Abstract
OBJECTIVE:
To assess the efficacy and safety of cilostazol on the progression and regression of symptomatic intracranial artery stenosis.
DATA RETRIVAL:
We searched the main databases for eligible trials including Medline (from 1966 to June 2014), Embase (from 1980 to June 2014), Cochrane Library (Issue 6, 2014), Chinese National Knowledge Infrastructure (from 1995 to June 2014), Current Controlled Trials (http://controlled-trials.com), Clinical Trials.gov (http://clinicaltrials.gov), and Chinese Clinical Trial Registry (http://www.chictr.org). All studies regarding prevention and treatment of symptomatic intracranial arterial stenosis by cilostazol were collected. The Mesh or text keywords were the English words: “cilostazol, phosphodiesterase 3 inhibitor, atherosclerosis, and ischemic stroke.” No restrictions were put on publications or publication language.
SELECTION CRITERIA:
Grade A or B randomized controlled trials were selected according to the quality of evaluation criteria from the Cochrane Collaboration, in which cilostazol and aspirin were used to evaluate the effects of cilostazol in the treatment of patients with symptomatic intracranial artery stenosis. The quality of study methodology was evaluated based on criteria described in Cochrane Reviewer's Handbook 5.0.1. RevMan 5.2 software was used for data analysis.
MAIN OUTCOME MEASURES:
Clinical efficacy and safety of cilostazol in stopping progression and promoting regression of symptomatic intracranial artery stenosis were measured by magnetic resonance angiography and transcranial Doppler.
RESULTS:
Two randomized controlled trials with a total of 203 patients were included in this study. The results showed that while cilostazol was associated with a significantly reduced progression of intracranial artery stenosis (OR = 0.21, 95%CI: 0.09–0.47, P < 0.01), it had no beneficial effect on symptom regression (OR = 1.42, 95%CI: 0.80–2.51, P = 0.24). During the follow-up period, although some adverse effects developed, including headache, gastrointestinal disturbance, and dizziness, incidences of bleeding were lower than in aspirin-treated patients.
CONCLUSION:
Cilostazol may prevent the progression of symptomatic intracranial artery stenosis, which could reduce the incidence of ischemic stroke.
Keywords: nerve regeneration; systemic review; cilostazol; atherosclerosis; aspirin; stroke, ischemic; magnetic resonance angiography; transcranial Doppler; intracranial artery stenosis; follow-up studies; neural regeneration
Introduction
Ischemic stroke is a major cause of death and disability worldwide (Hénon et al., 1995) and antiplatelet treatment is a mainstay in secondary stroke prevention. Atherosclerotic intracranial arterial stenosis is a leading cause of ischemic stroke (Sacco et al., 1995; Wong et al., 2003), and preventing its progression is important for reducing the risk of stroke in patients with symptomatic stenosis. Although aspirin is widely used as an antiplatelet agent, the development of drug resistance and the risk of bleeding (Szczeklik et al., 2005) have limited its clinical application.
Cilostazol, a selective phosphodiesterase III inhibitor, has been shown to be effective in the secondary prevention of stroke (Matsumoto, 2005), thus it has drawn much attention over the past few years. Along with other antiplatelet medications, it was shown not only to inhibit the proliferation of arterial smooth muscle cells and improve lipid metabolism (Park et al., 2009), but also to prevent re-stenosis after coronary angioplasty (Park et al., 2000). Additionally, it acted as a neuroprotective agent (Lee et al., 2006) by increasing cyclic adenosine monophosphate levels (Huang et al., 2008).
Based on these results, it has been hypothesized that cilostazol reduces the progression of intracranial arterial stenosis and thus prevents ischemic events. Here, we collected all studies about the prevention of intracranial arterial stenosis with cilostazol and analyzed them using RevMan 5.2 software. The analysis assessed the efficacy of cilostazol versus aspirin in ameliorating intracranial arterial stenosis, aiming to provide guidance for clinical application.
Data and Methods
Data retrieval
Electronic databases including PubMed (1966/2014-06), Embase (1980/2014-06), Cochrane Library (Issue 6, 2014), Chinese National Knowledge Infrastructure (1995/2014-06), Current Controlled Trials (http://controlled-trials.com), Clinical Trials.gov (http://clinicaltrials.gov), and Chinese Clinical Trial Registry (http://www.chictr.org) were searched by using the Mesh or text keywords of “cilostazol, phosphodiesterase 3 inhibitor, atherosclerosis, and ischemic stroke”. No restrictions were put on publications or publication language.
Criteria for study selection
Inclusion criteria
(1) All patients in the studies had a medical history of ischemic stroke or transient ischemic attack. (2) The published studies were all randomized controlled trials in which patients were randomly grouped, with each group receiving different interventions for comparison. (3) The primary outcome was the change from baseline in the degree of intracranial artery stenosis, evaluated by magnetic resonance angiography and transcranial Doppler. (4) All studies lasted at least 6 months.
Exclusion criteria
(1) Studies were non-randomized controlled trials. (2) Patients had a history of gastrointestinal bleeding, bloody urine, or conjunctival hemorrhage. (3) Patients had serious diseases such as malignant neoplasm, heart failure, or renal failure.
Assessment of study quality and data extraction
Data were extracted independently by two investigators. Discrepancies were resolved by consensus or third-author adjudication. The following data were abstracted from each study: details of participant characteristics (age, gender), interventions given to each group, outcome measures, and follow-up results.
Methodological quality of the randomized controlled trials was assessed by evaluating the quality of several domains: randomization, generation of the random sequence, allocation concealment, blinding, use of intention to treat analysis, description of withdrawals and completeness of follow-up.
Outcome measures
The outcome measure was the change from baseline of intracranial artery stenosis as evaluated by magnetic resonance angiography (MRA) and transcranial Doppler (TCD).
Statistical analysis
We used RevMan 5.2 software (http://tech.cochrane.org/revman) to perform this meta-analysis. Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs) for dichotomous outcomes. Heterogeneity across trials was assessed by standard Chi square test. With the I2 > 50%, P < 0.01 was considered evidence of substantial heterogeneity. If this was true, a random effects model was used for statistical analysis, otherwise we used a fixed model. In order to avoid potential sources of heterogeneity, we explored the data using subgroups. Publication bias was tested by funnel plot.
Results
Eligible trials
Two randomized controlled trials (Kwon et al., 2005; Guo et al., 2009) with a total of 203 patients were identified for inclusion from 126 potentially relevant publications (Figure 1).
Figure 1.
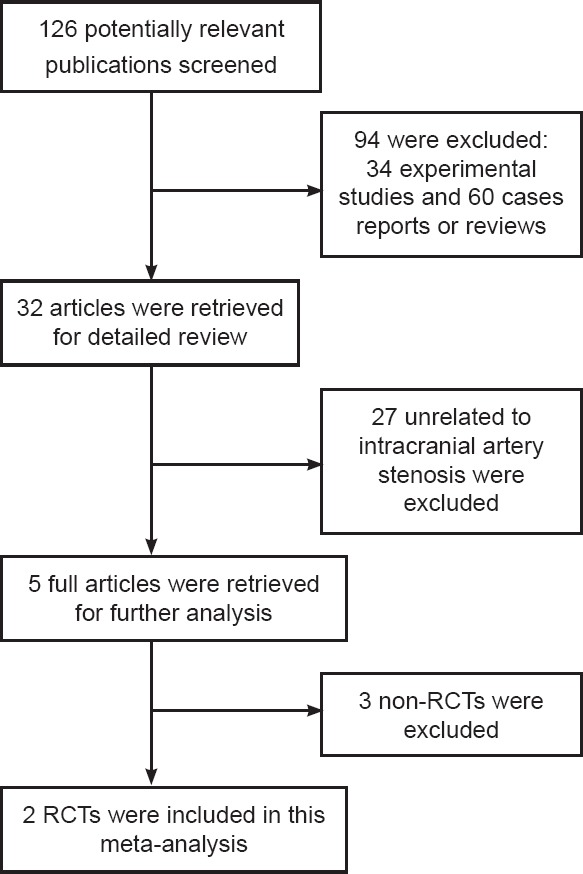
Process of study selection.
Of the initial 126 potentially relevant publications, 94 were excluded after primary reading, including 34 experimental animal studies and 60 case reports or reviews. The remaining 32 articles were examined further, and 27 studies unrelated to intracranial artery stenosis were excluded. Of the remaining five full articles, two were excluded because they were non-RCTs. Thus, two RCT studies were analyzed. RCTs: Randomized controlled trials.
Baseline characteristics and study quality
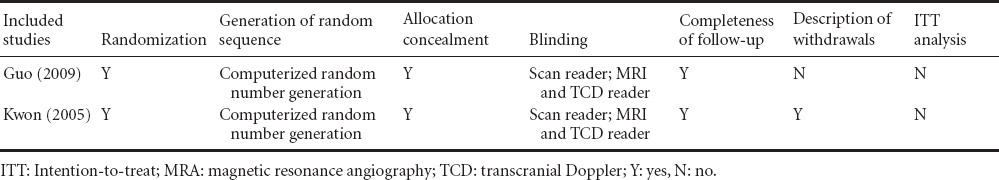
Using this retrieval method, two eligible trials (Kwon et al., 2005; Guo et al., 2009) published between 2005 and 2009 were found from different countries including Korea and China, containing a total of 203 patients. One study compared the efficacy of cilostazol and aspirin (Guo et al., 2009), and the other compared cilostazol with a placebo based on aspirin usage (Kwon et al., 2005). Table 1 summarizes the baseline characteristics of the two trials. Both trials analyzed the progression and regression of intracranial artery stenosis. Regarding side effects, both trials analyzed headaches, gastrointestinal disturbance, dizziness, skim rash and major bleeding. Both studies were of type scale A. Table 2 summarizes the study quality of the two trials.
Table 1.
Baseline characteristics of the two randomized controlled trials
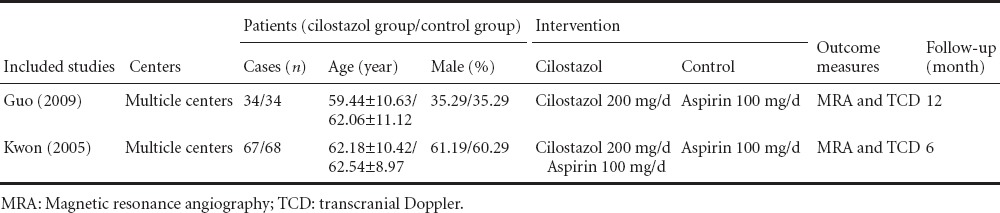
Table 2.
Study quality of the two randomized controlled trials
Meta analysis
Progression of intracranial artery stenosis
The included studies (Kwon et al., 2005; Guo et al., 2009) reported the progression of symptomatic intracranial artery stenosis, with aspirin and placebo as respective controls. MRA and TCD were used in both studies to examine arteriostenosis. There was no heterogeneity in MRA or TCD results across studies (P = 0.35, I2 = 9%), and a fixed-effects model was adopted. Meta-analysis revealed significant differences between the effect of cilostazol and aspirin (OR = 0.21, 95%CI: 0.09–0.47, Z = 3.76, P < 0.01), and similar results were obtained with a random-effects model (OR = 0.24, 95%CI: 0.10–0.60, Z = 3.08, P < 0.01). Therefore, according MRA and TCD data, cilostazol was superior to control treatment in preventing and treating the progression of intracranial artery stenosis (Figure 2).
Figure 2.
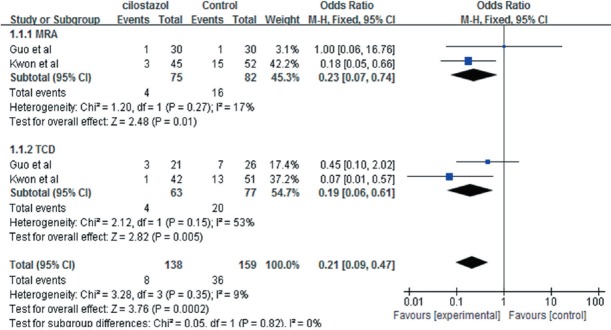
Forest plots comparing the progression of intracranial artery stenosis between cilostazol and control groups, as measured with magnetic resonance angiography (MRA) and transcranial Doppler (TCD).
Progression in cilostazol groups was significantly less than in control groups (P < 0.05).
Regression of intracranial artery stenosis
The two studies also reported the regression of symptomatic intracranial artery stenosis. There was no heterogeneity in the MRA or TCD results across studies (P = 0.49, I2 = 0%), and a fixed-effects model was again adopted. Meta-analysis results revealed no significant differences between the effects of cilostazol and aspirin (OR = 1.42, 95%CI: 0.80–2.51, Z = 1.19, P = 0.24), and similar results were obtained with a random-effects model (OR = 1.42, 95%CI: 0.79–2.55, Z = 1.18, P = 0.24) (Figure 3). Therefore, according MRA and TCD data, cilostazol was not superior to control treatment in inducing regression of intracranial artery stenosis.
Figure 3.
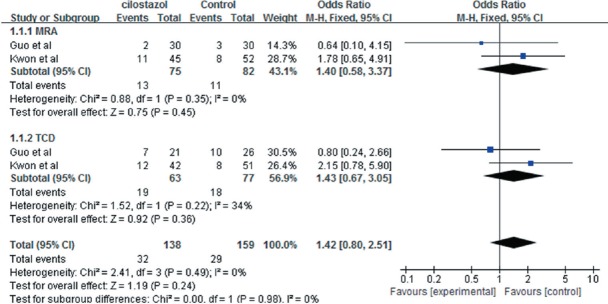
Forest plots comparing the regression of intracranial artery stenosis between cilostazol and control groups, as measured with magnetic resonance angiography (MRA) and transcranial Doppler (TCD).
Regression in cilostazol groups did not significantly differ from that in control groups (P > 0.05).
Adverse effects
Guo et al. (2009) reported that some adverse effects developed during the follow-up period, including headache (14.7%), gastrointestinal disturbance (7.4%), and dizziness (2.9%). Kwon et al. (2005) also found that patients suffered from headaches (24.4%), gastrointestinal disturbances (16.3%), and dizziness (12.6%). Headache developed more frequently in the cilostazol groups than that in the aspirin groups, while the frequency of bleeding events was much less in the cilostazol groups than in the aspirin groups. The incidences of other adverse events were not significantly different between groups.
Publication bias evaluation
We used a funnel plot to examine publication bias between the studies that were included. Results showed that plots centered on the OR, but were not symmetrical, indicating that there may have been publication bias (Figure 4). Because of the small number of articles included, this was inevitable. However, there was little heterogeneity between the two studies and the statistic results were reliable.
Figure 4.
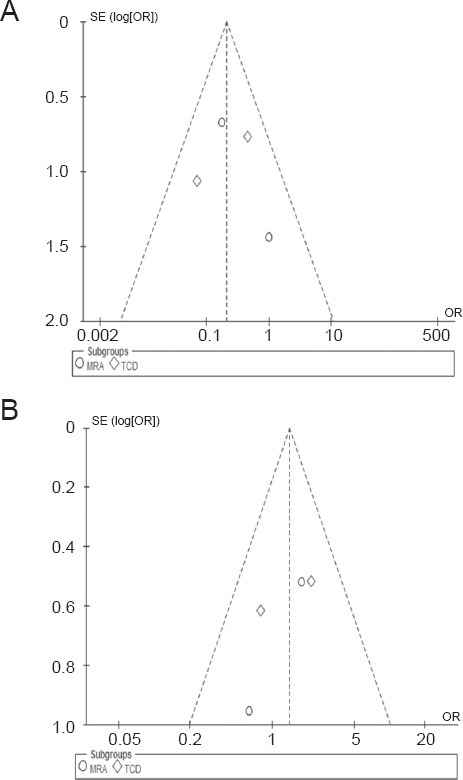
Funnel plots for progression (A) and regression (B).
Plots did not take an inverted funnel shape, suggesting that there may have been publication bias in the included studies. The vertical axis is a measure of the precision of the estimate of the treatment effect. Here the measure of precision is the standard error of the log OR. The horizontal axis measures the treatment effect (the OR).
Discussion
This meta-analysis suggests that cilostazol may reduce the progression of intracranial artery stenosis and that it is more effective that aspirin when given either alone or in combination with aspirin. There are four categories of drugs widely used in the clinical treatment of ischemic stroke: aspirin as a representative cyclooxygenase inhibitor, clopidogrel as a representative ADP-receptor antagonist, cilostazol as a representative phosphodiesterase inhibitor, and tirofiban as a representative IIb/IIIa receptor antagonist. Aspirin, cilostazol, and clopidogrel (Urbano et al., 2004; Furie et al., 2011) are currently recommended by the American Heart Association and the American Stroke Association (Symeonidis et al., 2002) as ischemic stroke secondary prevention drugs.
Cilostazol reversibly inhibits the activation of phosphodiesterase-3A and hinders degradation and transformation of adenylate cyclase. This increases adenylate cyclase levels in platelets and blood vessels, which helps prevent platelet aggregation (Ikeda, 1999) and inhibits pathologic proliferation of vascular intima (Porto et al., 2012). Moreover, cilostazol also plays a role in lowering serum triglycerides and raising the amount of high-density lipoproteins that make plaque stable (Yan et al., 2011), and significantly prevents thickening of the carotid artery intimamedia (Geng et al., 2012), which is an indicator of atherosclerosis and an established risk factor for stroke (Hollander et al., 2003).
Intracranial atherosclerosis is considered to be a high risk factor of recurrent ischemic stroke. In the GESICA (Groupe d’Etude des Sténoses Intra-Crâniennes Athéromateuses symptomatiques) study, stroke or transient ischemic attack occurred within 2 years in 38.2% of patients with symptomatic intracranial stenosis, and the risk was increased to 60.7% for thermodynamically significant stenosis (Mazighi et al., 2006). Randomized studies of symptomatic intracranial arterial stenosis showed that treatment with cilostazol significantly reduced progression of the stenosis (Sallustio et al., 2010; Yamada and Fujimoto, 2011).
Cilostazol had a remodeling effect on stenotic lesions due to arteriosclerotic changes and improved cerebral blood flow in some patients (Kai et al., 2011). The current results support these findings, with meta-analysis showing that cilostazol significantly prevented progression of intracranial artery stenosis. However, it did not appear to have any effect on regression of the stenosis. Because MRA images show regions of cerebral blood flow, rather than the true vascular diameter, the degree of stenosis is often overestimated, particularly in mild to moderate stenosis. In the evaluation of arterial stenosis, digital subtraction angiography is more sensitive and specific than MRA (Hirai et al., 2002).
Results of a preliminary study using both MRA and digital subtraction angiography showed that cilostazol induced improvement of symptomatic intracranial arterial stenosis (Yamada and Fujimoto, 2011). In addition to suppressing platelet aggregation and platelet adhesion, cilostazol also suppresses vascular smooth-muscle cell proliferation (Takahashi et al., 1992), improves vascular endothelial functions, and dilates blood vessels (Shin et al., 2004). Previous studies have reported that cilostazol was associated with significant decrease in high sensitive C-reactive protein (Agrawal et al., 2007), total leukocyte count, interleukin-6, platelet P-selectin, and neutrophil Mac-1 (Inoue et al., 2004). This evidence indicates that cilostazol has anti-inflammatory effects, which may play an important role in inhibiting the progression of atherosclerosis.
Recently, a meta-analysis revealed that cilostazol might be a more effective and safer alternative to aspirin for patients with ischemic stroke (Qian and Bi, 2013). Qian and Bi (2013) also reported fewer incidences of bleeding in patients treated with cilostazol than in those treated with aspirin. This difference is possibly related to cilostazol's pharmacological effect. Cilostazol cannot only prevent platelet aggregation and inhibit thrombosis, but it also can improve endothelial function and dilate blood vessels by increasing nitric oxide, an endogenous vasodilator factor, and by reducing intracellular calcium concentration (Shinohara et al., 2010). However, in addition to this positive side effect, cilostazol was also associated with minor adverse effects, such as gastrointestinal disorders dizziness, and headache. This result is quite consistent with that of another report (Huang et al., 2008). Because of the small sample size of studies investigating this issue, more clinical studies are still warranted for establishing the side effects of cilostazol.
Although cilostazol provides a new treatment option for aspirin-resistant patients, cilostazol's monthly cost is several times higher than aspirin (Feng et al., 2010). For developing countries, cost is a point that doctors must consider when they recommend a medication, and for patients in these regions, the cost of cilostazol it too high, especially compared with that of aspirin. Additionally, there are still very few studies related to cilostazol. All these factors contribute to limiting the breadth for cilostazol use. For patients who have failed to medically manage internal carotid artery disease, changing to other types of drugs or to combination therapy may be reliable options.
While actively controlling risk factors for cerebrovascular disease, combination therapy (such as cilostazol combined with aspirin or clopidogrel) could make full use of drugs and achieve the goal of reduced platelet aggregation, which should prevent deterioration and progression of the disease (Li and Huang, 2008). However, the safety and efficacy of these methods have yet to be assessed.
The present study may have the following limitations. First, although a comprehensive search was undertaken, the resulting sample size was small and publication bias was found. Thus, well designed, large studies are still warranted. Second, all patients were from East-Asian populations, and caution should be taken in generalizing the findings to more diverse populations. Third, follow-up periods were non-uniform in the trials of the sample studies, which could add clinical heterogeneity to this study.
In summary, although cilostazol does not seem to reverse intracranial artery stenosis, it may work to prevent further progression of the condition. Although publication bias resulted from the small number of studies, we avoided selection bias by collecting as many documents as possible and controlled study bias through discussions between two researchers. All the randomized controlled trials included were scale A. The quality of study methodological was strictly evaluated. The final statistical results showed that heterogeneity between the two studies was small and similar results were obtained using fixed-effect and random-effects models. Therefore, the final results were reliable. Further studies are required to confirm this beneficial effect in larger cohorts of patients with ischemic stroke caused by intracranial artery stenosis.
Footnotes
Conflicts of interest: None declared.
Copyedited by Phillips A, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Agrawal Neeraj K, Maiti Rituparna, Dash D, Pandey BL. Cilostazol reduces inflammatory burden and oxidative stress in hypertensive type 2 diabetes mellitus patients. Pharmacol Res. 2007;56:118–123. doi: 10.1016/j.phrs.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Feng HX, Yang M, Jiang HL, Hua WZ, He JF, Yao HX, Li YB, He LX, Shi X, Yuan JQ, Liu YL. Efficacy and safety in secondary prevention of ischemic stoke with cilostazol or aspirin: a systematic review. Zhongguo kangfu Lilun yu Shijian. 2010;16:961–965. [Google Scholar]
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research (2011) Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- Geng DF, Deng J, Jin DM, Wu W, Wang JF. Effect of cilostazol on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;220:177–183. doi: 10.1016/j.atherosclerosis.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Guo JJ, Xu E, Lin QY, Zeng GL, Xie HF. Effect of cilostazol on cerebral arteries in secondary prevention of ischemic stroke. Neurosci Bull. 2009;25:383–390. doi: 10.1007/s12264-009-6192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hénon H, Godefroy O, Leys D, Mounier-Vehier F, Lucas C, Rondepierre P, Duhamel A, Pruvo JP. Early predictors of death and disability after acute cerebral ischemic event. Stroke. 1995;26:392–398. doi: 10.1161/01.str.26.3.392. [DOI] [PubMed] [Google Scholar]
- Hirai T, Korogi Y, Ono K, Nagano M, Maruoka K, Uemura S, Takahashi M. Prospective evaluation of suspected stenoocclusive disease of intracranial artery: combined MR angiography compared with digital subtraction angiography. AJNR. 2002;23:93–101. [PMC free article] [PubMed] [Google Scholar]
- Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Witteman JC M, Breteler MMB. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam study. Stroke. 2003;34:2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- Huang YN, Cheng Y, Wu J, Li YS, Xu E, Hong Z, Li ZY, Zhang WW, Ding MP, Gao XG, Fan DS, Zeng JS, Wong KS, Lu CZ, Xiao JX, Yao C. Cilostazol versus Aspirin for Secondary Ischaemic Stroke Prevention cooperation investigators (2008) Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol. 7:494–499. doi: 10.1016/S1474-4422(08)70094-2. [DOI] [PubMed] [Google Scholar]
- Ikeda Antiplatelet therapy using cilostazol, a specific PDE3 inhibitor. Thromb Haemost. 1999;82:435–438. [PubMed] [Google Scholar]
- Inoue T, Uchida T, Sakuma M, Imoto Y, Ozeki Y, Ozaki Y, Hikichi Y, Node K. Cilostazol inhibits leukocyte integrin Mac-1, leading to a potential reduction in restenosis after coronary stent implantation. J Am Coll Cardiol. 2004;44:1408–1414. doi: 10.1016/j.jacc.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Kai Y, Watanabe M, Morioka M, Hirano T, Yano S, Ohmori Y, Kawano T, Hamada J, Kuratsu J. Cilostazol improves symptomatic intracranial artery stenosis - Evaluation of cerebral blood flow with single photon emission computed tomography. Surg Neurol Int. 2011;28:8–14. doi: 10.4103/2152-7806.76145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, Lee JH, Kim JS. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–786. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park SY, Shin YW, Hong KW, Kim CD, Sung SM, Kim KY, Lee WS. Neuroprotection by cilostazol, a phosphodiesterase type 3 inhibitor, against apoptotic white matter changes in rat after chronic cerebral hypoperfusion. Brain Res. 2006;1082:182–191. doi: 10.1016/j.brainres.2006.01.088. [DOI] [PubMed] [Google Scholar]
- Li JB, Huang L. The progress of research on aspirin resitance. Zhongguo Xunhuan Zazhi. 2008;23:314–316. [Google Scholar]
- Matsumoto M. Cilostazol in secondary prevention of stroke: impact of the cilostazol stroke prevention study. Atheroscler Suppl. 2005;6:33–40. doi: 10.1016/j.atherosclerosissup.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, Woimant F. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66:1187–1191. doi: 10.1212/01.wnl.0000208404.94585.b2. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee CW, Kim HS, Lee NH, Nah DY, Hong MK, Kim JJ, Park SJ. Effects of cilostazol on angiographic restenosis after coronary stent placement. Am J Cardiol. 2000;86:499–503. doi: 10.1016/s0002-9149(00)01001-8. [DOI] [PubMed] [Google Scholar]
- Park SY, Shin HK, Lee JH, Kim CD, Lee WS, Rhim BY, Hong KW. Cilostazol ameliorates metabolic abnormalities with suppression of proinflammatory markers in a db/db mouse model of type 2 diabetes via activation of peroxisome proliferator-activated receptor gamma transcription. J Pharmacol Exp Ther. 2009;329:571–579. doi: 10.1124/jpet.108.146456. [DOI] [PubMed] [Google Scholar]
- Porto I, D’Amario D, Crea F. Cilostazol and primary-PCI: mirage or good alternative? Curr Vasc Pharmacol. 2012;10:468–471. doi: 10.2174/157016112800812854. [DOI] [PubMed] [Google Scholar]
- Qian Y, Bi Q. Systematic study of cilostazol on secondary stroke prevention: a meta-analysis. Eur J Med Res. 2013;18:53. doi: 10.1186/2047-783X-18-53. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- Sallustio F, Rotondo F, Di Legge S, Stanzione P. Cilostazol in the management of atherosclerosis. Curr Vasc Pharmacol. 2010;8:363–372. doi: 10.2174/157016110791112331. [DOI] [PubMed] [Google Scholar]
- Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lection-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004;109:1022–1028. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, Kusuoka H, Nishimaru K, Tsushima M, Koretsune Y, Sawada T, Hamada C. CSPS 2 group (2010) Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 9:959–968. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- Symeonidis A, Kouraklis-Symeonidis A, Seimeni U, Galani A, Giannakoulas N, Fragopanagou E, Tiniakou M, Matsouka P, Zoumbos N. Ticlopidine-induced aplastic anemia: two new case reports, review, and meta-analysis of 55 additional cases. Am J Hematol. 2002;71:24–32. doi: 10.1002/ajh.10150. [DOI] [PubMed] [Google Scholar]
- Szczeklik A, Musial J, Undas A, Sanak M, Nizankowski R. Aspirin resistance. Pharmacol Rep. 2005;57(Suppl):33–41. [PubMed] [Google Scholar]
- Takahashi S, Oida K, Fujiwara R, Maeda H, Hayashi S, Takai H, Tamai T, Nakai T, Miyabo S. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of smooth muscle cell culture. J Cardiovasc Pharmacol. 1992;20:900–906. doi: 10.1097/00005344-199212000-00009. [DOI] [PubMed] [Google Scholar]
- Urbano LA, Bogousslavsky J. Antiplatelet drugs in ischemic stroke prevention: from monotherapy to combined treatment. Cerebrovasc Dis. 2004;17:74–80. doi: 10.1159/000074800. [DOI] [PubMed] [Google Scholar]
- Wong KS, Li H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke. 2003;34:2361. doi: 10.1161/01.STR.0000089017.90037.7A. [DOI] [PubMed] [Google Scholar]
- Yamada K, Fujimoto Y. Efficacy of cilostazol for intracranial arterial stenosis evaluated by digital subtraction angiography/magnetic resonance angiography. Adv Ther. 2011;28:866–878. doi: 10.1007/s12325-011-0060-y. [DOI] [PubMed] [Google Scholar]
- Yan Q, Shi ZH, Yang KH, Yian JH. Cilostazol for preventing ischemic stroke recurrence. Zhongguo Xunzheng Yixue Zazhi. 2011;11:540–544. [Google Scholar]


