 2002;7:129–131. doi: 10.1038/sj.mp.4000994. [DOI] [PubMed] [Google Scholar]
- Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki K, Suzuki SO, Matsushita T, Matsuoka T, Imamura S, Yamasaki R, Suzuki M, Suenaga T, Iwaki T, Kira JI. Connexin 43 astrocytopathy linked to rapidly progressive multiple sclerosis and neuromyelitis optica. PLoS One. 2013;8:e72919. doi: 10.1371/journal.pone.0072919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuuchi L, Naus CC. Gap junction proteins on the move: connexins, the cytoskeleton and migration. Biochim Biophys Acta. 2013;1828:94–108. doi: 10.1016/j.bbamem.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Mei X, Ezan P, Giaume C, Koulakoff A. Astroglial connexin immunoreactivity is specifically altered at β-amyloid plaques inβ-amyloid precursor protein/presenilin1 mice. Neuroscience. 2010;171:92–105. doi: 10.1016/j.neuroscience.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi SK, Gregory BG, Donglin B. The distribution and functional properties of Pelizaeus-Merzbacher-like disease-linked Cx47 mutations on Cx47/Cx47 homotypic and Cx47/Cx43 heterotypic gap junctions. Biochem J. 2013;452:249–258. doi: 10.1042/BJ20121821. [DOI] [PubMed] [Google Scholar]
- Moinfar Z, Dambach H, Faustmann PM. Influence of drugs on gap junctions in glioma cell lines and primary astrocytes in vitro. Front Physiol. 2014;5:186. doi: 10.3389/fphys.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y, Choi SY, Kim K, Kim H, Sun W. Expression of connexin29 and 32 in the penumbra region after traumatic brain injury of mice. Neuroreport. 2010;21:1135–1139. doi: 10.1097/WNR.0b013e32834051c7. [DOI] [PubMed] [Google Scholar]
- Mylvaganam S, Ramani M, Krawczyk M, Carlen PL. Roles of gap junctions, connexins, and pannexins in epilepsy. Front Physiol. 2014;5:172. doi: 10.3389/fphys.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Nagy J, Li W, Hertzberg E, Marotta C. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer's disease. Brain Res. 1996;717:173–178. doi: 10.1016/0006-8993(95)01526-4. [DOI] [PubMed] [Google Scholar]
- Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- O’Carroll SJ, Gorrie CA, Velamoor S, Green CR, Nicholson LF. Connexin43 mimetic peptide is neuroprotective and improves function following spinal cord injury. Neurosci Res. 2013;75:256–267. doi: 10.1016/j.neures.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer's disease. Glia. 2010;58:831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- Olbina G, Eckhart W. Mutations in the second extracellular region of connexin 43 prevent localization to the plasma membrane, but do not affect its ability to suppress cell growth. Mol Cancer Res. 2003;1:690–700. [PubMed] [Google Scholar]
- Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Sαez PJ, Jiang JX, Naus CC, Sαez JC, Giaume C. Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J Neurosci. 2011;31:4962–4977. doi: 10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Enriquez AD, Abrams CK, Scherer SS. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol Cell Neurosci. 2007;34:629–641. doi: 10.1016/j.mcn.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Salsano E, Abrams CK, Bizzi A, Uziel G, Freidin MM, Lamantea E, Zeviani M, Scherer SS, Pareyson D. Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain. 2009;132:426–438. doi: 10.1093/brain/awn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Paul DL. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986;103:123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell. 2007;18:337–347. doi: 10.1091/mbc.E06-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzuti A, Flex E, Mingarelli R, Salpietro C, Zelante L, Dallapiccola B. A homozygous GJA1 gene mutation causes a Hallermann-Streiff/ODDD spectrum phenotype. Hum Mutat. 2004;23:286. doi: 10.1002/humu.9220. [DOI] [PubMed] [Google Scholar]
- Pu P, Xia Z, Yu S, Huang Q. Altered expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg. 2004;107:49–54. doi: 10.1016/j.clineuro.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Schalper KA, Shoji KF, Orellana JA, Bennett MV, Sαez JC. Possible involvement of different connexin43 domains in plasma membrane permeabilization induced by ischemia-reperfusion. J Membr Biol. 2007;218:49–63. doi: 10.1007/s00232-007-9043-y. [DOI] [PubMed] [Google Scholar]
- Revel J, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33:C7–12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe WA, Kidder GM, Karlik SJ. Experimental allergic encephalomyelitis in connexin 43-heterozygous mice. Cell Commun Adhes. 2007;14:57–73. doi: 10.1080/15419060701459569. [DOI] [PubMed] [Google Scholar]
- Rufer M, Wirth S, Hofer A, Dermietzel R, Pastor A, Kettenmann H, Unsicker K. Regulation of connexin-43, GFAP, and FGF-2 is not accompanied by changes in astroglial coupling in MPTP-lesioned, FGF-2-treated Parkisonian mice. J Neurosci Res. 1996;46:606–617. doi: 10.1002/(SICI)1097-4547(19961201)46:5<606::AID-JNR9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Schalper KA, Carvajal-Hausdorf D, Oyarzo MP. Possible role of hemichannels in cancer. Front Physiol. 2014;5:237. doi: 10.3389/fphys.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Kleopa KA. X-linked Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2012;17:9–13. doi: 10.1111/j.1529-8027.2012.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Seifert G, Carmignoto G, Steinhäuser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Shovel KS, Slade AM, Powell T, Twist VW, Green CR. Fate of gap junctions in isolated adult mammalian cardiomyocytes. Circ Res. 1989;65:22–42. doi: 10.1161/01.res.65.1.22. [DOI] [PubMed] [Google Scholar]
- Shy M, Siskind C, Swan E, Krajewski K, Doherty T, Fuerst D, Ainsworth P, Lewis R, Scherer S, Hahn A. CMT1X phenotypes represent loss of GJB1 gene function. Neurology. 2007;68:849–855. doi: 10.1212/01.wnl.0000256709.08271.4d. [DOI] [PubMed] [Google Scholar]
- Silberstein S. Tonabersat, a novel gap‐junction modulator for the prevention of migraine. Cephalalgia. 2009;29:28–35. doi: 10.1111/j.1468-2982.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- Sin WC, Crespin S, Mesnil M. Opposing roles of connexin43 in glioma progression. Biochim Biophys Acta. 2012;1818:2058–2067. doi: 10.1016/j.bbamem.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Solan J, Lampe P. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Manning TJ, Sontheimer H. Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia. 2001;33:107–117. doi: 10.1002/1098-1136(200102)33:2<107::aid-glia1010>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sosinsky GE, Nicholson BJ. Structural organization of gap junction channels. Biochim Biophys Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Spray DC, Hanstein R, Lopez-Quintero SV, Stout RF, Jr, Suadicani SO, Thi MM. Gap junctions and Bystander Effects: Good Samaritans and executioners. Wiley Interdiscip Rev Membr Transp Signal. 2013;2:1–15. doi: 10.1002/wmts.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancanelli C, Taioli F, Testi S, Fabrizi GM, Arena MG, Granata F, Russo M, Gentile L, Vita G, Mazzeo A. Unusual features of central nervous system involvement in CMTX associated with a novel mutation of GJB1 gene. J Peripher Nerv Syst. 2012;17:407–411. doi: 10.1111/j.1529-8027.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- Steinhäuser C, Seifert G, Bedner P. Astrocyte dysfunction in temporal lobe epilepsy: K + channels and gap junction coupling. Glia. 2012;60:1192–1202. doi: 10.1002/glia.22313. [DOI] [PubMed] [Google Scholar]
- Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. discussion 24-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Döring B, Frisch C, Söhl G, Teubner B, Euwens C. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Ragavi BS, Raneesha P, Ahmed NA, Cynthia S, Manoharan D, Manoharan R. Hallermann-streiff syndrome. Indian J Dermatol. 2013;58:383. doi: 10.4103/0019-5154.117311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress O, Maglione M, Zlomuzica A, May D, Dicke N, Degen J, Dere E, Kettenmann H, Hartmann D, Willecke K. Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus-Merzbacher-like disease in humans. PLoS Genet. 2011;7:e1002146. doi: 10.1371/journal.pgen.1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RC, Dodson SC, Rosen CL, Huber JD. The science of cerebral ischemia and the quest for neuroprotection: navigating past failure to future success: A review. J Neurosurg. 2013;118:1072–1085. doi: 10.3171/2012.11.JNS12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenberg B, Schuelke M, Rüschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloğlu H, Nürnberg P, Hübner C, Weschke B, Gärtner J. Mutations in the gene encoding gap junction protein alpha 12 (connexin 46. 6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet. 2004;75:251–260. doi: 10.1086/422763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke JK, Musil LS. Dislocation and degradation from the ER are regulated by cytosolic stress. J Cell Biol. 2002;157:381–394. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega JL, Subiabre M, Figueroa F, Schalper KA, Osorio L, Gonzαlez J, Sαez JC. (2013) Role of gap junctions and hemichannels in parasitic infections. Biomed Res Int. 2013 doi: 10.1155/2013/589130. 589130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. 2013a;1828:35–50. doi: 10.1016/j.bbamem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma A, Zhu W, Zhu L, Zhao Y, Xi J, Zhang X, Zhao B, Becker DL. The role of connexin 43 and hemichannels correlated with the astrocytic death following ischemia/reperfusion insult. Cell Mol Neurobiol. 2013b;33:401–410. doi: 10.1007/s10571-013-9906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Song JH, Denisova JV, Park WM, Fontes JD, Belousov AB. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J Neurosci. 2012;32:713–725. doi: 10.1523/JNEUROSCI.3872-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Zhang W, Couldwell WT, Simard MF, Song H, Lin JH, Nedergaard M. Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res. 1999;59:1994–2003. [PubMed] [Google Scholar]


