Abstract
Inflammation is believed to play a central role in many of the chronic diseases that characterize modern society. In the past decade, our understanding of how dietary fats affect our immune system and subsequently our inflammatory status has grown considerably. There are compelling data showing that high-fat meals promote endotoxin [e.g., lipopolysaccharide (LPS)] translocation into the bloodstream, stimulating innate immune cells and leading to a transient postprandial inflammatory response. The nature of this effect is influenced by the amount and type of fat consumed. The role of various dietary constituents, including fats, on gut microflora and subsequent health outcomes in the host is another exciting and novel area of inquiry. The impact of specific fatty acids on inflammation may be central to how dietary fats affect health. Three key fatty acid–inflammation interactions are briefly described. First, the evidence suggests that saturated fatty acids induce inflammation in part by mimicking the actions of LPS. Second, the often-repeated claim that dietary linoleic acid promotes inflammation was not supported in a recent systematic review of the evidence. Third, an explanation is offered for why omega-3 (n–3) polyunsaturated fatty acids are so much less anti-inflammatory in humans than in mice. The article closes with a cautionary tale from the genomic literature that illustrates why extrapolating the results from inflammation studies in mice to humans is problematic.
Keywords: fatty acids, inflammation, endotoxin, linoleic acid, omega-3, microflora, lipopolysaccharide
Introduction
Inflammation is an essential component of the host response to infection or injury (1, 2). The inflammatory response involves the interactions among and between many different cell types. The classic symptoms associated with inflammatory responses include heat, redness, swelling, pain, and loss of function. Typically, inflammation is meant to be transient, but under some circumstances the acute response can become chronic (3). Excess chronic inflammation is an important etiologic factor in a wide range of common chronic diseases, including cardiovascular disease (4), diabetes (4), Alzheimer and other neurologic diseases (5), and cancer (6). Inflammatory responses result in local and systemic production of numerous soluble products, including C-reactive protein (CRP)4, TNF-α, IL-6, serum amyloid A, and plasminogen activator inhibitor 1, among others (7). Numerous epidemiologic studies have reported associations between one or more of these biomarkers and the risk of various chronic diseases. However, there is no consensus regarding which inflammatory biomarker is best. Instead, it appears that many researchers measure multiple biomarkers in any given study to increase the odds that associations with clinical outcomes will emerge. Importantly, recent reports provide support for the idea that different diseases are associated with specific inflammatory biomarker profiles, which relates to dysregulation of specific immune cell populations (8, 9). In the future one hopes that disease-specific inflammation biomarkers will be clearly defined. In the meantime, readers interested in gaining a better understanding of the many issues surrounding biomarker selection are encouraged to read the report of the European branch of the International Life Sciences Institute, which commissioned a review of the biomarkers for monitoring inflammation in human nutritional studies (10).
There exists a large body of evidence suggesting that a variety of dietary factors can enhance or diminish inflammation (11). The focus of this article is to describe how dietary fats affect inflammation and why it is an important human health consideration. Current dietary guidelines for fats provide information about both the amount as well as the types of fats that should be consumed (12). Because fats are energy dense, the guidelines set an upper limit of 35% of total calories from fats. The primary goal of setting this upper limit is to reduce the risk of developing obesity, a condition associated with elevated concentrations of several inflammatory biomarkers, including CRP and TNF-α (13).
Evidence suggests that, in addition to the amount of fat, the types of fats consumed can have a major impact on human health. Their impact on the risk of cardiovascular disease has dominated the rationale for these recommendations for the past 50 y (14–16). There is a growing recognition by leaders in the nutrition and health field that dietary fats can affect host inflammatory responses (17). After reviewing the evidence, it seems reasonable that the impact of dietary fats on inflammatory status be factored into future dietary guidelines for these nutrients.
Current Status of Knowledge
It appears that one way in which dietary fat is linked to inflammation is by promoting the translocation of microbial products from the gut into the bloodstream. Conservative estimates suggest that there are >100 trillion commensal (i.e., normal) organisms in the gut (18). These microorganisms, collectively referred to as the “gut microbiome,” contain >1 g of LPS. LPS is also referred to as endotoxin, because it is an endogenous component of the cell wall of all gram-negative bacteria and it can have “toxic” effects in most mammals (19). LPS is a very potent stimulus of inflammatory responses, with bioactivity in the microgram per liter concentration range. Importantly, there exists considerable diversity in the structure and bioactivity of LPS from microbial species (20). This diversity suggests that individual gut microbiomes may be more or less proinflammatory on the basis of how effectively innate immune receptors recognize these microbially derived agonists.
Researchers have known for decades that gut microbes could be a source of systemic bacterial infection leading to sepsis and organ failure under a variety of medical circumstances (21). Yet, it was only recently that researchers reported that dietary fat could promote endotoxin absorption. Cani et al. (22) reported that feeding mice a diet very high in fat (i.e., 72% of total energy) over 4 wk significantly elevated circulating endotoxin concentrations compared with mice fed a low-fat control diet. The data suggest that high-fat feeding results in a chronic elevation in circulating endotoxin throughout the day and night. The authors reported that the high-fat diet altered the distribution and numbers of some of the microbial populations found in the gut. Interestingly, the authors went on to demonstrate that high-fat feeding affected a number of metabolic processes associated with metabolic syndrome (e.g., hepatic TG accumulation, elevated fasting insulin, visceral adipose tissue accumulation) in a manner similar to infusion of LPS. Elevated expression of a number of inflammation biomarkers, such as TNF-α, IL-1, and IL-6, was observed in the liver, adipose, and muscle. These responses were surprisingly similar in mice infused with LPS compared with those fed the high-fat diet. However, it was the authors’ use of mice carrying a deletion of CD14, a critical component of the LPS receptor, which provided the most compelling evidence that many of the adverse effects of high-fat feeding may be a consequence of activation of inflammatory signaling pathways.
Current thinking is that the acute postprandial inflammatory response associated with fat consumption is mediated by endotoxin, primarily derived from gut microflora (23). Others suggested, however, that many foods contain endotoxin or related proinflammatory compounds that, upon absorption, directly stimulate inflammatory responses (24). Those foods with the most in vitro inflammatory activity included meats, cheeses, and dairy products. Regardless of source, once absorbed, endotoxin is shuttled between chylomicrons and HDL particles via a specific binding proteins and soluble receptors present in the circulation (25).
Rodent studies indicate that the structure/form of the dietary fat affects how much endotoxin is absorbed (26). There is a strong correlation between postprandial lipemia and net endotoxin absorption. Evidence suggests that chylomicrons play a critical role in the absorption and transport of endotoxin. Factors that promoted fat absorption, such as emulsification, also enhanced endotoxin absorption. Recently, Mani et al. (27) demonstrated that fat source affected postprandial endotoxin absorption and transport in the sera of domestic pigs. After overnight feed deprivation, pigs were fed a meal containing 12.5% by weight (∼25% of total energy) added fat or saline (control). The meal was consumed within 10 min, then blood samples were taken hourly for 5 h. Pigs fed coconut oil, rich in SFAs, had the highest circulating concentrations of endotoxin followed by those that were fed vegetable oil and then those fed fish oil. Additional testing on freshly isolated samples of ileum showed that the fats had not affected overall intestinal integrity or permeability. The authors suggested that the endotoxin absorbed was most likely transported by way of lipid raft–mediated endocytosis.
However, not all fat challenge studies reported significant findings. Tousoulis et al. (28) examined the impact of a single bout of fat/oil consumption on inflammation using soluble vascular cell adhesion molecule 1 assessment. Healthy subjects (n = 37) were randomly assigned to receive 50 mL of water or oil (e.g., corn oil, extra-virgin olive oil, soy oil, or cod liver oil). The authors found no statistical differences between treatment groups for circulating soluble vascular cell adhesion molecule 1 either pre- or post-treatment. The authors’ reliance on a single inflammatory biomarker is an important limitation of this study.
The selection of multiple inflammatory biomarkers, however, is still no guarantee that treatment effects will be observed. For example, Voon et al. (29) monitored circulating TNF-α, IL-1, IL-6, IL-8, and CRP in 45 healthy human subjects who participated in a randomized crossover intervention study. Three different test fats were examined for their impact on circulating cholesterol and inflammatory status. The test fats included a palmitic acid (16:0, hexadecanoic acid)–rich palm oil, coconut oil (rich in 12:0 + 14:0, dodecanoic + tetradecanoic acids), and virgin olive oil [rich in oleic acid (18:1, octadecenoic acid)]. The hypothesis was that fats rich in SFA, such as coconut and palm oils, would enhance circulating biomarkers of inflammation, and thus the inflammatory status of these healthy subjects. Fasting and nonfasting (2 h postprandial) blood samples were collected 5 wk after the start of the dietary interventions. The macronutrient content of the test diets were maintained at 20% of energy from protein, 30% from fat, and 50% from carbohydrates. The results failed to demonstrate any significant impact of fat source on any of these circulating biomarkers of inflammation. The most instructive aspect of these data was the significant inter- and intraindividual variation that was noted for these inflammation biomarkers. As a consequence of this variation, many such studies suffer from being underpowered.
Clearly, not all fats under all circumstances promote postprandial inflammation. There are insufficient data to predict when and how specific fat sources will affect inflammatory status in people. One possible explanation for the discrepancies in the literature is the variability in the types of microbes in the gastrointestinal tract of individuals being studied in these postprandial fat challenge studies. Recent advances in our understanding of how the gut microbiome adapts to changes in human ecology over time are particularly relevant in this context (30). It was suggested that the increasing incidence of allergic and metabolic disorders (e.g., obesity, type 2 diabetes) in immigrant populations as they adopt Western dietary patterns is linked to shifts in gut microbial populations. Whether this can explain why some individuals are more sensitive to systemic inflammation upon a fat challenge is unknown.
Recent findings suggest that dietary fats can influence gut microflora composition and that this can affect inflammatory status in vivo (31). In a recent study the effects of 3 different dietary fat sources on the normal microflora of C57BL/6 mice were examined (32). When compared with lard, milk fat (MF) and a PUFA-rich fat had similar effects on Bacteroidetes and Firmicutes; however, a large increase in a member of the Deltaproteobacteria, Bilophila wadsworthia, was consistently observed only with MF. Interestingly, these microbiota changes differed from those induced by lard-based SFAs.
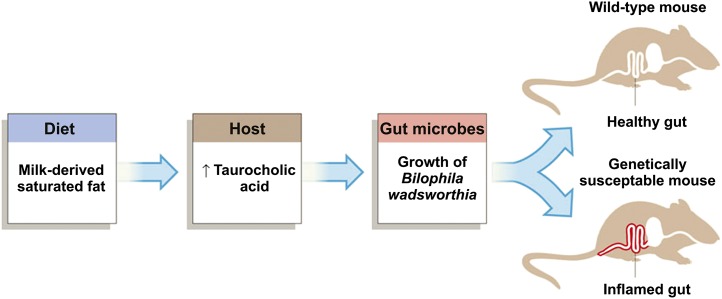
To demonstrate the clinical relevance of such a shift in gut microflora, the researchers conducted a follow-up study with a strain of mice that are susceptible to developing inflammatory bowel disease (i.e., Il10–null mice). Only MF consumption promoted colitis and inflammatory cytokine expression in the distal colonic mucosa of these Il10–null mice. These researchers went on to show that the mechanism by which MF affected gut microflora was related to changes in hepatic bile acid production (see Figure 1) (33). Whether dietary fats substantially affect inflammatory status of people by altering their gut microflora remains untested, but with the rapid advances in the field, answers should be forthcoming.
FIGURE 1.
Pathway from consuming milk fat to colitis by way of altered bile acid production and subsequent shifts in gut microbial populations. Reproduced from reference 33 with permission.
Apart from their role in promoting the uptake of endotoxin in the gut, many researchers believe that dietary fats are able to affect inflammation by more direct means. For example, it has long been known that SFAs are an essential structural component of bacterial endotoxins (19). The lipid A portion of all pathogenic LPS contain 6 ester- and/or amide-linked saturated fatty acyl groups. The length of the acyl chains in lipid A ranges from 12 to 16 carbons. The substitution of SFA with MUFA or PUFA eliminates the proinflammatory activity of LPS. Macrophages, and other cells of the innate immune system, possess receptors [i.e., toll-like receptor (TLR) 4] that recognize LPS (34). LPS-mediated signaling through TLR4 leads to the activation of NF-κB, a transcription factor, that subsequently turns on the expression of numerous proinflammatory cytokines, such as TNF-α, IL-1, IL-6, and IL-8. TLR4 is part of a larger family of receptors responsible for recognizing pathogen-associated molecular patterns (35). More than a dozen TLRs have been identified in humans and mice. These receptors are expressed throughout the body and are critical for host defense against invading pathogens.
In 2001, Lee et al. (36) were the first to demonstrate that SFAs were able to directly stimulate inflammatory gene expression by way of TLR4 signaling in vitro. The relative potency of various SFAs varied with chain length, with lauric acid (12:0) showing the greatest activity, whereas myristic acid (14:0) and stearic acid (18:0, octadecanoic acid) appeared to have surprisingly little proinflammatory activity. In contrast to SFAs, MUFAs and PUFAs failed to activate TLR4 signaling. Interestingly, these researchers were able to show that pretreatment of cells for 3 h with a variety of PUFAs or oleic acid (octadecaenoic acid; 18:1n–9) significantly reduced the subsequent proinflammatory effect of lauric acid treatment. They went on to show that the ability of PUFAs to block inflammatory responses induced by LPS or lauric acid was dependent on TLR4.
A few years later, this same group examined the impact of FAs on TLR2 signaling (37). TLR2, like TLR4, is a potent stimulator of inflammatory responses. Microbial components that are potent agonists for TLR2 include di- and triacylated lipoproteins, peptidoglycans, and lipoteichoic acid. It turns out that TLR2 only functions as part of a heterodimer with either TLR1 or TLR6. The rationale for exploring the potential of FAs to affect TLR2 signaling arose from the results of a study these researchers conducted with innate immune cells from TLR4-mutant mice. When bone marrow cells from TLR4-null mice were differentiated into macrophages and then treated with lauric acid the authors observed upregulation of cyclooxygenase 2 Ptgs2 expression, an inflammatory gene. Because these cells did not express TLR4, LPS treatment was without effect on cyclooxygenase 2 expression. These authors found that lauric acid stimulated TLR2-mediated signaling only when TLR2 was coexpressed with TLR1 or TLR6. In contrast to lauric acid, DHA (22:6n–3, an n–3 PUFA) treatment tended to diminish microbial agonist-mediated signaling of a wide variety of TLRs. More will be said about the anti-inflammatory activity of DHA in the section on n–3 FAs.
Linoleic acid (LA; 18:2n−6, octadecadienoic acid) is an n–6 PUFA and an essential nutrient (38). LA comprises ≥50% of the most widely consumed vegetable oils in Western societies. For many decades it has been known that LA helps reduce blood cholesterol concentrations and that substituting LA for SFAs lowers the risk of heart disease (39). Therefore, current recommendations from numerous expert bodies, including the Institute of Medicine and the American Heart Association, are that people should consume between 5% and 10% of total energy as LA for a heart-healthy diet (40).
However, a few members of the lipid research community have expressed concerned that LA-rich diets are unhealthy and promote inflammation (41, 42). The theoretical basis for this concern over LA’s proinflammatory actions involve a number of putative interrelated metabolic processes, including the following: 1) dietary LA promoting tissue arachidonic acid (AA; 20:4n−6, eicosatetraenoic acid) accumulation, 2) enhanced synthesis of proinflammatory eicosanoids derived from AA, 3) reduced conversion of α-linolenic acid (ALA; 18:3n−3, octadecatrienoic acid) into EPA (eicosapentaenoic acid; 20:5n–3) and/or DHA, and 4) diminished synthesis of anti-inflammatory eicosanoids from EPA and DHA. The experimental evidence supporting each step of this paradigm originated primarily from rodent and cell culture studies. More recently, and in contrast with the multistep process described above, it was suggested that various oxidized forms of LA are directly responsible for stimulating inflammation (43).
Fortunately, the first evidence-based review of all the human clinical data available that addressed the impact of dietary LA on inflammation in healthy adults was recently published (44). Fifteen studies (8 parallel and 7 crossover) met the inclusion criteria. The most important inclusion criterion was that the only FA other than LA that was allowed to differ substantially between the experimental and control dietary interventions was oleic acid. The reason for this was the existing evidence that suggested that oleic acid had no impact on inflammation (45). In contrast, it is believed that SFAs promote and n–3 PUFAs reduce inflammation; thus, simultaneous changes in the intake of those FAs along with LA could confound interpretation of the results. Heterogeneity between these studies prevented meta-analysis. Regardless of this limitation, not one of the studies reported a significant positive association between LA intake and circulating concentrations for a wide variety of inflammatory markers. More often than not, higher LA intake was associated with lower, not higher, inflammatory status in healthy adults. In addition, the results from a randomized controlled trial, which was completed and published after the systematic review, indicated that increasing LA intake from 4% to 13% of energy improved (i.e., diminished) biomarkers of inflammation in obese subjects (46). Not surprisingly, those subjects consuming extra SFAs from butter showed elevations in plasma markers of inflammation.
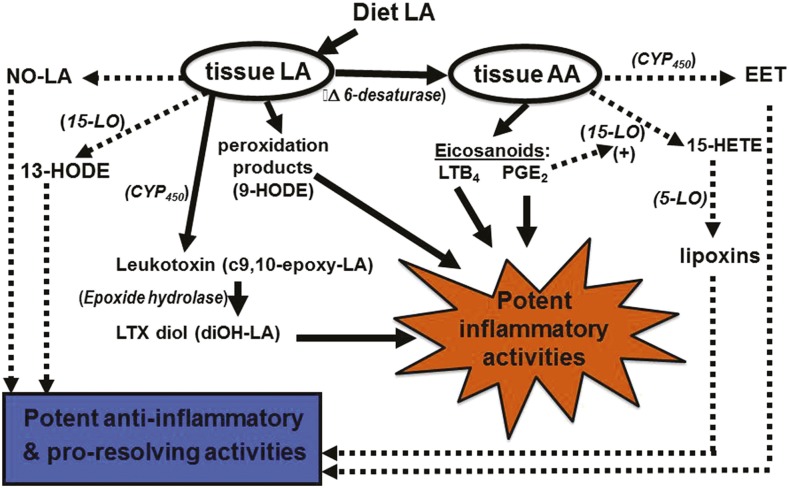
So an important question is why did the evidence from human clinical trials fail to support the theory that dietary LA promotes inflammation? One reason might be that the “LA-proinflammatory paradigm” relies on an overly simplified model of LA metabolism. Originally, most of the proinflammatory activity of dietary LA was thought to be a consequence of an accumulation of AA, which leads to greater production and release of proinflammatory eicosanoids, such as PGE2 and leukotriene B4 (LTB4). A review of the clinical literature by Rett and Whelan (47) indicated that increasing LA up to 6-fold within the context of a typical Western diet failed to increase tissue AA. Surprisingly, reducing dietary LA down to 10% of control was without effect on circulating AA. Therefore, for those who currently advocate for large reductions in dietary LA, their emphasis has shifted to the potential adverse effects of oxidized forms of this PUFA. Recent advances in analytical capabilities (i.e., lipidomics) have greatly expanded our knowledge of LA-derived metabolites (48). Yet, our understanding of the bioactivity and physiologic role of each of these novel metabolites remains incomplete. Figure 2 is meant to capture some of this complexity, at least as it relates to LA. Unfortunately, this author is unaware of a single publication that describes a research study in which all of the possible bioactive metabolites of LA and other important FAs have been measured and accounted for. Two excellent reviews related to this topic were recently published (49, 50).
FIGURE 2.
LA and AA metabolites play roles in both inflammation and resolution. Solid lines indicate proinflammatory pathways, and dotted/dashed lines represent anti-inflammatory/proresolving pathways. AA, arachidonic acid; CYP450, cytochrome P450; EET, epoxyeicosatrienoic acids; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; LA, linoleic acid; LO, lipoxygenase; LTB4, leukotriene B4; LTX, leukotoxin; NO, nitrosylated; PGE2, prostaglandin E2.
Much has been written about the potential health benefits associated with increasing our intake of n–3 PUFAs, including improved neurologic and cardiovascular health and diminished inflammation (51–53). n–3 PUFAs are a group of structurally related FAs. ALA is an 18-carbon n–3 PUFA from which all other n–3 PUFAs can be produced through a series of metabolic steps. Like LA, ALA is considered to be an essential nutrient for humans and most animals. DHA is the final end product of ALA elongation and desaturation (54). Although DHA is found in the highest concentrations in the brain and retina, it can be found in every cell membrane. Although consumption of ALA is an inefficient means for enriching cellular DHA content, consuming preformed DHA from various marine products (e.g., fish oil) can result in substantial increases in cellular DHA and EPA content (55).
Importantly, DHA modulation of immune cell function and subsequent inflammatory response are thought to be a result of one or more of these 3 actions. First, DHA (and EPA) are precursors for anti-inflammatory, proresolving lipid mediators known as resolvins, docosatrienes, and protectins (56). The anti-inflammatory activity of these dual-acting lipid mediators is a consequence of their promotion of neutrophil apoptosis and monocyte recruitment. These monocytes differentiate into macrophages that efficiently engulf the apoptotic neutrophils and depart the inflammatory site by way of the lymphatic system. The novel concept that resolution is not simply the absence of inflammation but a complex process that involves a programmed series of steps has only recently become widely accepted. Importantly, in addition to DHA and EPA, AA-derived lipid mediators (i.e., lipoxins) also play an important role in programming resolution.
Second, DHA is believed to affect lipid microdomains within cell membranes (i.e., lipid rafts) that play a role in immune cell signaling pathways critical to inflammation (57). These researchers reported that, although lauric acid promoted, DHA diminished the recruitment of TLR4 into lipid raft fractions after LPS treatment. This DHA action reduced TLR4 homodimerization and subsequent signaling through this key proinflammatory pathway. The ability of DHA to affect the physical properties of cellular membrane microdomains has been shown for a variety of cell types (58). DHA-mediated alterations in membrane structure can induce apoptosis in cancer cells, but its effects are highly dependent on the cell type and the molar concentration of DHA within membrane phospholipids.
A third possible mechanism of action for DHA (and EPA) relative to their anti-inflammatory activity recently emerged from the laboratory of Olefsky and coworkers (59). They reported that a novel G protein–coupled receptor 120 (GPR120) serves as a receptor/sensor for DHA (and to a lesser extent, EPA). Using a mouse macrophage cell line (i.e., RAW 264.7 cells) they demonstrated that stimulation of GPR120 with DHA or a chemical agonist resulted in a greatly diminished inflammatory response from these cells. By using small interfering RNA–mediated knockdown of GPR120, they were able to completely abrogate the anti-inflammatory activity of in vitro DHA treatment. The fact that most of the data generated in these studies used 100 μmol/L DHA, a concentration that greatly exceeds physiologic norms, raises some concerns about the actual role of GPR120 in vivo. The evidence that GPR120 is specific for DHA (and EPA) remains uncertain in light of the limited nature of the actual dose-response curves presented and the fact that palmitoleic acid (16:1n–7, hexadecenoic acid) was just about as effective as DHA (59).
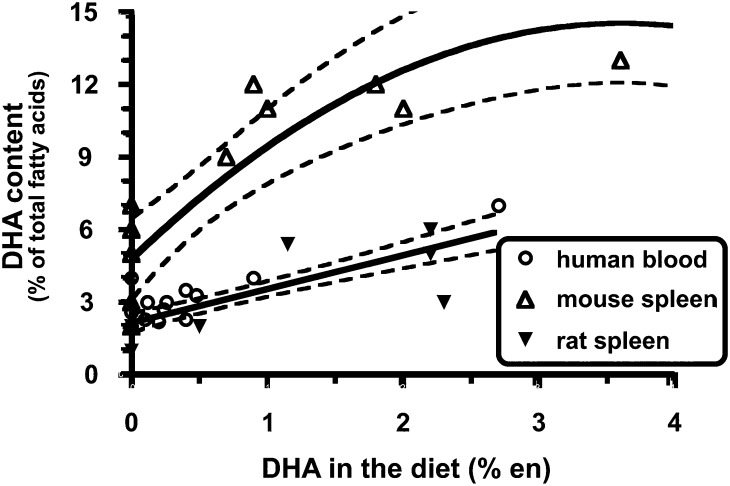
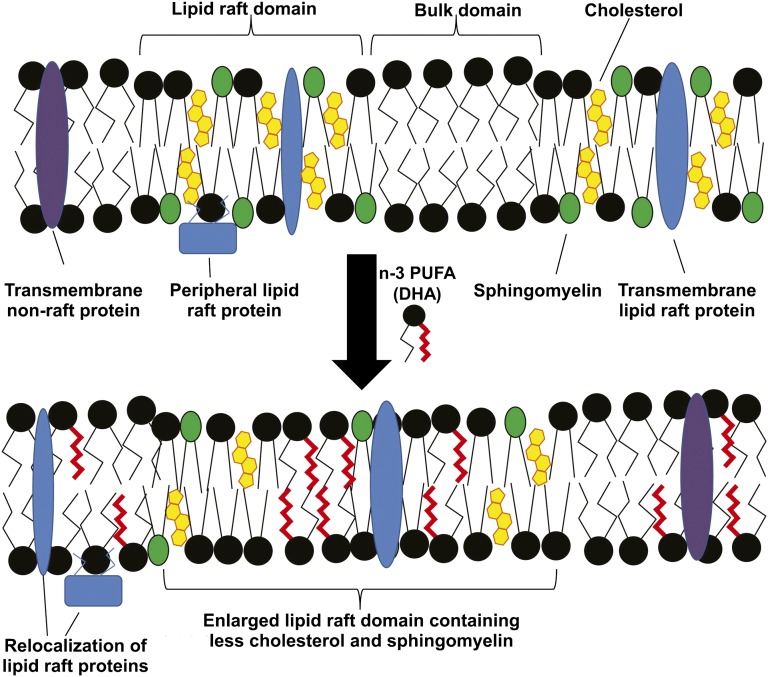
Regardless of which mechanism might explain the anti-inflammatory role of DHA, it would be reasonable to presume that when exploring the impact of dietary interventions on DHA-mediated immune and inflammatory responses, the molar concentration and the magnitude of change in membrane DHA content would be critical factors in determining to what extent cellular responses are altered. It is here where there is an important difference between mice and humans. Specifically, immune cells from mice start out with higher concentrations of DHA and, upon exposure to dietary DHA, the levels of enrichment in immune cell membranes far exceed what is possible in human immune cells (see Figure 3) (60). The dramatic accumulation of DHA in murine immune cells likely affects lipid rafts and cell signaling in ways that are not reproduced in the more modestly enriched human immune cells. Turk and Chapkin (61) illustrated how DHA enrichment might affect membrane-based lipid rafts (see Figure 4). These and other experts in the field (62) have discussed the dose-dependent nature of n–3 FAs on a number of health conditions, including inflammation. In addition, the lower concentrations of cellular EPA and DHA in human immune cells would in all likelihood result in more modest production of the anti-inflammatory lipid mediators than that produced by murine cells. Unfortunately, quantitative analyses of these lipid mediators have not been reported to date. These key species-dependent differences may help explain why dietary fish oil, a rich source of DHA, has such a powerful beneficial impact on a variety of inflammatory conditions in mice, whereas human clinical trials have shown much more modest benefits, if any.
FIGURE 3.
Quantitative comparison of dietary DHA with immune cell DHA from mice, rats, and humans. The data were from studies that met the following criteria: 1) dietary n–3 PUFA (i.e., EPA and/or DHA) intake was a dependent variable in the study design, 2) the FA profile of an identifiable immune cell population was reported, and 3) data were published and identified in PubMed (National Library of Medicine) through December 2005. n–3 PUFA intake is expressed as a percentage of total energy consumed (i.e., en%). In most studies, daily caloric intake was not reported. Thus, the following assumptions were made: 1) human subjects consumed 2000 kcal/d and 2) rodents consumed the same calories across diet treatment groups. Best-fit lines/curves with 95% CI displayed by dotted lines were generated by using Prism software version 4.0b (GraphPad). Reproduced from reference 60 with permission.
FIGURE 4.
Putative model for the effect of n–3 PUFAs on lipid rafts. Lipid rafts are nanoscale regions of the plasma membrane, enriched in cholesterol, sphingomyelin, and phospholipids containing saturated acyl chains. Both transmembrane and peripheral membrane proteins can be localized to lipid rafts. Upon treatment with a combination of n–3 PUFAs or DHA alone, these PUFAs are incorporated into phospholipids, which are inserted into both raft and nonraft regions of the plasma membrane. This results in enhanced clustering of lipid raft regions, which are depleted of cholesterol and sphingomyelin. In addition, many lipid raft–associated proteins “mislocalize” to the bulk membrane domain. This results in a suppression of lipid raft–mediated processes, including T cell activation and downstream signal transduction. Reproduced from reference 61 with permission.
Recently, a comprehensive analysis of the disparity between mouse and human responses relative to the potency of dietary n–3 PUFAs to affect inflammatory conditions was published (63). Consistent with the refractory nature of human immune cells to incorporate DHA into their membranes, one would predict that dietary intake of DHA would need to be much higher than what would be required to modulate immune cell function in mice, whose immune cells are more easily enriched with n–3 PUFAs. In fact, that is exactly what the author concluded: “In adult humans, an EPA plus DHA intake >2 g/d seems to be required to elicit anti-inflammatory actions.” Because such intake amounts cannot readily be obtained through dietary means, these effects should be considered pharmacologic and not nutritional in nature.
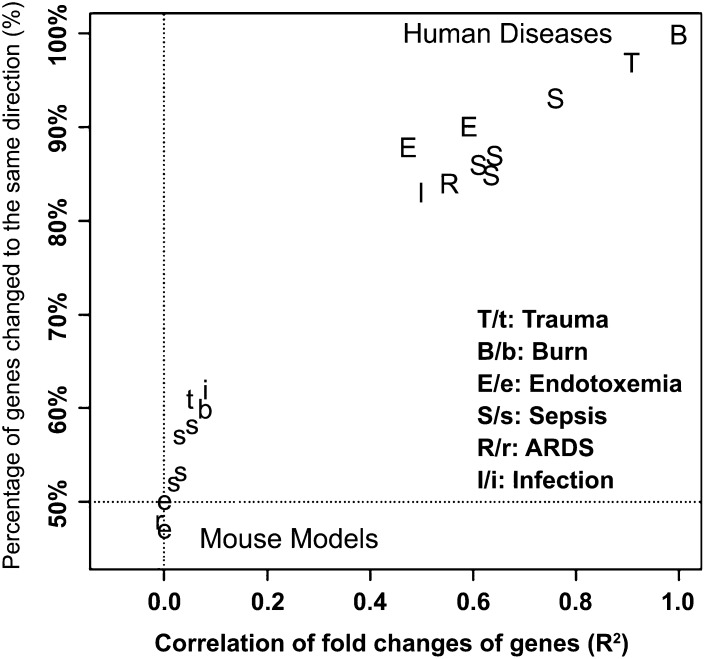
Recent data from genomic screening experiments in mice and humans suggest that there is another reason that results from mouse feeding studies with n–3 PUFA as well as other fat sources may have led to findings that were not predictive of responses in humans (64). In this report, the authors compared the temporal changes in the expression of thousands of genes from blood leukocytes isolated from humans or mice after 3 forms of serious trauma: burns, endotoxemia, and blunt injury. The genomic responses in circulating human leukocytes to these diverse forms of trauma were surprisingly similar. Yet, among the genes that were changed significantly in humans, the mouse orthologs failed to reflect similar changes (R2 between 0.0 and 0.1; see Figure 5). These data suggest that mouse models poorly reflect the physiologic responses seen in humans to systemic inflammatory challenges. In fact, similar conclusions were drawn in a 2007 article, in which the clinical outcomes (e.g., circulating cytokines, leukopenia, fever, changes in respiration) associated with sepsis in humans and the various “relevant” mouse models were compared (65). Researchers should therefore exercise caution when relying solely on mouse models for investigating the impact of dietary fats on inflammatory responses/status in humans.
FIGURE 5.
Comparison of the genomic response in circulating leukocytes to severe acute inflammation from 6 distinct causes in human and murine models. GEO was queried for studies in the white blood cells of severe acute inflammatory diseases (i.e., burns, endotoxemia, trauma, sepsis, ARDS, and infection) in humans and mice. The fold-change of each gene measured was calculated between patients and controls in a human study or between treated and control groups in a murine model study; and for a time-course data set, the maximum fold-change was calculated. The gene response in each data set was then compared with the 5554 genes that were significantly changed in human trauma, burns, and endotoxemia. Shown are correlations (x axis) and directionality (y axis) of gene response from the resulting multiple published data sets in GEO compared with human burn injury. ARDS, acute respiratory distress syndrome; GEO, Gene Expression Omnibus. Reproduced from reference 64 with permission.
Conclusions
Dietary fats have a major impact on human health. A growing body of evidence suggests that inflammatory status should be included as one of the characteristics for which dietary fats are evaluated relative to their impact on human health. At this time, it is uncertain how dietary fats might affect inflammatory status, but current evidence suggests that the gut microbiome is important in this regard. Studies should account for the possibility that fats can have both acute as well as chronic effects on host inflammatory responses. Whereas cell culture and animal models play an important role in biomedical research, limitations inherent in these models suggest that data from human clinical trials will continue to have primacy in setting dietary recommendations for fats and FAs. In light of the lack of consensus regarding which biomarker is best for monitoring inflammatory status, it is recommended that as many inflammation biomarkers be measured as feasible and that studies be appropriately powered in recognition of the highly variable nature of these biomarkers.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; ALA, α-linolenic acid; CRP, C-reactive protein; GPR120, G protein–coupled receptor 120; LA, linoleic acid; LTB4, leukotriene B4; MF, milk fat; TLR, toll-like receptor.
References
- 1.Henson PM. Dampening inflammation. Nat Immunol 2005;6:1179–81. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature 2002;420:846–52. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C, Ding A. Nonresolving inflammation. Cell 2010;140:871–82. [DOI] [PubMed] [Google Scholar]
- 4.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7. [DOI] [PubMed] [Google Scholar]
- 5.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 2007;7:161–7. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen GL, Henson PM. Mediators of inflammation. Annu Rev Immunol 1983;1:335–59. [DOI] [PubMed] [Google Scholar]
- 8.Nikolajczyk BS, Jagannathan-Bogdan M, Denis GV. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev 2012;249:253–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol 2014;222:R113–27. [DOI] [PubMed] [Google Scholar]
- 10.Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, Holgate ST, Jonsson LS, Latulippe ME, Marcos A. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr 2013;109(Suppl 1):S1–34. [DOI] [PubMed] [Google Scholar]
- 11.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol 2006;26:995–1001. [DOI] [PubMed] [Google Scholar]
- 12.Kris-Etherton PM, Innis S, Ammerican DA. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc 2007;107:1599–611. [PubMed] [Google Scholar]
- 13.Trayhurn P, Wood I. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 2005;33:1078–81. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 2001;20:5–19. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. . Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Zhao L, Hwang DH. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev 2010;68:38–61. [DOI] [PubMed] [Google Scholar]
- 18.Guarner F, Malagelada J-R. Gut flora in health and disease. Lancet 2003;361:512–9. [DOI] [PubMed] [Google Scholar]
- 19.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Di Padova F. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J 1994;8:217–25. [DOI] [PubMed] [Google Scholar]
- 20.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 2005;3:36–46. [DOI] [PubMed] [Google Scholar]
- 21.Deitch EA. Bacterial translocation of the gut flora. J Trauma 1990;30:S184–89. [DOI] [PubMed] [Google Scholar]
- 22.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Touhy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–72. [DOI] [PubMed] [Google Scholar]
- 23.Berg RD. Bacterial translocation from the gastrointestinal tract. In: Mechanisms in the pathogenesis of enteric diseases. Vol. 2. Springer Science+Business Media, New York; 1999. p. 11–30.
- 24.Erridge C. The capacity of foodstuffs to induce innate immune activation of human monocytes in vitro is dependent on food content of stimulants of Toll-like receptors 2 and 4. Br J Nutr 2011;105:15–23. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Hailman E, Wright SD. Lipopolysaccharide binding protein and soluble CD14 catalyze exchange of phospholipids. J Clin Invest 1997;99:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laugerette F, Vors C, Géloën A, Chauvin M-A, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 2011;22:53–9. [DOI] [PubMed] [Google Scholar]
- 27.Mani V, Hollis JH, Gabler NK. Dietary oil composition differentially modulates intestinal endotoxin transport and postprandial endotoxemia. Nutr Metab (Lond) 2013;10:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tousoulis D, Papageorgiou N, Antoniades C, Giolis A, Bouras G, Gounari P, Stefanadi E, Miliou A, Psaltopoulou T, Stefanadis C. Acute effects of different types of oil consumption on endothelial function, oxidative stress status and vascular inflammation in healthy volunteers. Br J Nutr 2010;103:43–9. [DOI] [PubMed] [Google Scholar]
- 29.Voon PT, Ng TKW, Lee VKM, Nesaretnam K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0+14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am J Clin Nutr 2011;94:1451–7. [DOI] [PubMed] [Google Scholar]
- 30.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol 2011;65:411–29. [DOI] [PubMed] [Google Scholar]
- 31.Burcelin R, Garidou L, Pomié C, editors. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. In: Seminars in immunology. Elsevier; 2012. [DOI] [PubMed]
- 32.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in IL10−/− mice. Nature 2012;487:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnbaugh PJ. Microbiology: fat, bile and gut microbes. Nature 2012;487:47–8. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol 1999;162:3749–52. [PubMed] [Google Scholar]
- 35.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003;21:335–76. [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 2001;276:16683–9. [DOI] [PubMed] [Google Scholar]
- 37.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem 2004;279:16971–9. [DOI] [PubMed] [Google Scholar]
- 38.Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J 2006;1:420–39. [DOI] [PubMed] [Google Scholar]
- 39.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902–7. [DOI] [PubMed] [Google Scholar]
- 40.Flock MR, Kris-Etherton PM. Dietary Guidelines for Americans 2010: implications for cardiovascular disease. Curr Atheroscler Rep 2011;13:499–507. [DOI] [PubMed] [Google Scholar]
- 41.Lands WE. Dietary fat and health: the evidence and the politics of prevention: careful use of dietary fats can improve life and prevent disease. Ann N Y Acad Sci 2005;1055:179–92. [DOI] [PubMed] [Google Scholar]
- 42.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999;70(Suppl):560S–9S. [DOI] [PubMed] [Google Scholar]
- 43.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013;346;e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet 2012;112(7):1029–41, e15. [DOI] [PubMed]
- 45.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr 2004;79:969–73. [DOI] [PubMed] [Google Scholar]
- 46.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M. Effects of n−6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 2012;95:1003–12. [DOI] [PubMed] [Google Scholar]
- 47.Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond) 2011;8:36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dennis EA. Lipidomics joins the omics evolution. Proc Natl Acad Sci USA 2009;106:2089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delmastro-Greenwood M, Freeman BA, Gelhaus Wendell S. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Ann Rev Physiol 2014;76:79–105. [DOI] [PMC free article] [PubMed]
- 50.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 2013;53:37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 2010;68:280–9. [DOI] [PubMed] [Google Scholar]
- 52.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins J, Capps NE. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ 2006;332:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc 2009;109:668–79. [DOI] [PubMed] [Google Scholar]
- 54.Brenna JT. Efficiency of conversion of α-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care 2002;5:127–32. [DOI] [PubMed] [Google Scholar]
- 55.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am J Clin Nutr 2006;83(Suppl):1467S–76S. [DOI] [PubMed] [Google Scholar]
- 56.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong SW, Kwon M-J, Choi AM, Kim H-P, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 2009;284:27384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev 2005;45:559–79. [DOI] [PubMed] [Google Scholar]
- 59.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fritsche K. Important differences exist in the dose–response relationship between diet and immune cell fatty acids in humans and rodents. Lipids 2007;42:961–79. [DOI] [PubMed] [Google Scholar]
- 61.Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 2013;88:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol 2011;668:S50–8. [DOI] [PubMed] [Google Scholar]
- 63.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr 2012;142(Suppl):592S–9S. [DOI] [PubMed] [Google Scholar]
- 64.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 2013;110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 2007;81:137–43. [DOI] [PubMed] [Google Scholar]