Abstract
Over the past few decades, the Dietary Guidelines for Americans has consistently recommended that consumers decrease consumption of saturated fatty acids due to the correlation of saturated fatty acid intake with coronary artery disease. This recommendation has not been easy to achieve because saturated fatty acids play an important role in the quality, shelf life, and acceptability of foods. This is because solid fats are critical to producing desirable textures (e.g., creaminess, lubrication, and melt-away properties) and are important in the structure of foods such as frozen desserts, baked goods, and confectionary products. In addition, replacement of saturated fats with unsaturated fats is limited by their susceptibility to oxidative rancidity, which decreases product shelf life, causes destruction of vitamins, and forms potentially toxic compounds. This article will discuss the fundamental chemical and physical properties in fats and how these properties affect food texture, structure, flavor, and susceptibility to degradation. The current sources of solid fats will be reviewed and potential replacements for solid fats will be discussed.
Keywords: fats, oils, shortbread cookies, hydrogenation, oxidation, health, cholesterol, dietary, baked goods
Introduction
Food lipids can be categorized into fats and oils, with fats being solid at room temperature. Fats are typically higher in SFAs, which have higher melting points than do unsaturated FAs. Over the past few decades, the Dietary Guidelines for Americans (1) has consistently recommended that consumers decrease their intake of SFAs because of the correlation of dietary SFAs with increased concentrations of LDL cholesterol and increased risk of coronary artery disease. This recommendation has more recently been focused on decreased consumption of solid fats because this can be an easier way for consumers to identify fats that are high in SFAs (1).
One might expect that with these consistent recommendations to limit the consumption of solid fats, our diet would have shifted more toward the use of highly unsaturated oils. However, such a switch is not easily accomplished in many types of foods because fats and oils play different roles in the quality, shelf life, and acceptability of foods. For example, naturally occurring solid fats are important in the textural properties of dairy, meat, and some plant foods (2, 3). Solid fats can play a role in creaminess (e.g., yogurt and milk), lubrication (ice cream and meats), and the feeling of solid fat converting to liquid oil in the mouth (melt-away; e.g., chocolate and butter). Solid fats are also important in prepared foods because they play a role in forming solid crystal networks that are important to food texture (frozen desserts, chocolate, and butter), allowing the incorporation of air into baked products to produce fluffy textures (cookies), minimizing oil migration out of the foods (crackers and cookies), and producing flaky textures by inhibiting gluten network formation (pastries) (4–6). On the other hand, incorporation of unsaturated fats into prepared foods can be challenging because they are susceptible to oxidative deterioration (7, 8). Lipid oxidation is problematic because it causes the formation of “off” flavors that decrease shelf life, the destruction of vitamins, and the formation of potentially toxic compounds (7–9). Therefore, in many foods the use of highly unsaturated oils is limited by their chemical liability.
Food lipids are also a nutritional concern due to their high caloric density (9 kcal/g vs. 4 kcal/g for carbohydrates and proteins) (2). Therefore, foods high in fats are typically high in calories and thus could be linked to obesity (1). Several attempts have been made by the food industry to decrease calories for fats, such as the physical removal of fats, use of noncaloric fat substitutes, and the addition of nonlipids that can mimic the sensory properties of lipids (2).
Because solid fats are so important in both food quality and nutrition, the food industry has tried for many years to decrease the use of SFAs in foods. Originally, many foods were produced with animal fats such as lard and tallow (the original fat in McDonald’s French fries). However, concerns about cholesterol consumption led to the elimination of animal fats in many foods (10). The removal of a critical food ingredient requires a replacement additive with similar chemical and physical properties, which prompted the food industry to respond to this challenge by increasing the use of tropical oils (palm, coconut, and palm kernel) to provide a solid and stable fat. Tropical fats can be substituted for animal fats because of their high amounts of unsaturated FAs. However, nutrition research has also suggested for some years that dietary saturated fats cause an increase in LDL cholesterol (1, 10). This led the food industry to switch to hydrogenated fats, which could be made with high amounts of unsaturated FAs, were still solid at room temperature, and had good oxidative stability due to a small fraction of saturated fats and the elimination of the most unsaturated FAs such as α-linolenic acid (2). However, the discovery that the trans FAs formed during hydrogenation increased LDL cholesterol and also decreased the “good" HDL cholesterol (11) prompted labeling requirements for trans FAs in foods in the United States and thus eliminated the use of hydrogenated FAs in many foods. These numerous changes in recommendations for dietary fats over the past several decades have led to broad consumer confusion over which fats are healthiest.
This review provides an overview of how the chemical structure of lipids affects their physical and chemical properties and thus their use in foods. The development of new technologies to provide alternatives to solid fats will be discussed. Selected technologies to decrease lipid concentrations in foods will also be covered both in this article as well as in the subsequent article by McClements (57), which is also included in this supplement issue.
Melting Properties of Fats and Oils
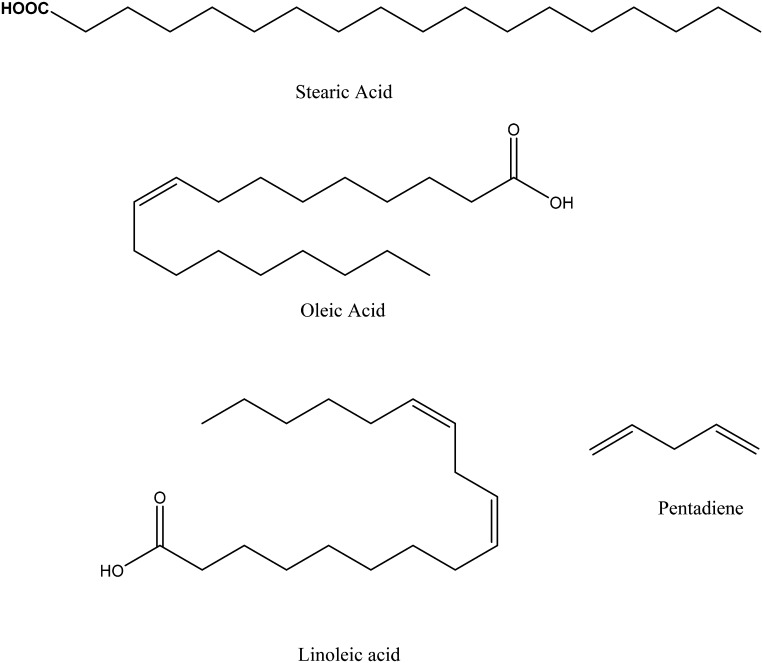
Lipids are defined as compounds that are soluble in organic solvents. Food lipids consist of many compounds including free and esterified FAs, sterols (e.g., cholesterol), carotenoids, and fat-soluble vitamins (2). The large majority of lipids in foods are in the form of TGs. TGs consist of glycerol esterified to 3 FAs. FAs contain a carboxylic acid with an aliphatic chain. The chain length is most commonly <22 carbons, and the large majority of FAs have even carbon numbers (12). FAs can be saturated, meaning that they are fully hydrogenated, or unsaturated, meaning that they contain double bonds. The double bonds on natural unsaturated FAs are typically in the cis configuration. PUFAs contain more than one double bond in a pentadiene structure (Figure 1) with the double bonds being 3 carbons apart (12).
FIGURE 1.
Examples of SFAs, MUFAs, and PUFAs.
TG molecules are oriented so that the 2 FAs at the ends of the glycerol molecule point in one direction, and the FA in the middle point in the opposite direction (2, 12). As with other nonpolar molecules, the most important types of molecular interaction between TGs are van der Waals attraction and steric repulsion (2, 13). The van der Waals interactions favor association of the molecules, whereas steric repulsion determines how closely the molecules can pack together. The physical state (e.g., solid or liquid) of TG molecules is primarily determined by calculating the change in energy (G) due to the balance between enthalpy (H) temperature (T), and entropy (S) (ΔG = ΔH – TΔS). The enthalpy effects are mainly governed by changes in the strength of the molecular interactions (e.g., van der Waals attraction) associated with the phase change, whereas the entropy effects are mainly determined by the structural organization of the molecules. At low temperatures, the enthalpy term dominates (ΔH), which favors the crystalline state (stronger attractive interactions), whereas at high temperatures, the entropy term dominates (TΔS), which favors the liquid state (more disorder). The temperature at which the free energy change is zero (ΔH = TΔS) is the melting point of the TG molecule. Typically, the melting point increases with increasing molecular weight of the TG because the entropy contribution that opposes crystallization becomes smaller for bigger molecules (2, 14).
A major factor that affects the melting point of FAs is the geometric shape of the molecules. SFAs are more linear than unsaturated FAs with cis double bonds (12), allowing them to pack together closely and thus have the potential for more attractive molecular interactions. This means that a higher amount of energy is needed to disrupt the attractive molecular interactions and convert a solid fat to a liquid oil. Thus, SFAs have higher melting points than unsaturated FAs with the same carbon number. The trans double bonds cause the shape of FAs to be intermediate between SFAs and unsaturated FAs, thus producing intermediate melting points. For example, stearic (18:0), eliadic (trans-18:1), and oleic (cis-18:1) FAs have melting points of 70°C, 45°C, and 13°C, respectively (15).
Although the melting points of individual FAs are important, it is the combination of FAs on a TG that defines the melting properties of food lipids because almost all FAs in foods are in the form of TGs (2). TGs can have their FAs randomly distributed on the glycerol (e.g., tallow) or they can have the FAs in specific locations (16). For example, cocoa butter contains primarily oleic cis 18:1, stearic (18:0), and palmitic (16:0) acids with oleic acid mainly at the middle sn-2 position and stearic and palmitic at sn-1 and sn-3. A fat with many different types of FAs in a random configuration has many different TGs and a broad melting range. TGs with only a few FAs in very specific locations has a narrow melting range (2, 13). This is the case for cocoa butter, which has a very narrow melting range, and because its combination of FAs produces a melting range near body temperature (33°C), it melts quickly in the mouth, producing a very desirable melt-away texture. Tallow has a broad melting range, with some TGs melting above body temperature. This produces a fat that would melt slowly and partially remain solid in the mouth, creating a sandy texture.
Role of Solid Fat in the Physical Properties of Foods
There is probably no better example of the role of solid fat in the quality of foods than is seen in chocolate. The lipids in chocolate have a melting range at or below the temperature of the mouth, allowing them to melt in the mouth to produce very desirable melt-away properties. The melting properties in fats such as cocoa butter are not just dependent on their TG composition but also on their crystal structure (17). TGs have a polymorphic crystal structure, meaning that they have multiple crystal forms. These crystals have different packing densities; therefore, the number of van der Waals interactions can vary and thus melting points of the different crystals will be different. Food processors use techniques such as tempering or seeding to achieve the desired crystal type and thus melting properties (18).
Solid fats are also used to form structures in products such as margarines, spreads, and butter. In this case, solid-fat crystals form networks of gel-like structures that can entrap liquid oil (2). These networks allow products such as margarine to be made with small amounts of high-melting SFAs that entrap large amounts of unsaturated FAs to produce a solid fat that contains >60% unsaturated FAs (19).
Solid fats also form structures in products such as ice cream, whipped cream, and baked goods by aiding in the incorporation of air (2, 4, 13). This occurs during the mixing of these products; the air produces a light texture that gives a desirable mouthfeel and changes the appearance of the product. In the case of ice cream, the product is both mixed and cooled simultaneously allowing air to be mixed in the product. The fat, sugar, and water crystallize, entrapping air in a gel-like network (4). In the case of whipped cream, a similar process occurs, but because there is no cooling and thus no ice crystal formation, higher amounts of fats are required to form the fat crystal network. Solid fats are critical in the formation of the gel network in whipped cream, which is why many recipes have instructions to keep the cream as cold as possible, including cooling the bowl used to whip the cream (5).
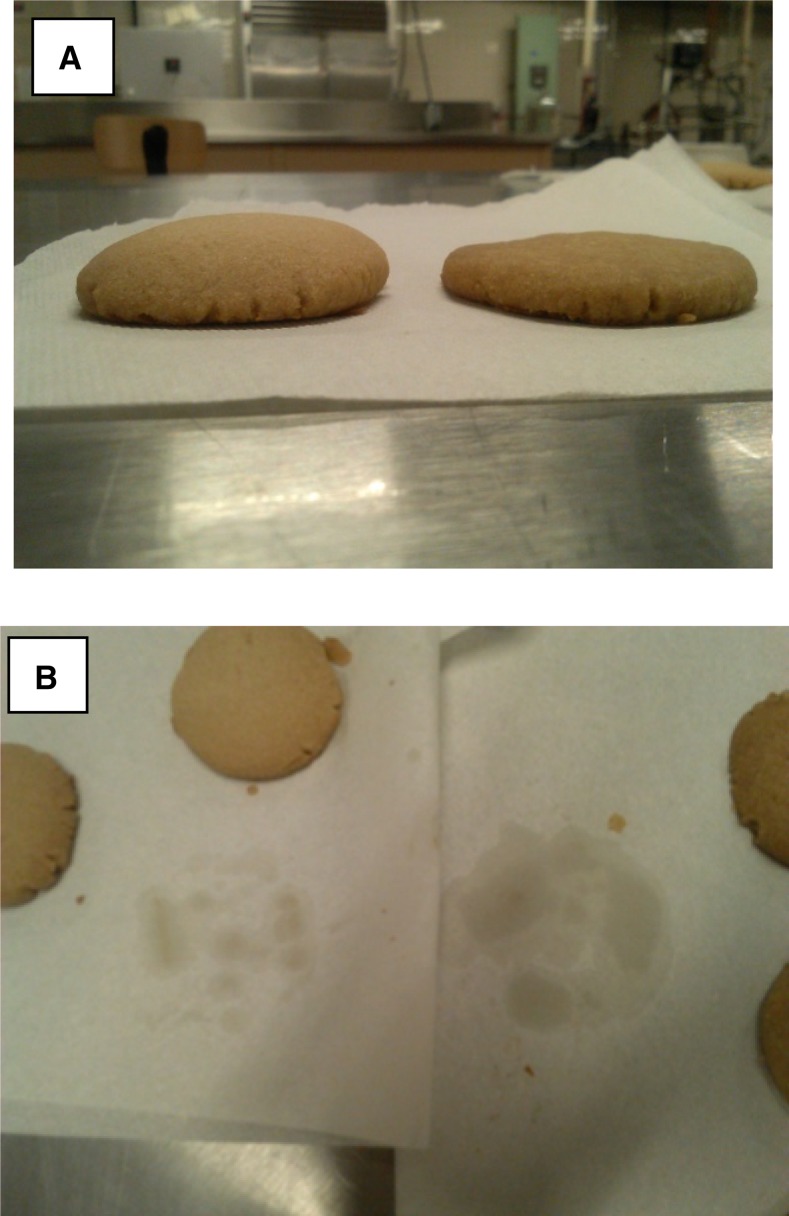
In baked goods, solid fat is mixed with sugar in a process known as creaming. Creaming imparts air into the product, helps entrap gases from leavening agents, and produces a fluffy texture (6). Preparing baked goods, such as cookies, with liquid oil produces a flat product, and because the fat is liquid, it can migrate to the surface of the cookie to produce a greasy texture (Figure 2). Pastry is a baked good that also has a large amount of fat (25–30%; 19) mixed into the flour. Bread typically contains much lower amounts of fat, which allows the gluten in flour to form strong disulfide crosslinks, producing very elastic dough that can entrap large amounts of carbon dioxide (from either yeast or chemical leavening agents) and results in an airy, yet chewy product. Pastry, cookies, and crackers compared with bread have higher amounts of fats, resulting in lipid-gluten interactions that inhibit gluten disulfide crosslinking, which results in a flaky and/or soft texture (5). In these products, kneading and rolling of the dough are critical to ensure the gluten is coated with solid fat during product formation. Solid fats can also play a similar role in products such as cookies and biscuits to produce soft and crumbly textures vs. the elastic properties of bread. If fat content is high enough, little to no water is required and an extremely soft texture is produced, which melts in the mouth (5). Combinations of fat and sugar will result in a syrupy mixture that does not harden upon cooling, thus making soft cookie textures (5).
FIGURE 2.
Short-bread cookies prepared with solid shortening (left side of panels) and liquid vegetable oils (right side of panels). Note the increased height of the cookie prepared with shortening due to higher air incorporation (A) and less oil migration in the cookie produced with solid fat as seen by less oil absorbed into the paper below the cookie (B).
Solid Fats for Use in Foods
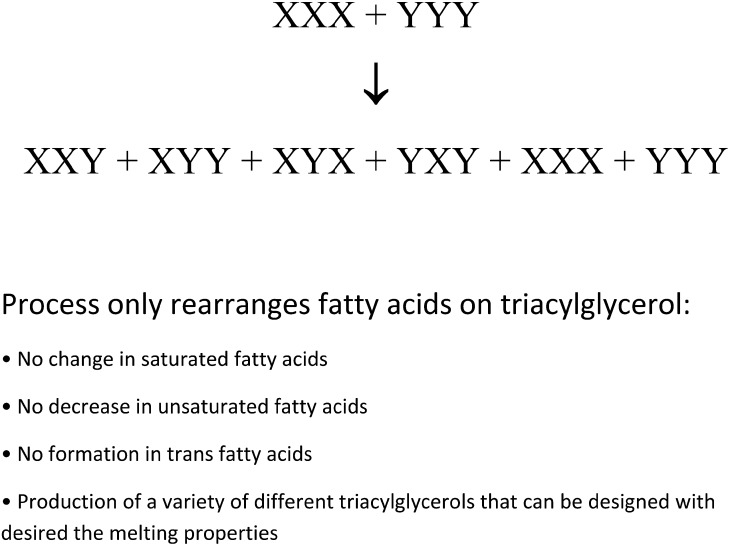
As mentioned earlier, the original solid fats in foods were animal fats such as butter, lard, and tallow, but their use has been curtailed in the food industry because of their cholesterol content and price (butter). Hydrogenated fats were also commonly used as a source of solid fat, but due to trans labeling requirements, this is now rarely the case (20). Current sources of solid fats include fat blends in which manufacturers use fats high in SFAs such as palm oil and blend with liquid oils such as canola, cottonseed, and soybean (21, 22). These blended fats are useful in many foods, but they sometimes produce uneven melting curves due to distinct differences in the melting of the solid and liquid fat fractions. To overcome this problem, interesterification can be used to produce a fat with a gradual melting profile. In this process, a blend of lipids high in saturated (e.g., tropical or 100% hydrogenated fats) and unsaturated FAs is mixed with a catalyst such as sodium methoxide (23) or various lipase sources (24). This process initially produces a diacylglycerol anion (2). The diacylglycerol anion then accepts an FA from another TG and produces a second diacylglycerol anion (Figure 3). This process is called transesterification, and when it is conducted with a chemical catalyst, the result consists of randomized TGs containing a combination of the FAs from both the original highly saturated and highly unsaturated FA lipid sources. Enzymes (lipases) can be used to direct interesterification because of the specificity of the lipases for FAs at difference positions on the glycerol (e.g., positions 1 and 3) (25). Interesterification is quite expensive because of the processing cost and lower yields of TGs (mono- and diacylglycerol byproducts are formed, which must be removed from the final product). Because of the increased cost, interesterified fats were initially used in higher-cost products such as margarines but are now more commonly available for numerous food products (21, 22).
FIGURE 3.
Chemical mechanisms and consequences in the chemical interesterification of lipids. X and Y indicate different FAs.
A new potential source of food ingredients that could replace solid fats are oleogels or organogels (26). These products are formed by the addition of compounds (organogelators) to liquid oils where they form crystalline networks at low concentrations and entrap liquid oils. The organogelators must have a balance between solubility in the oil (so they can interact with the oil components) while maintaining insolubility (so they can crystalize and self-assemble into a gel network). The subsequent solid fats can contain liquid oil fractions of up to 99%, meaning that they can be very high in unsaturated FAs with minimal trans FA concentrations. Organogelators that are suitable for foods include phytosterols, ceramides, lecithin, plant waxes, and monoacylglycerols. Phytosterols and ceramides have a higher cost compared with lecithin, plant waxes, and monoacylglycerols. One example of a food-approved organogelator is candelilla wax, which comes from the Yerba evergreen shrub. Candelilla wax contains several potential organogelators including alkanes, sterols, FAs, fatty alcohols, and waxy esters. Among these, the primary organogelator seems to be n-alkanes that range from 29 to 33 carbons and make up >40% of the candelilla wax. Co and Marangoni (26) published an excellent review of the properties of oleogels produced from ethylcellose. Oleogels have been successfully incorporated into products such as sausages (23); however, their potential to be incorporated into other food products is largely unknown.
As mentioned above, partial hydrogenation using traditional technologies can produce high amounts of trans FAs. Because trans FAs have negative health effects and their appearance on Nutrition Facts panels is undesired, research has been conducted to find alternate hydrogenation technologies that produce low amounts of trans FAs. Nickel is the most extensively used metal catalyst due to its high activity, low cost, and easy removal after hydrogenation (27). The development of alloy nano-catalysts from platinum, palladium, or nickel-boron was shown to reduce the concentration of trans FAs by half compared with the commercial nickel catalyst (27). These technologies are mainly limited by the high cost of these alternative catalysts.
Electrochemical hydrogenation is a novel method of reducing the double bonds in unsaturated FAs. Electrochemical hydrogenation has a high reaction rate, which leads to a high degree of full hydrogenation of the unsaturated FAs, resulting in lower reformation of double bonds into trans-isomer configurations (28). The process involves a cathode made of an electrically conducting metal catalyst and an anode for oxidation reactions. The cathode adsorbs hydrogen reduction products from water or H2 that react with the double bonds on FAs, forming SFAs. The concentration of atomic hydrogen on the cathode surface can be controlled by the applied current, which allows the process to occur at moderate temperature and pressure while minimizing the isomerization of double bonds into trans FAs (29).
Supercritical fluid state hydrogenation is an additional novel method used to reduce formation of trans FAs. The supercritical fluid state (e.g., supercritical carbon dioxide) has a good homogeneous phase, which improves the transfer of the hydrogen to the metal catalysts surface, thus increasing the rate at which unsaturated FAs are fully hydrogenated and decreasing the formation of trans FAs (28).
Utilization of Highly Unsaturated Oils in Foods
The Dietary Guidelines for Americans (1) not only recommends decreased consumption of solid fats but also replacement of solid fats with liquid oils. This recommendation can be very helpful for fats used in cooking (e.g., butter vs. olive oil), but as mentioned above, liquid oils will often not produce the desirable textural properties of products such as cookies, pastry, confectionaries, ice cream, and whipped cream. The Dietary Guidelines for Americans (1) also recommends increased consumption of fish containing omega-3 (ω-3) FAs due to their benefits to heart health.
The challenge of incorporating polyunsaturated oils and ω-3 FAs into foods is their susceptibility to oxidative deterioration (rancidity), resulting in changes in both negative alteration in food quality and nutrition (7–9). This is especially true of ω-3 FAs that not only oxidize quickly but also have oxidation products which humans can detect at concentrations as low at 1 μg/L (8). Therefore, food manufacturers must not only be concerned about how the physical properties of fats and oils affect foods but also the chemical stability of these nutritionally beneficial lipids. If lipids are not protected from oxidation, the resulting rancidity will prevent their consumption, thus decreasing their health benefits.
Chemical Deterioration of Lipids in Foods
Lipids can chemically degrade by numerous mechanisms to negatively affect food quality, including thermal degradation, hydrolytic rancidity, and oxidative rancidity. Thermal heating of oils causes TGs to hydrolyze into FFAs and glycerol, which can volatilize and cause oil to smoke (30). Smoke points decrease with increasing FFA concentrations (31). This means that unrefined oils (e.g., olive oil), which have not gone through the neutralization step of refining and thus have high FFA concentrations, have lower smoke points and may not be suitable for frying. In addition, the high temperature of frying in combination with the addition of water into the oil from the frying of the food will accelerate hydrolysis of TGs, thus increasing FFA concentrations and decreasing smoke points. This problem can be decreased by occasionally filtering the oil with a filter aid that removes FFAs and prolongs the shelf life of the oil (30).
Optimal frying temperatures are ∼180°C. Unrefined oils such as extra-virgin olive oil have a smoke point of 191°C (31), which means that it could be used for frying. However, if this oil is used for prolonged frying and FFAs increase, the smoke point can decrease to the frying temperature. This creates a problem because thermal degradation of frying oils can produce acrolein, a potentially toxic compound (9). In addition, the smoke point is followed by the flash point, and if the oil is heated too long it could catch on fire (31).
Oils can also chemically degrade by nonthermal hydrolytic rancidity. This occurs in the presence of water at high temperatures and at extremes in pH (2). In addition, many biological materials from which the oils are extracted contain lipases that convert TGs to FFAs and glycerol. Lipases can cause FFA accumulation during the extraction of oil because extraction can cause the release of lipase and/or oil from subcellular organelles allowing the enzyme and lipid to interact (32). Microbial lipases can also hydrolyze lipids in dairy products such as cheeses that contain microbial starter cultures (33). In most foods, FFAs negatively affect quality because they can produce soapy flavors and decrease smoke points (31). However, in some cases, small amounts of FFAs can be important positive flavor notes such as in cheeses (34).
Lipid oxidation is a free radical chain reaction between unsaturated fats and oxygen (8). Free radicals are highly reactive molecules that have unpaired electrons (2, 8). These unpaired electrons seek an electron pair, thus promoting reactions such as addition, transfer, and scission. Lipid radicals can react with the unpaired electrons in oxygen, resulting in the addition of oxygen onto the FA to form peroxyl radicals. The peroxyl radical can then abstract a hydrogen from one unsaturated FA and transfer the radical to another FA, thus resulting in the formation of lipid hydroperoxides (2, 8). The hydroperoxides are susceptible to decomposition by light and metals to form alkoxyl radicals. These alkoxyl radicals are very high in energy, allowing them to promote the scission of FAs into low-molecular-weight volatile compounds that produce rancid off-flavors and aromas.
Rancidity in food occurs when the concentration of unsaturated FA decomposition products exceed its sensory threshold levels. Most FA decomposition products have negative aromas such as grassy and fishy, thus causing degradation in food quality (8). However some, such as 2,4-decadienal, have positive flavor notes and in this case produce the typical flavor of fried foods (8). FA decomposition products and free radicals can cause additional food quality problems. Free radicals can react with other molecules, resulting in degradation of vitamins, loss of color, and changes in protein functionality (2, 35). FA decomposition products, especially unsaturated aldehydes, can react with proteins to alter their function and have been postulated to alter protein function in vivo, promoting diseases such as atherosclerosis (9).
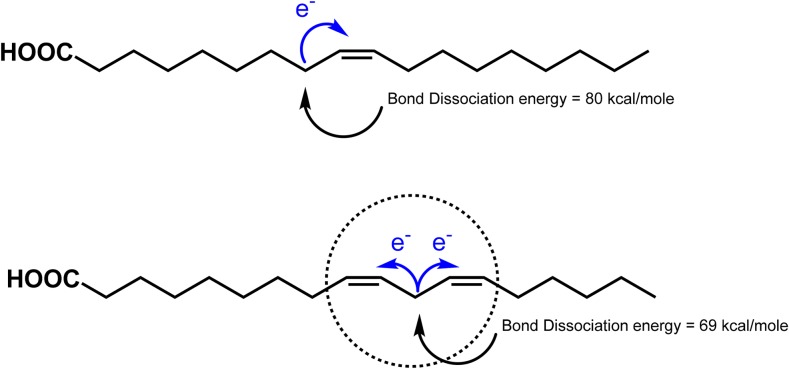
The susceptibility of oils and foods to lipid oxidation is influenced by numerous factors. Probably the most important factor is the chemical structure of the FAs and in particular the number, position, and geometry of double bonds (2, 8). Saturated lipids are much more stable to oxidation than unsaturated lipids, which makes them desirable in foods very susceptible to oxidation such as frying oils (2, 8, 36). Double bonds increase the susceptibility of FAs to oxidation, with an increasing number of double bonds increasing oxidative susceptibility. For example, the oxidation rates of oleate (cis 18:1), linoleate (cis 18:2), and linolenate (cis 18:3) have been reported to be 1:40–50:100 on the basis of oxygen uptake and 1:12:25 on the basis of hydroperoxide formation (8). The ω-3 FAs EPA (20:5) and DHA (22:6) are even more susceptible to oxidation, meaning that they are extremely difficult to incorporate into foods. Increasing susceptibility to oxidation in unsaturated FAs is due to the ability of double bonds to attract electrons from neighboring carbons. This makes the hydrogen on the carbon more susceptible to abstraction and thus free radical formation. Two double bonds make the FA even more susceptible to oxidation because 2 double bonds attract the electrons from the neighboring carbon and thus the ease of hydrogen abstraction increases dramatically (Figure 4) (2, 8, 36). This is why MUFAs are often preferable to PUFAs in processed foods.
FIGURE 4.
Differences in the bond dissociation energies of the hydrogen adjacent to double bonds in oleic (cis 18:1) and linoleic (cis 18:2) acids. Decreased bond dissociation energies increase susceptibility to oxidation due to easier hydrogen abstraction.
The detrimental effect of lipid oxidation in foods and nutrition can be controlled by numerous strategies. Because oxygen is one of the main components of the reaction, its removal can decrease oxidation rates. Unfortunately, FA radical-oxygen reactions are very fast because they are both free radicals (2, 8, 36). This means that almost complete oxygen removal, in processes such as vacuum packaging, is needed to effectively control oxidation.
The rate of oxidation of lipids, similar to most chemical reactions, approximately doubles for every 10°C increase in temperature (8). However, temperature also decreases oxygen solubility and thus can alter oxidation rates (31). For example, oxidation rates are slow in heated frying oils and only increase dramatically when foods are added to the oil and the subsequent steam generation increases oxygen incorporation into the oil (2, 8). Low temperature storage can increase oxidation rates when SFAs crystalize out of the oil, resulting in a more highly unsaturated oil that can be more prone to oxidation (38).
Water is a key factor affecting lipid oxidation, with oxidation rates being highest at both high (0.6–0.8) and low water activities (0–0.2). Water affects lipid oxidation by hydrogen bonding with hydroperoxides to increase hydroperoxide stability and by altering the hydration, solubility, and mobility of pro-oxidants such as metal (8).
Lipid oxidation is often referred to as auto-oxidation because it can be a self-propagating reaction. However, the reality is that most foods contain pro-oxidants that can be the primary drivers of oxidation, thus increasing reaction rates and changing kinetics such that auto-oxidation is often only a minor component of the cause of rancidity (36, 38).
Transition metals are likely the most important lipid oxidation pro-oxidants in foods (38). Transition metals are weak pro-oxidants on their own, but in the presence of lipid and hydrogen peroxides, they become highly reactive because they can decompose peroxides into alkoxyl, hydroxyl, and peroxyl radicals, which can attack unsaturated FAs. Metals are active at μg/L levels and they are found in essentially all foods at this concentration (38). Lipid hydroperoxides are also ubiquitous to all fats and oils because some oxidation occurs during lipid isolation and refining (39). The rate of hydroperoxide breakdown is dependent on the concentration, chemical state, and type of metal. Iron is generally more important to control than copper due to its higher prevalence. However, copper is 50-fold more reactive than iron, so in some cases, it can be an important pro-oxidant. The state of the metal is also important because reduced iron (Fe2+) is much more efficient at decomposing hydroperoxides and is much more soluble (40). Because of the high reactivity of reduced metals, some antioxidants (e.g., ascorbic acid) can sometime act as pro-oxidants because they increase the reactivity of metals (41).
Pro-oxidants such as lipoxygenases (42, 43) and singlet oxygen (44) can promote the formation of lipid hydroperoxides. These hydroperoxides are the substrate of other pro-oxidants such as iron and copper, which break down the peroxide into free radicals that attack other unsaturated FAs (40). The prevention of hydroperoxide decomposition is probably the most important step in food quality because this reaction leads to the cleavage of FAs to produce the low-molecular-weight, volatile compounds that are responsible for rancid aromas (36, 41).
Light and irradiation also promote lipid oxidation. UV light can promote hydroperoxide decomposition to form free radicals. Irradiation, on the other hand, can produce hydroxyl radicals (•OH) from water, leading to the initiation of lipid oxidation. Light is also important in singlet oxygen formation when it excites photosensitizers such as chlorophyll and riboflavin (44).
Antioxidants
Improving the health and wellness of foods by increasing the amount of unsaturated lipids can often be accomplished through the addition of antioxidants. The most common type of antioxidants are those that scavenge free radicals (7, 41). This reaction results in the transfer of the free radical from the FA to the antioxidant to form an antioxidant radical. This reaction inhibits lipid oxidation because the phenolic structure of the antioxidant can delocalize the free radical throughout the molecule, which lowers the energy to the free radical. This decreases oxidation rates because the antioxidant free radical no longer has enough energy to abstract a hydrogen atom from unsaturated FAs, thus stopping free radical propagation (41).
The effectiveness of free radical scavenging antioxidants is dependent on both their chemical properties and their physical location within a food (7, 8, 41). Many studies have compared the effectiveness of various antioxidants with differing solubility properties in different food systems such as bulk oil and in oil-in-water emulsions (45–48). These studies showed that hydrophilic antioxidants are most effective in bulk oil, whereas hydrophobic antioxidants are most effective in oil-in-water emulsions. This observation is known as the “antioxidant polar paradox” polar antioxidants are effective in oil, and nonpolar antioxidants are effective in foods with high water contents (45–49). The reason for this effect is that hydrophobic antioxidants are retained in the lipid phase of high-moisture food where they are able to interact with lipid phase free radicals. Conversely, hydrophilic antioxidants are effective in bulk oils because they can concentrate at the water-oil interface of association colloids (50).
The most common commercially available free radical scavenging hydrophobic antioxidants are the synthetics butylated hydroxytoluene (BHT) and butylated hydroxylanisole (BHA), ascorbyl palmitate, the tocopherol homologs, and carnosol and carnosic acid, which are found in rosemary (41). Commercially available hydrophilic antioxidants include the synthetic propyl gallate and naturally occurring ascorbic acid, catechins (green tea), and rosmarinic acid (rosemary). Tertiary butyl hydroxyquinone (TBHQ) has intermediate polarity and tends to be one of the most effective antioxidants (7, 41).
Research on the antioxidant polar paradox has led to the development of a new generation of antioxidants. These free radical scavenging antioxidants are conjugates of phenolic compounds with aliphatic chains of varying carbon number (51). In “phenolipids,” the antioxidant components have been phenolic, such as chlorogenic acid, rosmarinic acid, and caffeic acid. These antioxidants are unique because they can be made with varying hydrophobicity but similar free radical scavenging activity. The phenolic antioxidant portion of the molecule does not change, but the aliphatic chain can range from 0 to 20 carbons. Although these antioxidants are not currently approved for food use, they have been instrumental in providing information on how the physical location of antioxidants affects their ability to control lipid oxidation. Research by several groups has shown that antioxidants with intermediate polarity (8- to12-carbon aliphatic chains) are the most effective antioxidants in oil-in-water emulsions, and polar antioxidants (no added aliphatic chains) are most effective in bulk oils (51–53). Interestingly, in oil-in-water emulsions, the most hydrophobic antioxidants were not the most effective, which is contrary to the antioxidant polar paradox (51). Recent research has shown that rosmarinic acid with a 20-carbon ester has very little antioxidant activity in oil-in-water emulsions, even though almost all of the antioxidant is in the emulsion droplet (51). The addition of surfactant to the emulsions increased the amount of the antioxidant at the emulsion droplet interface and increased antioxidant activity. This suggested that the concentration of antioxidants at the oil-water interface increases their activity, which is most likely due to the ability to locate at the same location as lipid hydroperoxides and surface-active free radicals (54).
The second most common antioxidant strategy is to control the reactivity of pro-oxidant metals with chelators. The most common commercially available chelators are citric acid, phosphates, and EDTA (2, 38). It is believed that carboxyl groups of citric acid bind with metals and form complexes, thus retarding lipid oxidation. Not all compounds capable of binding metals are antioxidative because chelation can increase metal solubility and thus reactivity (41, 55). In addition, combinations of chelators and free radical scavenging antioxidants are often more effective than the individual antioxidants (41) due to the ability of the chelator to reduce metal-promoted generation of free radicals that can decompose phenolic antioxidants.
Antioxidants are sometimes multifunctional, acting as both free radical scavengers and metal chelators. A good example of this phenomenon is proteins. Proteins contain amino acids such as tyrosine, phenylalanine, histidine, cysteine, methionine, and lysine that can scavenge free radicals (35). However, at pH values above their isoelectric point, proteins are negatively charged and thus can also chelate metals. In fact, in emulsions, this chelating activity is often the major antioxidant mechanism. This is because many of the free radical scavenging amino acids are buried in the interior of the protein where they cannot interact with free radicals. Exposing these amino acids by denaturing or hydrolyzing the protein can often increase antioxidant activity [for review see (35)].
Conclusions
Nutritional recommendations for dietary fats and oils continue to evolve as we learn more about the impact of FAs on health. However, most nutritional organizations agree that the consumption of saturated fats should be decreased and polyunsaturated fats and ω-3 FA consumption should be increased. Making major alterations in the lipid composition of foods can be quite challenging because solid fats have important physical properties that allow the formation of foods such as baked goods, butter, and ice cream. In addition, polyunsaturated oils and ω-3 FAs are very susceptible to oxidation, leading to development of off-flavors, loss of nutrients, and formation of potentially toxic compounds. Therefore, the substitution of highly unsaturated fats for solids fats could have negative nutritional consequences unless technologies are utilized to prevent their oxidation. These challenges, along with the removal of hydrogenated fats from the food supply, are driving food manufacturers to utilize oils high in MUFAs because these FAs have higher melting points and are more oxidatively stable. MUFAs tend to be neutral with regard to heart health so this change in fat source could lead to further unintended consequences in consumer health.
Acknowledgments
All authors read and approved the final manuscript.
References
- 1.USDA; US Department of Health and Human Services. Dietary guidelines for Americans. 7th ed. Washington (DC): US Government Printing Office; 2010.
- 2.McClements DJ, Decker EA. Lipids. In: Srinivasan D, Parkin KL, Fennema OR, editors. Fennema's food chemistry. 4th ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. p. 155–216. [Google Scholar]
- 3.Marangoni A, Narine S. Physical properties of lipids. New York: Marcel Dekker; 2002. [Google Scholar]
- 4.Goff HD, Hartel RW. Ice cream. 7th ed. New York: Springer; 2013. [Google Scholar]
- 5.McGee H. Cereal doughs and batters. In: On food and cooking: the science and lore of the kitchen. 2nd ed. New York: Scribner; 2004. p. 515–69. [Google Scholar]
- 6.Pareyt B, Delcour JA. The role of wheat flour constituents, sugar, and fat in low moisture cereal based products: a review on sugar-snap cookies. Crit Rev Food Sci Nutr 2008;48:824–39. [DOI] [PubMed] [Google Scholar]
- 7.Decker EA, Chen B, Panya A, Elias RJ. Understanding mechanisms of oxidation and antioxidant activity. In: Decker EA, Chen B, Panya A, Elias RJ, editors. Oxidation in foods and beverages and antioxidant applications. Vol.1. London: Woodhead Publishing; 2010. p. 225–43. [Google Scholar]
- 8.Frankel EN. Lipid oxidation. 2nd ed. Bridgewater (United Kingdom): The Oily Press; 2005. [Google Scholar]
- 9.Kubow S. Routes of formation and toxic consequences of lipid oxidation-products in foods. Free Radic Biol Med 1992;12:63–81. [DOI] [PubMed] [Google Scholar]
- 10.Kritchevsky D. History of recommendations to the public about dietary fat. J Nutr 1998;128(Suppl):449S–52S. [DOI] [PubMed] [Google Scholar]
- 11.Katan MB, Zock P, Mensink R. Trans-fatty-acids and their effects on lipoproteins in humans. Annu Rev Nutr 1995;15:473–93. [DOI] [PubMed] [Google Scholar]
- 12.Lobb K, Chow CK. Fatty acids classification and nomenclature. In: Chow CK, editor. Fatty acids in foods and their health implications. 3rd ed. New York: Marcel Dekker; 2008. p. 1–16. [Google Scholar]
- 13.Marangoni AG, Acevedo N, Maleky F, Co E, Peyronel F, Mazzanti G, Quinn B, Pink D. Structure and functionality of edible fats. Soft Matter 2012;8:1275–300. [Google Scholar]
- 14.Israelachvili J. Intermolecular and surface forces. 3rd ed. London: Academic Press; 2011. [Google Scholar]
- 15.O'Neil MJ. editor. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. 14th ed. Whitehouse Station (NJ): Merck; 2006. [Google Scholar]
- 16.Decker EA. The role of stereospecific saturated fatty acid positions on lipid nutrition. Nutr Rev 1996;54:108–10. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo NC, Peyronel F, Marangoni AG. Nanoscale structure intercrystalline interactions in fat crystal networks. Curr Opin Colloid Interface Sci 2011;16:374–83. [Google Scholar]
- 18.Koyano T, Hachiya I, Sato K. Fat polymorphism and crystal seeding effects on fat bloom stability of dark chocolate. Food Structure. 1990;9:231–40. [Google Scholar]
- 19.USDA; Agricultural Research Service. USDA National Nutrient Database for Standard Reference, release 26. 2013 [cited 2014 Jan 1]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 20.Downs SM, Thow AM, Leeder SR. The effectiveness of policies for reducing dietary trans fat: a systematic review of the evidence. Bull World Health Organ 2013;91:262–9H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ADM (Archers Daniel Midland). 2013. Lower trans blends. [cited 2014 Jan 1]. Available from: http://www.adm.com/en-US/products/food/oils/Pages/LowerTransBlends.aspx.
- 22.Bunge Oils. 2013. Bakery applicable lab. [cited 2014 Jan 1]. Available from: http://www.bungebiic.com/bakery-applications-lab/.
- 23.Zeitoun M, Neff W, List G, Mounts T. Physical-properties of interesterified fat blends. J Am Oil Chem Soc 1993;70:467–71. [Google Scholar]
- 24.Ghosh S, Bhattacharyya D. Utilization of high-melting palm stearin in lipase-catalyzed interesterification with liquid oils. J Am Oil Chem Soc 1997;74:589–92. [Google Scholar]
- 25.Abigor R, Marmer W, Foglia T, Jones K, DiCiccio R, Ashby R, Uadia P. Production of cocoa butter-like fats by the lipase-catalyzed interesterification of palm oil and hydrogenated soybean oil. J Am Oil Chem Soc 2003;80:1193–6. [Google Scholar]
- 26.Co ED, Marangoni AG. Organogels: an alternative edible oil-structuring method. J Am Oil Chem Soc 2012;89:749–80. [Google Scholar]
- 27. Li T, Zhang W, Lee RZ, Zhong Q. Nickel-boron alloy catalysts reduce the formation of trans fatty acids in hydrogenated soybean oil. Food Chem 2009;15;114(2):447–52.
- 28.Jang E, Jung M, Min D. Hydrogenation for low trans and high conjugated fatty acids. Comp Rev Food Sci Food Safety 2005;4:22–30. [DOI] [PubMed] [Google Scholar]
- 29.List GR, Warner K, Pintauro P, Gil M. Low-trans shortening and spread fats produced by electrochemical hydrogenation. J Am Oil Chem Soc 2007;84:497–501. [Google Scholar]
- 30.Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci 2007;72:R77–86. [DOI] [PubMed] [Google Scholar]
- 31.Formo MW, Jungermann E, Norris FA, Sonntag NOV. Bailey’s industrial oil and fats products. 4th ed. New York: John Wiley & Sons; 1979. [Google Scholar]
- 32.Matthaeus B. Oil technology. Adv Bot Res 2007;45:483–527. [Google Scholar]
- 33.Jooyandeh H, Kaur A, Minhas KS. Lipases in dairy industry: a review. J Food Sci Technol Mysore 2009;46:181–9. [Google Scholar]
- 34.Murtaza MA, Ur-Rehman S, Anjum FM, Huma N, Hafiz I. Cheddar cheese ripening and flavor characterization: a review. Crit Rev Food Sci Nutr 2014;54:1309–21. [DOI] [PubMed] [Google Scholar]
- 35.Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 2008;48:430–41. [DOI] [PubMed] [Google Scholar]
- 36.McClements D, Decker E. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci 2000;65:1270–82. [Google Scholar]
- 37.Okuda S, McClements D, Decker E. Impact of lipid physical state on the oxidation of methyl linolenate in oil-in-water emulsions. J Agric Food Chem 2005;53:9624–8. [DOI] [PubMed] [Google Scholar]
- 38.Decker EA, McClements DJ, Chaiyasit W, Nuchi C, Sivestre MPC, Mancuso JR, Tong LM, Mei L. Factors influencing free radical formation in food emulsions. In: Ho CT, Shahidi F, editors. Free radicals in health and food. Washington (DC): ACS Press; 2002. p. 83–97. [Google Scholar]
- 39.Nuchi CD, McClements DJ, Decker EA. Impact of Tween 20 hydroperoxides and iron on the oxidation of methyl linoleate and salmon oil dispersions. J Agric Food Chem 2001;49:4912–6. [DOI] [PubMed] [Google Scholar]
- 40.Dunford HB. Free-radicals in iron-containing systems. Free Radic Biol Med 1987;3:405–21. [DOI] [PubMed] [Google Scholar]
- 41.Decker EA. Antioxidant mechanisms. In: Akoh CC, Min DB, editors. Food lipids, chemistry, nutrition and biotechnology. 3rd ed. New York: Marcel Dekker; 2008 p. 475–98. [Google Scholar]
- 42.Zhuang H, Barth MM, Hildebrand D. Fatty acid oxidation in plant lipids. In: Akoh CC, Min DB, editors. Food lipids, chemistry, nutrition and biotechnology. 2nd ed. New York: Marcel Dekker; 2002. p. 413–64. [Google Scholar]
- 43.Montillet J, Agnel J, Ponchet M, Vailleau F, Roby D, Triantaphylides C. Lipoxygenase-mediated production of fatty acid hydroperoxides is a specific signature of the hypersensitive reaction in plants. Plant Physiol Biochem 2002;40:633–9. [Google Scholar]
- 44.Paillous N, Feryforgues S. Interest of photochemical methods for induction of lipid-peroxidation. Biochimie 1994;76:355–68. [DOI] [PubMed] [Google Scholar]
- 45.Frankel E, Huang S, Prior E, Aeschbach R. Evaluation of antioxidant activity of rosemary extracts, carnosol and carnosic acid in bulk vegetable oils and fish oil and their emulsions. J Sci Food Agric 1996;72:201–8. [Google Scholar]
- 46.Hopia A, Huang S, Schwarz K, German J, Frankel E. Effect of different lipid systems on antioxidant activity of rosemary constituents carnosol and carnosic acid with and without alpha-tocopherol. J Agric Food Chem 1996;44:2030–6. [Google Scholar]
- 47.Huang S, Frankel E, Schwarz K, German J. Effect of pH on antioxidant activity of alpha-tocopherol and trolox in oil-in-water emulsions. J Agric Food Chem 1996;44:2496–502. [Google Scholar]
- 48.Huang S, Hopia A, Schwarz K, Frankel E, German J. Antioxidant activity of alpha-tocopherol and trolox in different lipid substrates: Bulk oils vs oil-in-water emulsions. J Agric Food Chem 1996;44:444–52. [Google Scholar]
- 49.Porter WL. Paradoxical behavior of antioxidants in food and biological-systems. Toxicol Ind Health 1993;9:93–122. [DOI] [PubMed] [Google Scholar]
- 50.Chen B, Han A, Laguerre M, McClements DJ, Decker EA. Role of reverse micelles on lipid oxidation in bulk oils: impact of phospholipids on antioxidant activity of alpha-tocopherol and Trolox. Food Funct 2011;2:302–9. [DOI] [PubMed] [Google Scholar]
- 51.Laguerre M, Bayrasy C, Lecomte J, Chabi B, Decker EA, Wrutniak-Cabello C, Cabello G, Villeneuve P. How to boost antioxidants by lipophilization? Biochimie 2013;95:20–6. [DOI] [PubMed] [Google Scholar]
- 52.Laguerre M, Giraldo LJL, Lecomte J, Figueroa-Espinoza M, Barea B, Weiss J, Decker EA, Villeneuve P. Relationship between hydrophobicity and antioxidant ability of "phenolipids" in emulsion: a parabolic effect of the chain length of rosmarinate esters. J Agric Food Chem 2010;58:2869–76. [DOI] [PubMed] [Google Scholar]
- 53.Laguerre M, Giraldo LJL, Lecomte J, Figueroa-Espinoza M, Barea B, Weiss J, Decker EA, Villeneuve P. Chain length affects antioxidant properties of chlorogenate esters in emulsion: the cutoff theory behind the polar paradox. J Agric Food Chem 2009;57:11335–42. [DOI] [PubMed] [Google Scholar]
- 54.Panya A, Laguerre M, Bayrasy C, Lecomte J, Villeneuve P, McClements DJ, Decker EA. An investigation of the versatile antioxidant mechanisms of action of rosmarinate alkyl esters in oil-in-water emulsions RID B-9787-2009. J Agric Food Chem 2012;60:2692–700. [DOI] [PubMed] [Google Scholar]
- 55.Mahoney J, Graf E. Role of alpha-tocopherol, ascorbic-acid, citric-acid and EDTA as oxidants in model systems. J Food Sci 1986;51:1293–6. [Google Scholar]
- 56.McClements D. Reduced-fat foods: the complex science of developing diet-based strategies for tackling overweight and obesity. Adv Nutr 2015;6:338–52. [DOI] [PMC free article] [PubMed] [Google Scholar]