Abstract
Fat plays multiple roles in determining the desirable physicochemical properties, sensory attributes, nutritional profile, and biologic response of food products. Overconsumption of fats is linked to chronic diseases, such as obesity, coronary heart disease, diabetes, and cancer. There is therefore a need to develop reduced-fat products with physicochemical properties and sensory profiles that match those of their full-fat counterparts. In addition, foods may be redesigned to increase the feelings of satiety and satiation, and thereby reduce overall food intake. The successful design of these types of functional foods requires a good understanding of the numerous roles that fat plays in determining food attributes and the development of effective strategies to replace these attributes. This article provides an overview of the current understanding of the influence of fat on the physicochemical and physiologic attributes of emulsion-based food products and highlights approaches to create high-quality foods with reduced-fat contents.
Keywords: reduced-fat, obesity, overweight, food design, food matrix effects, satiety, functional foods, emulsions
Introduction
Overconsumption of calorie-rich foods has been linked to increasing incidences of chronic human diseases, such as obesity, coronary heart disease, diabetes, hypertension, and cancer (1–3). The food industry is responding to this problem by developing reduced-calorie products that consumers can incorporate into their regular diet. However, the creation of reduced-calorie foods that consumers find desirable is challenging because of the important role that calorie-rich ingredients play in determining the overall physicochemical, sensory, and physiologic properties of foods. There is therefore a pressing need to develop reduced-calorie products that are convenient, affordable, and desirable to consumers.
The focus of this review will be on the design and fabrication of reduced-calorie foods that fall into the category of oil-in-water (O/W)5 emulsions, such as many beverages, dips, desserts, dressings, sauces, and soups (4, 5). These types of products are a major source of calories in the human diet, and therefore they are an important target for calorie reduction. O/W emulsions consist of small fat droplets dispersed within an aqueous medium, which may contain a variety of water-soluble or water-dispersible ingredients, such as salts, sugars, thickening agents, and gelling agents. The overall calorie content of emulsion-based food products is governed by the type and amount of ingredients they contain. Fats have the highest number of calories per gram of the major food components, and therefore there has been considerable effort in reducing the fat content of emulsion-based foods. An understanding of the impact of fats on the physicochemical, sensory, and physiologic properties of full-fat foods is essential for the development of successful reduced-calorie versions.
The desirable quality attributes of emulsion-based foods is largely governed by the properties of the fat droplets they contain—e.g., their amount, composition, size, interfacial properties, and interactions (5–8). These fat droplets contribute to the optical properties, texture, mouthfeel, flavor profile, and biologic response (e.g., hunger, satiety, satiation, and pleasure) of emulsion-based products. Consequently, there are major challenges to maintaining the desirable quality attributes of food emulsions once the fat droplets are removed (5, 9–13). The numerous roles that fat droplets play in food quality usually means that a single fat replacement strategy cannot be used to create reduced-calorie products with the same desirable attributes as their full-fat counterparts. Instead, a combination of different fat replacement strategies is needed to mimic their physicochemical, sensory, and biochemical properties.
This article provides an overview of the multiple roles that fat droplets play in determining the properties of food emulsions and highlights how this knowledge can be used to develop effective strategies to create reduced-calorie foods.
Emulsion-Based Foods
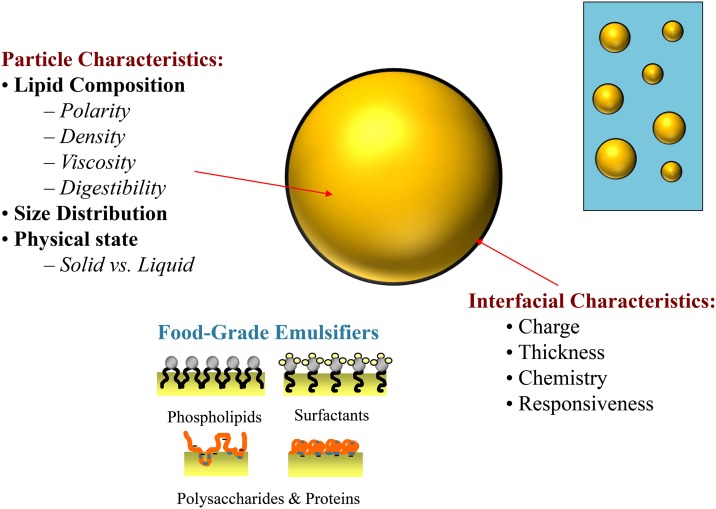
The fats in many commercial food and beverage products are present as O/W emulsions that consist of small fat droplets dispersed within an aqueous medium (e.g., mayonnaise, dressings, sauces, condiments, milk, cream, cheese, yogurt, nutritional beverages, and desserts) (5, 14). Emulsions are compositionally and structurally heterogeneous materials consisting of at least 2 immiscible liquids, with 1 of them being dispersed as fine particles in the other. In the food industry, these 2 immiscible liquids are usually oil and water. The fat droplets in O/W emulsions are covered by a protective coating of emulsifier molecules, such as small molecule surfactants, phospholipids, proteins, or polysaccharides (Figure 1). Emulsifiers facilitate the formation of small fat droplets during homogenization by adsorbing to oil-water interfaces, thereby lowering the interfacial tension and facilitating droplet disruption. Emulsifiers also facilitate the stability of fat droplets to aggregation by forming a protective coating that inhibits flocculation and coalescence. However, the type and concentration of emulsifier used must be carefully selected to ensure appropriate physicochemical properties, good product stability, and a desirable biologic response. The nature of ingredients and processing operations used to fabricate an emulsion determine fat droplet characteristics, such as composition, concentration, size, charge, physical state, interfacial properties, and interactions. In turn, fat droplet characteristics influence the physicochemical properties, sensory perception, and physiologic behavior of emulsion-based food products (Figure 2) (5, 7). This article primarily focuses on calorie reduction of emulsion-based food products by using fat replacement strategies, but it also highlights the role of other key nonfat ingredients on emulsion properties. Emulsion-based products are the main focus of this article, because they are an integral part of many commonly consumed high-calorie foods, such as creams, sauces, dressings, dips, and desserts.
FIGURE 1.
The properties of oil-in-water emulsions depend on the characteristics of the droplets they contain (e.g., concentration, composition, particle size, physical state, and interfacial characteristics).
FIGURE 2.
Overview of the relations between emulsion properties, sensory perception, and biologic response of food emulsions.
Influence of Fat Droplets on Physicochemical and Sensory Properties
The physicochemical properties of emulsions, such as optical properties, rheology, stability, and molecular distribution, are important because they influence food processing and quality attributes. The most important physicochemical properties of food emulsions are briefly discussed below, with emphasis on the role that fat droplets play in influencing these properties. Potential strategies that can be used to maintain the desirable physicochemical properties of food emulsions when fat droplets are removed are also highlighted.
Rheology.
Emulsions may exhibit a range of rheological (textural) characteristics depending on their composition, structure, and interactions. They have textures that include low-viscosity fluids (e.g., milk), high-viscosity fluids (e.g., creams), viscoelastic solids (e.g., mayonnaise), and solids (e.g., cheese) (5, 15, 16). Fat droplets make an important contribution to the overall rheological attributes of many emulsion-based foods. However, nonfat ingredients may also contribute to their texture, such as hydrocolloids, starch granules, air bubbles, fat crystals, or ice crystals, depending on the precise nature of the product.
The rheology of fluid emulsions is characterized by their apparent shear viscosity (η), which is primarily determined by the continuous phase viscosity (ηC), the fat droplet concentration (φ), and the nature of the droplet-droplet interactions (w): η = ηC × f(φ,w) (5, 16). In a simple O/W emulsion containing only fat droplets dispersed in water, the viscosity increases with increasing fat content, gradually at first and then more steeply as the droplets become more closely packed together (Figure 3). Around and above the fat content where close packing of the fat droplets occurs (φcrit ∼ 50–60%), the emulsion exhibits elastic-like characteristics, such as viscoelasticity and plasticity (5). The critical fat droplet content where this steep increase in viscosity occurs depends on droplet-droplet interactions, decreasing for either strong attractive or repulsive interactions (5). The viscosity of an emulsion increases when the fat droplets are flocculated because the effective particle concentration is increased due to water being trapped within the floc structure. In addition, strong shear-thinning behavior is observed in flocculated emulsions because of deformation and breakdown of the floc structure at high shear stresses (17). Controlling the colloidal interactions between fat droplets can therefore be used to modulate their texture and is a useful strategy for developing reduced-calorie foods in some applications.
FIGURE 3.
Dependence of the shear viscosity of an oil-in-water emulsion on fat droplet content. It is assumed that the fat droplets are not flocculated and that there are no thickening agents in the continuous phase.
The impact of droplet characteristics on rheology is an important consideration when designing reduced-fat versions of emulsion-based food products. When the fat droplets are removed there is a decrease in viscosity, and there may even be a conversion from a gel-like to a fluid product (Figure 3). For example, the desirable elastic-like textural attributes of dressings and mayonnaises (such as “thickness” and “spoonability”) are lost when the fat droplet content is reduced below a certain level, unless an effective fat replacement strategy is adopted. A number of approaches may be used to compensate for the loss of desirable textural attributes when fat droplets are removed. The viscosity of a reduced-calorie O/W emulsion can be increased by incorporating thickening agents in the aqueous phase, such as starch granules or hydrocolloids. Alternatively, emulsion viscosity can be increased by adding nonfat particles that mimic the flow characteristics of fat droplets, such as protein particles, hydrogel particles, air bubbles, or crystalline cellulose. The texture of some reduced-fat products may be increased by inducing fat droplet flocculation because this can lead to the formation of a three-dimensional network with a higher viscosity or even elastic-like properties (18, 19).
Optical properties.
One of the most desirable attributes of many emulsion-based products is their milky or creamy appearance, which is associated with light scattering by fat droplets (20, 21). The optical properties of food emulsions can be quantitatively described by using tristimulus color coordinates, such as the L*a*b* system: L* represents lightness, a* represents red-green color, and b* represents yellow-blue color. The opacity of an emulsion can be characterized by L*, whereas the color intensity can be characterized by the chroma C = (a*2 + b*2)1/2. In general, these optical properties of emulsions are determined by droplet concentration, size, and refractive index contrast (5, 20). The lightness of an emulsion tends to increase with increasing droplet concentration and refractive index contrast and has a maximum value when the droplet diameter is approximately equal to the wavelength of light (typically around 500 nm). For O/W emulsions, the lightness increases steeply as the fat content increases from ∼0% to 5% of weight, but then increases more gradually as the amount of fat is increased further (Figure 4). This has important implications for production of reduced-calorie emulsion-based products, such as sauces, desserts, and dressings. There is a steep decrease in lightness when the fat content is reduced below a certain level, which may reduce consumer liking of the product (e.g., due to loss of the desirable “creamy” appearance).
FIGURE 4.
Dependence of the lightness of an oil-in-water emulsion on fat droplet content. The lightness of an emulsion increases steeply from ∼0% to 5% fat but then increases more gradually as the fat content is increased further.
A number of approaches are available to compensate for the alterations in the desirable optical properties of food emulsions when fat droplets are removed. The light-scattering efficiency of the droplets in a reduced-fat product may be enhanced by altering their particle size distribution (21, 22). For example, if a product normally contains large droplets that do not scatter light strongly, then it may be possible to create a lower-fat version with the same lightness by homogenizing it to produce smaller fat droplets that scatter light more efficiently. Alternatively, it is possible to incorporate nonfat particles that scatter light so as to increase the overall opacity of a reduced-fat product [e.g., titanium dioxide (23), biopolymer particles (24), or indigestible fats (25)].
Stability.
Emulsions are thermodynamically unfavorable systems that tend to break down over time due to a variety of physicochemical mechanisms (Figure 5), including gravitational separation, flocculation, coalescence, and Ostwald ripening (4, 5, 26). Gravitational separation is one of the most common forms of instability in food emulsions and may take the form of either creaming or sedimentation depending on the relative densities of the dispersed and continuous phases. Creaming is the upward movement of droplets due to the fact that they have a lower density than the surrounding liquid, whereas sedimentation is the downward movement of droplets due to the fact that they have a higher density than the surrounding liquid. Oils normally have lower densities than water and so creaming is more prevalent in O/W emulsions.
FIGURE 5.
Schematic diagram of most common instability mechanisms that occur in colloidal delivery systems: gravitational separation, flocculation, coalescence, Ostwald ripening, and phase inversion.
The creaming velocity within an O/W emulsion tends to increase as the fat content decreases (Figure 6), which can be attributed to a reduction in the droplet-droplet interactions that inhibit fat droplet movement in concentrated systems (5). This phenomenon has important implications for the formulation of certain types of reduced-fat products (e.g., sauces, dips, desserts, or dressings). Creaming instability increases when the fat droplet concentration is decreased, thereby leading to a reduction in product shelf life. Thus, it may be necessary to reformulate reduced-fat products to prevent this phenomenon from occurring. A number of strategies are available to inhibit or prevent creaming in O/W emulsions containing low amounts of fat droplets (5), as follows: 1) adding thickening or gelling agents to increase the aqueous phase rheology, 2) decreasing the fat droplet size to retard droplet movement, 3) promoting droplet flocculation so as to form a three-dimensional network of aggregated droplets that extends throughout the system, and 4) decreasing the density contrast (e.g., by using partially crystalline fats or adding weighting agents).
FIGURE 6.
Calculated dependence of the creaming velocity of the fat droplets in an oil-in-water emulsion on fat droplet concentration. The rate of creaming decreases with increasing fat content because of droplet-droplet interactions.
Molecular distribution and flavor characteristics.
The flavor profile of an emulsion-based food product is governed by the distribution of volatile and nonvolatile flavor molecules between the different phases (e.g., oil, water, and gas phases), which is determined by equilibrium partition coefficients and kinetics of molecular motion (5, 27). Altering the fat content of a food alters the distribution of the flavor molecules within the system, as well as their release rates. In an emulsion, release is usually characterized in terms of the increase in concentration of a flavor compound in the aqueous phase (taste) or headspace (flavor) as a function of time (flavor intensity-time relation). The influence of fat content on the distribution and release of flavor molecules has an important impact on the development of reduced-fat products. For example, the influence of fat content on the number of volatile flavor molecules present within the headspace above an emulsion is shown in Figure 7. As the fat content increases, the concentration of nonpolar flavors in the headspace above the product decreases, whereas the opposite is true for polar flavors. This phenomenon obviously has important consequences for the formation of reduced-fat emulsions and means that the type and amount of the flavor components present in a reduced-fat system must be carefully reformulated to better mimic those of a full-fat product. This is often quite complicated because food flavors typically contain a complex mixture of different constituents with different volatilities and partition coefficients.
FIGURE 7.
Schematic representation of the calculated dependence of the flavor intensity above an oil-in-water emulsion on fat content. Nonpolar flavors decrease in intensity as the fat content increases, whereas polar flavors increase in intensity.
It should be noted that the nature of the fat replacement strategy used to create a reduced-fat food will have an important effect on the flavor profile. If fat droplets are replaced by a nondigestible fat (e.g., olestra), then there may not be a big impact on the overall flavor profile. On the other hand, if the fat droplets are replaced by a protein or polysaccharide that cannot solubilize flavor molecules, then removing the fat may have a major impact on the flavor profile.
Influence of Fat Droplets on Oral Processing
An important attribute of emulsion-based food products is their behavior within the mouth after ingestion (Figure 8). There has been considerable progress in understanding the physicochemical and physiologic processes involved in the oral processing of food emulsions and how this influences flavor perception (28–33).
FIGURE 8.
The behavior of an emulsion-based product within the oral cavity depends on the nature of the particles it contains (e.g., particle concentration, composition, charge, size, and interfacial properties). Particles may adhere, coalesce, flocculate, and spread when they encounter the oral cavity depending on their properties.
Oral processing
After ingestion, a food product is processed within the mouth until it reaches a form (the bolus) that is suitable for swallowing. The type and degree of oral processing required to create a bolus depends on the characteristics of the ingested food (e.g., composition, structure, and mass) and typically involves a combination of physicochemical and physiologic processes such as mechanical disruption, mixing, dilution, and chemical degradation (7, 34). Oral processing can be divided into a series of steps: first bite, size reduction, bolus formation, swallowing, and residual mouthfeel (7, 28, 35, 36). Oral processing involves coordinated movements of jaw, tongue, cheeks, and lips that are controlled by the brain (37, 38). Sensory feedback from various receptors in the oral cavity enables the motor program to adapt to the properties of the bolus throughout oral processing (7, 39). Ingested foods undergo various changes in their composition and structure during oral processing that contribute to the desirable mouthfeel and flavor perception of a product (7, 36, 40).
The fat droplets in an emulsion-based food product play an important role in oral processing because they influence texture, lubrication, coating, and flavor release properties (28, 33). Fat droplets influence the perceived texture of a product when it first enters the mouth, they alter the way the food product breaks down within the mouth, they alter the way the food interacts with the surfaces of the oral cavity, and they influence the after-feel of the product after the bolus has been swallowed. Consequently, the removal of fat droplets from reduced-calorie products often has a major influence on their desirable sensory attributes. Again, the nature of the fat replacement strategy used to formulate a reduced-calorie product will have an important impact on oral processing. If fat droplets are replaced by nondigestible fats (e.g., olestra), then there may not be a major impact on the overall perception of the product within the oral cavity. On the other hand, if fat droplets are replaced by nonfat molecules or particles (e.g., proteins or polysaccharides) that behave very differently in the oral cavity, then the perceived mouthfeel may be very different. For example, fat droplets may coalesce and spread over the tongue, whereas protein particles will not, which will affect the mouthfeel.
Flavor perception
The overall perceived flavor of a food product is a result of the combined attributes of mouthfeel, taste, and aroma (41) and usually involves integration of information from all 3 of these attributes during mastication (42, 43). The sensorial inputs to the brain that govern the overall flavor profile of a food product result from interactions of volatile and nonvolatile components with aroma, taste, and tactile receptors in the nasal and oral cavities before, during, and after mastication (44–46). Aroma is a result of the interactions of volatile constituents with flavor receptors in the nose. Taste is a result of interactions of certain nonvolatile compounds (bitter, sweet, acid, salty, and umami) with receptors within the mouth (45, 47, 48). Mouthfeel is a result of interactions of a food with tactile receptors within taste buds that are sensitive to mechanical stimuli (49). The overall flavor profile of a food product depends on the type and amount of flavor constituents in the original product, as well as their ability to reach the appropriate sensory receptors in the nasal and oral cavities (50, 51). The presence of fat droplets within an emulsion-based product influences flavor perception through both direct and indirect effects.
Flavor source.
Fats from different sources have different flavor profiles depending on their origin, processing, and storage (e.g., fish, corn, and olive oils all have different flavors). Each type of fat has different characteristic volatile and nonvolatile flavor constituents that determine its unique flavor profile. Recent studies suggest that there are taste receptors in the mouth and gastrointestinal tract (GIT) that detect and respond to the presence of the digestion products of dietary fats (i.e., FAs) (52). These chemoreceptors for fatty perception may play an important role in determining the desirable flavor perception of fatty foods. When fat droplets are removed from a product, these desirable attributes may be lost.
Flavor partitioning.
The amount of fat in a product may also have a more indirect influence on the overall flavor profile. The partitioning of nonvolatile and volatile flavor molecules between different phases within a food (e.g., oil, water, and headspace) depends on their molecular weight and polarity, as well as the type and amount of fat present (53). Nonpolar flavors preferentially partition into the oil phase, whereas polar flavors preferentially partition into the aqueous phase. As the fat content decreases, the amount of nonpolar flavors in the aqueous phase and headspace increases, whereas the amount of polar flavors decreases (Figure 7). Thus, the overall flavor profile of an emulsion-based food product depends on the type and amount of fat present, which has major implications for the formulation of reduced-fat products. When fat droplets are removed, the partitioning of flavor molecules among the different phases is altered, and so the product needs to be reformulated to achieve the desired flavor profile.
Flavor release profile.
The fat content of an emulsion-based product also influences the rate of flavor release. Nonpolar flavor molecules typically have a sustained release profile from high-fat products but a burst release profile from low-fat products (11, 27, 54). Burst release may occur from a low-fat product because an appreciable fraction of the nonpolar flavor molecules are initially present within the aqueous phase rather than the oil phase (11). A major challenge in producing reduced-fat products is therefore to match their flavor release profiles to those of full-fat products. This may be achieved by reformulating the type and amount of flavor molecules in a product and/or by using encapsulation technologies that modulate release profiles (11, 55, 56).
Mouthfeel.
A number of the desirable sensory attributes of emulsion-based food products are associated with their mouthfeel characteristics, such as “creaminess,” “fattiness,” “thickness,” “smoothness,” and “richness” (57–61). The mouthfeel of a product depends on the way it behaves in the mouth during mastication and is influenced by rheological (bulk and thin film), lubrication, and coating properties (28, 58, 60, 62). An important aspect of many emulsion-based products is the after-feel, which is the aspect or aspects of the mouthfeel that persist in the mouth after the bolus has been swallowed, such as coating and astringency (60). The overall mouthfeel of emulsion-based products depends on the concentration, size, interfacial characteristics, physical state, and interactions of fat droplets (63–65). Fat droplets may influence perceived mouthfeel in numerous ways, as follows: 1) they alter texture, 2) they influence lubrication, and 3) they impact coating of oral surfaces. During mastication, fat droplets may remain as individual entities, flocculate, or coalesce within the mouth (Figure 8), which affects their influence on texture, lubrication, and coating (36, 66). A coating of fat droplets on the tongue after swallowing contributes to the desirable flavor profile and mouthfeel of some emulsion-based products. These fat droplets increase the residence time of flavor compounds in the oral cavity thereby producing a prolonged flavor profile (53, 59, 67). When fat droplets are removed from a food product to create a reduced-calorie version, many of these desirable mouthfeel attributes are lost.
Thermal properties.
The transfer of heat from a food to oral surfaces (or vice versa) is also an important factor governing the overall flavor perception, with high-fat products being perceived as warmer than corresponding low-fat ones (68, 69). This phenomenon may be attributed to the lower thermal conductivity of fat compared with water, which leads to different temperature profiles within the mouth during mastication. Thus, when fat droplets are removed or replaced with nonfat ingredients, the thermal characteristics of an emulsion-based food within the oral cavity may be adversely affected.
Influence of Fat on Physiologic Responses
After ingestion, fats are physically and chemically processed within the GIT so as to facilitate their subsequent absorption by the human body (Figure 9). Fats also act as carriers for beneficial lipophilic components in foods, such as oil-soluble vitamins and nutraceuticals, and their presence in the GIT influences the bioavailability of these components. Ingested fats also promote the release of specific hormones and neurotransmitters that regulate food consumption and nutrient processing. The presence of fats within foods therefore plays an important role in determining the pleasurable sensory experience associated with eating, as well as affecting the health and wellness of individuals. Consideration of the physiologic response that a food elicits is important to take into account when formulating reduced-fat products. The reformulated product should not contain fat replacement ingredients that stimulate overconsumption of foods or biologic responses that lead to adverse health outcomes. Some examples of potential problems that may arise when foods are reformulated are as follows: the use of hydrogenated fats to increase the solidity of liquid oils (due to the associated increase in trans FAs) (70, 71) and replacement of fats with easily digestible carbohydrates (due to their potential to promote overeating and diabetes) (72, 73). In this section, the gastrointestinal fate of fats is briefly discussed and then the hormonal response to ingested fats is highlighted.
FIGURE 9.
The behavior of an emulsion-based product in the gastrointestinal tract plays an important role in satiety/satiation and delivery of nutrients. Emulsified TGs are digested by lipases; the resulting FAs are incorporated into mixed micelles, then absorbed by epithelium cells, and may be reassembled into chylomicrons before entering the systemic circulation.
Gastrointestinal fate of fats
After ingestion, a food experiences a complex range of physiochemical and physiologic environments as it travels through the GIT (74, 75). Understanding these processes is important for the design of reduced-fat foods that do not have adverse effects on human health. Within the mouth, the food is mixed with saliva and may undergo changes in pH, ionic composition, and temperature. It also experiences a complex range of mechanical forces and fluid flows during mastication, which may break any large structures into smaller ones. The amylase in saliva may promote breakdown of any starch-based ingredients. After mastication is completed, the resulting bolus is swallowed and passes through the esophagus and into the stomach. Within the stomach, the food is exposed to acidic gastric fluids that contain digestive enzymes capable of breaking down fats (lipases) and proteins (proteases). The food also experiences mechanical forces and fluid flows that further alter its structure and composition. The resulting chyme passes through the pylorus sphincter and enters the small intestine, where it is mixed with alkaline pancreatic and bile fluids. The intestinal fluids cause an appreciable increase in solution pH (to a pH of ∼6–7), and they contain digestive enzymes (lipases, proteases, amylase) and biologic surfactants (bile salts and phospholipids) that further breakdown the nutrients into a form that can easily be absorbed by the human body.
Ingested TGs are broken down to FFAs and monoacylglycerols that interact with bile salts and phospholipids to form mixed micelles (a complex mixture of micelles and vesicles). Any oil-soluble vitamins or nutraceuticals in the original food may be incorporated into these mixed micelles. The mixed micelles then transport the lipid digestion products and bioactive agents to the epithelium cells where they are absorbed. Short- and medium-chain TGs are usually transported directly from the epithelium cells into the portal vein and then into the systemic circulation. On the other hand, long-chain TGs and other highly nonpolar lipids (such as oil-soluble vitamins and nutraceuticals) are usually packaged into lipoprotein particles (chylomicrons) within the epithelium cells and then transported to the systemic circulation via the lymphatic system. After entering the systemic circulation, the lipoprotein particles may be broken down within specific tissues and their contents undergo the following different fates: used as an immediate energy source, stored for later use, used to modulate biochemical pathways, or excreted.
Reducing the fat content of foods may have a number of undesirable effects on human nutrition and health. Decreasing the total amount of digestible fat ingested may directly or indirectly influence the amount of bioactive lipids absorbed by the human body, such as oil-soluble vitamins and nutraceuticals (76, 77). Many sources of fats naturally contain bioactive lipids (e.g., tocopherols in corn oil), and therefore consuming less fat will reduce the amount of them ingested. In addition, the mixed micelles formed from digestion of fats help to solubilize and transport bioactive lipids present in other foods (e.g., carotenoids or vitamins in fruits and vegetables) (76, 78, 79). The amount of bile salts and phospholipids released by the body increases as the total amount of fat ingested increases (80–82). Thus, just by ingesting less fat there may be fewer mixed micelles available to solubilize and transport any bioactive lipids present. The transport of bioactive lipids into the systemic circulation via the lymphatic system will also be decreased if there are fewer long-chain FAs available to fabricate chylomicrons within epithelium cells, which may also alter the extent of metabolism (because passage through the liver is altered).
Hormonal and neurological response
The ingestion of foods elicits a number of hormonal and neurological responses within the human body that affect eating pleasure, food intake, and nutrient processing. The biochemical pathways involved in these processes are coordinated by the central nervous system (83–87). Normally, these biochemical pathways are highly regulated to maintain an optimum body weight and composition, with the amount of calories consumed balancing the amount of calories expended. In this section, only a brief overview of the hormonal and neurological responses to fat ingestion is given to highlight their importance in the development of reduced-calorie foods, and the reader is referred to more comprehensive reviews on this subject (88–90).
At certain times during the day, the body generates biochemicals that stimulate the feeling of hunger, thereby promoting food consumption (91, 92). After a certain quantity of food has been ingested, the body generates another set of biochemicals that suppress appetite, thereby reducing the drive for further food consumption (Figure 10). The decreased desire to consume food during a meal is known as satiation, whereas the reduced desire to consume food after a meal has been completed is known as satiety. Satiation therefore influences the total amount of food consumed during a meal, whereas satiety influences the length of time before a person feels hungry again. Designing foods that control satiation and satiety is therefore an important strategy to combat obesity by ensuring that overconsumption of food does not occur (83, 85, 91, 93, 94). As a food passes through the GIT the major food components (fats, proteins, and carbohydrates) are broken down into their digestion products (FAs, monoacylglycerols, amino acids, peptides, and sugars) that are detected by chemosensory receptors (52). These interactions trigger the release of biochemicals that regulate sensory pleasure and satiation/satiety, as well as nutrient processing, utilization, and storage. A number of the key biochemicals involved in the regulation of food intake and processing are summarized in Table 1, and more detailed information about these processes can be found in recent review articles (52).
FIGURE 10.
Schematic representation of changes in hunger and fullness responses before, during, and after consumption of a meal. The peptides associated with these responses and their interactions with the brain are also shown. CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; OXM, oxyntomodulin; PYY, peptide YY.
TABLE 1.
Summary of some important hormones and neurotransmitters related to food consumption and processing1
| Biochemical | Function | |
| Hunger | Ghrelin | Stimulates food consumption |
| Satiation | CCK | Suppresses hunger during eating |
| Satiety | PYY | Suppresses hunger after eating |
| GLP-1 | ||
| OXM | ||
| Pleasure | Dopamine | Reinforces the desire to consume |
| Nutrient regulation | Insulin leptin | Regulates metabolism and storage of sugars and lipids |
CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; OXM, oxyntomodulin; PYY, peptide YY.
Composition effects.
The different components within an ingested food have different effects on the various biochemical pathways that regulate hunger, pleasure, satiety, and satiation (52, 95). Altering the type and concentration of the major food components (fat, protein, and carbohydrate) within a food may therefore alter the physiologic and psychological responses it elicits, which may have undesirable consequences, such as overeating and associated health problems. When developing reduced-fat foods it is therefore important to take into account the physiologic and psychological effects that fats normally generate, and how these effects are altered when fat is removed and other ingredients are used to replace it. A good strategy for developing reduced-fat foods may therefore be to incorporate nonfat ingredients that are known to promote satiety and satiation.
The influence of fats, proteins, and carbohydrates on the biologic and psychological responses of the human body is currently a major area of research (95). The knowledge generated from this research has major implications for the formulation of reduced-calorie foods. This is a particularly complex area, and there are currently no definitive guidelines about the optimum food composition required to maintain normal body weight and good health (2, 96). In the remainder of this section, a brief outline of some of the effects of fats and other major foods components on the biochemical response of the body to food ingestion is given.
The detection of lipid digestion products (FFAs) within the GIT by chemoreceptors stimulates the release of hormones that suppress appetite [e.g., cholecystokinin (CCK), peptide YY (PYY), and glucagon-like peptide 1 (GLP-1)], and therefore decrease further energy intake (95). The consumption of fats therefore plays an important role in modulating the total amount of food consumed. Thus, even though fat has more calories per gram than digestible carbohydrates, it may still lead to lower overall food consumption if it is better at suppressing appetite or promoting satiety/satiation. Diets rich in digestible carbohydrates have been linked to increases in diseases such as obesity, diabetes, and metabolic syndrome (97). Increasing the protein content of foods may be beneficial in the development of reduced-calorie products because proteins may increase satiety and therefore reduce overeating (98). Indeed, some proteins have been claimed to be more effective at reducing appetite than either carbohydrates or fats (99). Proteins may also play a number of other important roles in maintaining body weight and health: they are a source of essential amino acids, they facilitate muscle development, they play a key role in energy metabolism, and they help maintain desirable blood sugar concentrations (99–101). Consequently, protein-based ingredients may be particularly suitable for the development of effective fat or carbohydrate replacement strategies in reduced-calorie foods.
A better understanding of the relation between food composition (e.g., the type and amount of fat, protein, and carbohydrate present) and biochemical responses may lead to the development of reduced-calorie food products that leave consumers feeling less hungry, more satisfied, and fuller, thereby reducing overconsumption (94). For example, foods could be fortified with those components known to have a high capacity to induce satiation and/or satiety. Chronic overconsumption of high-fat diets may cause potential defects in hormone signaling (102), which may therefore be alleviated by reducing the fat content of foods or by replacing the fats with other ingredients.
Structure effects.
The initial structural organization of the components within a food product, as well as how this structure changes within the GIT, also plays an important role in determining satiety/satiation (103–105). Foods can be designed to have different structural organizations of the major food components, such as fats, proteins, and carbohydrates. For example, the fats may be present in a continuous form (as in margarine or butter), in an emulsified form (as in milk, dressings, and sauces), or embedded in a solid network (as in cookies, crackers, and other baked goods). In addition, fat may be surrounded by other components in a food matrix that alter its fate within the GIT. For example, fat droplets may be coated with dietary fiber, or they may be embedded within dietary fiber hydrogel particles, which delays TG digestion by lipase in the GIT (106–108). Alternatively, fat droplets may be coated with certain types of small molecule surfactants that delay lipid digestion by inhibiting lipase adsorption to the fat droplet surfaces (109, 110). The amounts of lipid digestion products present within different locations of the GIT influence the satiety response through the ileal brake mechanism (105, 111). This mechanism regulates the rate at which foods pass through the GIT so as to allow efficient digestion to occur. When the amounts of undigested nutrients reaching the distal regions of the small intestine increase, hormones are released to slow down the transport of foods through the GIT so as to allow more time for digestion to occur. Indeed, DSM (a Dutch ingredient manufacturer) has introduced an emulsion-based ingredient (Fabuless) that has been claimed to have an appetite-suppressing effect, which is reported to work by slowing down the transit time of the fat through the GIT thereby inducing the ileal brake mechanism (112). Nevertheless, other studies reported no substantial effect of this structured emulsion on food intake or appetite in human trials, which may have been due to the effects of the food matrix or processing (113, 114). Clearly, further studies are needed to better understand the roles of fat droplet properties, the surrounding food matrix, and processing operations on the digestion of emulsified lipids in foods.
Studies have also indicated that the stability of emulsified lipids within the stomach influences the satiety response (115–118). Emulsified lipids that are stable to aggregation and creaming within the stomach reduce the rate of gastric emptying and increase the satiety response compared with those that are unstable. This phenomenon has been attributed to the rate of calorie transport from the stomach to the small intestine. Unstable emulsified lipids move to the top of the stomach due to creaming or oiling off, and hence the rate of calorie transport to the small intestine is relatively low because the liquid passing through the pylorus sphincter is mainly water (Fig. 11). Under these circumstances the body has a tendency to consume more food to increase the calorie uptake. Conversely, if the emulsified lipids are evenly distributed throughout the volume of the stomach, then the rate of calorie transport into the small intestine will be greater. The body then wants to slow down gastric emptying (so that all food components can be efficiently digested) and one feels fuller (because the body is detecting calories). There is therefore an opportunity for food manufacturers to control the satiety response by altering the structural organization of the ingredients in food products, thereby reducing the total amount of calories consumed per meal and increasing the length of time between food consumption. This can be achieved by controlling the nature of the emulsifier used to stabilize the fat droplets in a food product.
FIGURE 11.

The stability of fat droplets to sedimentation and creaming in the stomach may alter gastric emptying because the body detects the energy density of the food leaving the stomach. This mechanism has been proposed as a means of controlling satiety.
Development of Reduced-Fat Products
Nutritional recommendations to decrease the total number of calories from fat in the human diet led the food industry to create a range of reduced-fat products (10, 42, 59, 119–123). These products should reduce the total amount of calories consumed, but they should also have desirable physicochemical and sensory attributes; otherwise, consumers will not purchase them (59, 91). In addition, they should be carefully designed so that they do not have undesirable health consequences associated with their different behavior within the GIT (e.g., reducing the bioavailability of oil-soluble vitamins and nutrients or stimulating overeating by reducing satiety or satiation). Various strategies that can be used by the food industry to create reduced-fat foods are highlighted in this section.
Fat-based ingredients
A number of reduced-calorie ingredients can serve as direct substitutes for fats in foods. These ingredients have physical characteristics similar to conventional fats (e.g., polarity, viscosity, density, refractive index), but they have appreciably fewer calories because they are digested differently. The physicochemical and sensory properties of emulsions made from fat substitutes are therefore similar to those made from conventional fats, because the droplets they contain are fairly similar (Figure 12). Two of the most important ingredients that fall into this category are Olestra (Procter & Gamble) and Salatrim (Danisco).
FIGURE 12.
Some of the desirable food attributes provided by fat droplets (opacity, texture, and mouthfeel) can be provided by polymeric or particulate substances, such as thickening agents, hydrogel particles, indigestible fat droplets, starch granules, titanium dioxide, or protein particles.
Olestra is a sucrose polyester consisting of numerous long-chain FAs esterified to a sucrose molecule (124–126). These ester bonds are not hydrolyzed by digestive enzymes because of steric hindrance, and so olestra is not absorbed by the human body and does not contribute calories. However, olestra may retard the absorption of fat-soluble vitamins and nutraceuticals and may be associated with undesirable GIT symptoms, which led the US FDA to limit the amount that can be used in foods (124, 126).
Salatrim is another fat substitute used to reduce the calorie content of foods, which is prepared by interesterification of short-chain TGs with hydrogenated long-chain TGs (127). The resulting TG molecules contain a mixture of short- and long-chain SFAs esterified to a glycerol backbone. Long-chain FAs are primarily attached to the glycerol in the sn-2 position, which leads to the generation of lipid digestion products that are not readily absorbed, thereby reducing the effective calorie content (128, 129). Typically, the calorie content of Salatrim is approximately half that of conventional fats. Salatrim may also be an effective fat replacer due to its ability to induce satiety, because there is a greater concentration of undigested fat within the lower GIT (130).
The calorie content and satiety/satiation-inducing effects of the fat phase may also be altered by controlling the type and form of FAs present in foods (93, 131–133). FAs delivered in the form of diacylglycerols may be more effective at suppressing appetite and food intake than similar FAs delivered in the form of TGs (134). The effectiveness of TGs at reducing appetite and food intake has also been shown to increase as their chain length decreases (93, 131, 135–138) and their unsaturation increases (139). Consumption of appreciable quantities of CLA has been shown to reduce body fat (87, 140, 141). However, a meta-analysis of randomized clinical trials in humans showed that this effect is relatively small and of uncertain clinical significance (142). These differences in the response of the human body can be attributed to differences in the way that different lipids are metabolized. Consequently, it may be possible to design foods that regulate food intake and body mass by carefully controlling lipid composition.
Biopolymer-based ingredients
A number of food ingredients developed to replace some of the physicochemical and sensory properties normally supplied by fats are based on proteins and/or polysaccharides (143–145). Typically, these biopolymer-based ingredients are present either as soluble polymers or as colloidal particles. Additional potential advantages of using these ingredients as fat replacers are as follows: 1) some of them are indigestible dietary fibers that have relatively low-calorie contents and may have added beneficial health effects and 2) some of them may be able to induce greater satiety than fats.
Polymers.
A number of biopolymers are hydrophilic water-soluble molecules that act as effective thickening agents, including some proteins (e.g., gelatin) and many polysaccharides (e.g., agar, alginate, carrageenan, locust bean gum, pectin, starch, and xanthan). These biopolymers occupy an effective volume in solution that is much larger than the volume occupied by the polymer chains, due to their ability to trap water molecules (146). Some biopolymers are capable of forming physical or covalent crosslinks leading to gelation under appropriate solution or environmental conditions (e.g., alginate or pectin in the presence of calcium, agar or gelatin at low temperatures, or whey protein at high temperatures) (145). Thickening and gelling agents are typically used as fat replacers due to their ability to compensate for some of the textural changes that occur when fat droplets are removed from foods. Nevertheless, they may not be able to compensate for other changes in food properties that occur when fats are removed, such as changes in appearance, mouthfeel, or flavor profile.
Particles.
Certain types of biopolymer-based ingredients exist in foods in the form of colloidal particles with dimensions in the range from ∼100 nm to 100 μm (Figure 12). These biopolymer particles can simulate some of the desirable physicochemical and sensory attributes normally provided by fat droplets. First, they increase the viscosity or gel strength of aqueous solutions and therefore compensate for alterations in texture caused by fat reduction. Second, they scatter light and therefore compensate for the loss of creaminess that can occur upon fat reduction. Third, they may simulate some of the mouthfeel characteristics normally associated with the interaction of fat droplets with the tongue and oral cavity. Fourth, they can prevent creaming or sedimentation of fat droplets and other ingredients due to their ability to increase the rheology of the aqueous phase.
Protein microspheres with dimensions similar to those of fat droplets can be formed through controlled aggregation of globular proteins after thermal denaturation (147). Food ingredients based on this principle are commercially available as fat replacers, such as Simplesse (CP Kelco). Protein microspheres have been used as fat replacers in the development of numerous reduced-fat products, including cheese, ice cream, and yogurt (148–151).
Starch granules are another form of biopolymer particle that can be used as a fat replacer (152, 153). Raw starch granules swell when they are heated in water, which causes a large increase in viscosity and may lead to gel formation. Starch granules are commonly used in reduced-fat versions of dressings and sauces to replace some of the textural attributes that are normally lost when fat droplets are removed (154). One of the potential disadvantages of using starch as a fat replacer is that it contains calories, and that its overconsumption may lead to problems with overweight and diabetes (97). Some of these problems may be overcome by using resistant starch (155) or cellulose (156) biopolymer particles.
Inorganic ingredients
Some of the desirable physicochemical attributes normally provided by fat droplets can be mimicked by indigestible inorganic substances. For example, titanium dioxide particles are often used to increase the opacity (“whiteness”) of foods (157). Titanium dioxide particles have a relatively high refractive index contrast and therefore scatter light strongly, which makes them highly effective lightening agents. Thus, titanium dioxide can be used to replace some of the desirable creamy appearance of a product when the fat droplet content is reduced (23). However, food manufacturers often prefer to have “clean” labels, and therefore want to replace titanium dioxide with more natural forms of lightening agents, such as biopolymer particles made from proteins or polysaccharides (158, 159).
Structuring approaches
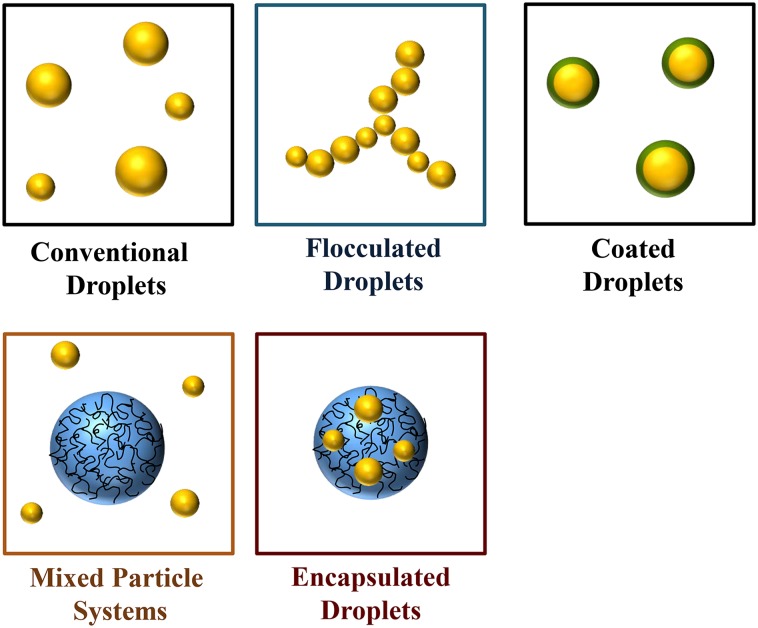
Structural design principles can be used to create specific structures in foods that mimic some of the desirable physicochemical, sensory, and physiologic attributes normally associated with fat droplets (e.g., creaminess, richness, satiety, and satiation) (74, 75, 160, 161). A variety of approaches based on structural design principles that can be used in emulsion-based products are highlighted in Figure 13. Fat droplets can be coated with substances that alter their digestibility within the GIT, such as certain surfactants and dietary fibers (156). Similarly, they can be embedded within biopolymer particles that alter their biologic fate within the GIT (106). Coatings or hydrogel particles that retard lipid digestion in the stomach and small intestine may be able to induce satiety through the ileal brake mechanism. Fat droplets can be made to form microclusters by inducing droplet flocculation either inside or outside of the human body. Flocculated droplets have a higher viscosity than do nonflocculated ones and so it may be possible to create emulsion-based products that have similar textural properties but lower fat contents by inducing droplet aggregation (18, 19). Aggregated fat droplets may also behave differently within the GIT, which may be useful for developing products that can induce satiety (162). Fat droplets may be combined with other kinds of colloidal particles (e.g., biopolymer particles, inorganic particles, or air bubbles) to form reduced-fat products with physicochemical and sensory attributes similar to high-fat products (163–165). The principles and utilization of various kinds of structural design approaches for altering the properties of foods were reviewed recently (108, 159, 162, 166, 167).
FIGURE 13.
Structural design principles may be used to create reduced-fat emulsion-based products with physicochemical attributes similar to conventional products.
Conclusions
In general, the fats in food products play numerous roles in determining their overall appearance, texture, flavor, and biologic response. For this reason, it is often challenging to create high-quality reduced-fat products with physicochemical, sensory, and physiologic properties similar to their high-fat counterparts. A number of ingredient and processing technology approaches have therefore been developed to create reduced-fat foods. The strategy adopted for a particular product depends on consumer expectations for product appearance, texture, and flavor, as well as manufacturers’ concerns about cost, production, and labeling. This article provides an overview of the numerous roles that fat droplets play in determining the properties of emulsion-based foods and has highlighted some of the strategies that can be used to compensate for the loss of desirable food properties when fat is removed. This information should provide useful guidance in the design of high-quality reduced-fat products with improved physicochemical, sensory, and biologic properties. Of course, it is important to use these technologies wisely so as not to promote passive overconsumption of foods. For example, it may be more beneficial to reduce the calorie content of a food product that is already an integral part of a diet (e.g., a spread, dressing, or sauce) rather than to promote greater consumption of less healthy food items, such as milkshakes, smoothies, snacks, or desserts.
Acknowledgments
The author thanks Cheryl Chung, Eric Decker, Ware Flora, and Gordon Smith for valuable discussions on reduced-fat products. The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: CCK, cholecystokinin; GIT, gastrointestinal tract; GLP-1, glucagon-like peptide 1; O/W, oil-in-water; PYY, peptide tyrosine tyrosine.
References
- 1.Brawer R, Brisbon N, Plumb J. Obesity and cancer. Prim Care 2009;36(3):509. [DOI] [PubMed]
- 2.Schrauwen P, Saris WHM. Dietary fat and obesity. In: Williams C, Buttriss J, editors. Improving the fat content of foods. Bocan Raton (FL): CRC Press; 2006. p. 141–61.
- 3.Swinburn BA, Caterson I, Seidell JC, James WPT. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr 2004;7(1A):123–46. [DOI] [PubMed] [Google Scholar]
- 4.Friberg S, Larsson K, Sjoblom J. Food emulsions. 4th ed. New York: Marcel Dekker; 2004.
- 5.McClements DJ. Food emulsions: principles, practices, and techniques. 2nd ed. Boca Raton (FL): CRC Press; 2005.
- 6.Druaux C, Voilley A. Effect of food composition and microstructure on volatile flavor release. Trends Food Sci Technol 1997;8:364–8. [Google Scholar]
- 7.Foster KD, Grigor JMV, Cheong JN, Yoo MJY, Bronlund JE, Morgenstern MP. The role of oral processing in dynamic sensory perception. J Food Sci 2011;76:R49–61. [DOI] [PubMed] [Google Scholar]
- 8.Talbot G. Reducing saturated fats in foods. Oxford (United Kingdom): Woodhead Publishing; 2011.
- 9.Bayarri S, Taylor JA, Joanne H. The role of fat in flavor perception: effect of partition and viscosity in model emulsions. J Agric Food Chem 2006;54:8862–8. [DOI] [PubMed] [Google Scholar]
- 10.Bayarri S, Smith T, Hollowood T, Hort J. The Role of Rheological Behavior in Flavor Perception in Model Oil/Water Emulsions. Eur Food Res Technol 2007;226:161–8. [Google Scholar]
- 11.Malone ME, Appelqvist IAM. Gelled emulsion particles for the controlled release of lipophilic volatiles during eating. J Control Release 2003;90:227–41. [DOI] [PubMed] [Google Scholar]
- 12.Brauss MS, Linforth RST, Cayeux I, Harvey B, Taylor AJ. Altering the fat content affects flavor release in a model yogurt system. J Agric Food Chem 1999;47:2055–9. [DOI] [PubMed] [Google Scholar]
- 13.Benjamins J, Vingerhoeds MH, Zoet FD, de Hoog EHA, van Aken GA. Partial coalescence as a tool to control sensory perception of emulsions. Food Hydrocoll 2009;23:102–15. [Google Scholar]
- 14.Le Reverend BJD, Norton IT, Cox PW, Spyropoulos F. Colloidal aspects of eating. Curr Opin Colloid Interface Sci 2010;15:84–9. [Google Scholar]
- 15.Walstra P. Physical chemistry of foods. New York: Marcel Decker; 2003. [Google Scholar]
- 16.Genovese DB, Lozano JE, Rao MA. The rheology of colloidal and noncolloidal food dispersions. J Food Sci 2007;72:R11–20. [DOI] [PubMed] [Google Scholar]
- 17.Quemada D, Berli C. Energy of interaction in colloids and its implications in rheological modeling. Adv Colloid Interface Sci 2002;98:51–85. [DOI] [PubMed] [Google Scholar]
- 18.Mao Y, McClements DJ. Fabrication of viscous and paste-like materials by controlled heteroaggregation of oppositely charged lipid droplets. Food Chem 2012;134:872–9. [DOI] [PubMed] [Google Scholar]
- 19.Mao Y, McClements DJ. Fabrication of reduced fat products by controlled heteroaggregation of oppositely charged lipid droplets. J Food Sci 2012;77:E144–52. [DOI] [PubMed] [Google Scholar]
- 20.McClements DJ. Theoretical prediction of emulsion color. Adv Colloid Interface Sci 2002;97:63–89. [DOI] [PubMed] [Google Scholar]
- 21.McClements DJ. Colloidal basis of emulsion color. Curr Opin Colloid Interface Sci 2002;7:451–5. [Google Scholar]
- 22.McClements DJ. Nanoparticle- and microparticle-based delivery systems: encapsulation, protection and release of active components. 2014, CRC Press, Boca Raton (FL).
- 23. Chantrapornchai W, Clydesdale FM, McClements DJ. Optical properties of oil-in-water emulsions containing titanium dioxide particles. Colloids Surf A Physicochem Eng Asp 2000;166(1–3):123–31.
- 24.Matalanis A, Jones OG, McClements DJ. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll 2011;25:1865–80. [Google Scholar]
- 25.Wang Y, Corwin R, Jaramillo DP, Wojnicki FJ, Coupland JN. Acceptability and digestibility of emulsions in a rat model: effects of solid fat content and lipid type. J Am Oil Chem Soc 2011;88:235–41. [Google Scholar]
- 26.Dickinson E. Introduction to food colloids. Cambridge (United Kingdom): Royal Society of Chemistry; 1992. [Google Scholar]
- 27.Frank D, Appelqvist I, Piyasiri U, Wooster TJ, Delahunty C. Proton transfer reaction mass spectrometry and time intensity perceptual measurement of flavor release from lipid emulsions using trained human subjects. J Agric Food Chem 2011;59:4891–903. [DOI] [PubMed] [Google Scholar]
- 28.Stokes JR, Boehm MW, Baier SK. Oral processing, texture and mouthfeel: from rheology to tribology and beyond. Curr Opin Colloid Interface Sci 2013;18:349–59. [Google Scholar]
- 29.van Aken GA, Vingerhoeds MH, de Wijk RA. Textural perception of liquid emulsions: role of oil content, oil viscosity and emulsion viscosity. Food Hydrocoll 2011;25:789–96. [Google Scholar]
- 30.Chen J, Eaton L. Multimodal mechanisms of food creaminess sensation. Food Funct 2012;3:1265–70. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Stokes JR. Rheology and tribology: two distinctive regimes of food texture sensation. Trends Food Sci Technol 2012;25:4–12. [Google Scholar]
- 32.Chen J, Engelen L. Food oral processing: fundamentals of eating and sensory perception. Hoboken (NJ): Wiley-Blackwell; 2012. [Google Scholar]
- 33.van Aken GA, Vingerhoeds MH, de Hoog EHA. Food colloids under oral conditions. Curr Opin Colloid Interface Sci 2007;12:251–62. [Google Scholar]
- 34.Chen JS. Food oral processing—a review. Food Hydrocoll 2009;23:1–25. [Google Scholar]
- 35.Ranc H, Servais C, Chauvy PF, Debaud S, Mischler S. Effect of surface structure on frictional behavior of a tongue/palate tribological system. Tribol Int 2006;39:1518–26. [Google Scholar]
- 36.van der Bilt A. Oral physiology, mastication and food perception. In: McClements DJ, Decker EA, editors. Designing functional foods: measuring and controlling food structure breakdown and nutrient absorption. Cambridge (United Kingdom): Woodhead Publishing; 2009. p. 3–37.
- 37.Dellow PG, Lund JP. Evidence for central timing of rhythmical mastication. J Physiol 1971;215:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada Y, Yamamura K, Inoue M. Coordination of cranial motorneurons during mastication. Respir Physiol Neurobiol 2005;147:177–89. [DOI] [PubMed] [Google Scholar]
- 39.Woda A, Foster K, Mishellany A, Peyron MA. Adaptation of healthy mastication to factors pertaining to the individual or to the food. Physiol Behav 2006;89:28–35. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen AM, Bardow A, Beier Jensen S, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis 2002;8:117–29. [DOI] [PubMed] [Google Scholar]
- 41.British Standards Institute. British Standards glossary of terms relating to the sensory analysis of food. London: British Standards Institute; 1975. [Google Scholar]
- 42.Gonzalez-Tomas L, Bayarri S, Taylor AJ, Costell E. Rheology, flavor release and perception of low-fat dairy desserts. Int Dairy J 2008;18:858–66. [Google Scholar]
- 43.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res 2005;166:345–57. [DOI] [PubMed] [Google Scholar]
- 44.Bell GA. Molecular mechanisms of olfactory perception: their potential for future technologies. Trends Food Sci Technol 1996;7:425–31. [Google Scholar]
- 45.Taylor AJ. Volatile flavor release from foods during eating. Crit Rev Food Sci Nutr 1996;36:765–84. [DOI] [PubMed] [Google Scholar]
- 46.Brown WE, Eves D, Ellison M, Braxton D. Use of combined electromyography and kinethesiology during mastication to chart the oral breakdown of food stuffs: relevance to measurement of food texture. J Texture Stud 1998;29:145–67. [Google Scholar]
- 47.Duran L, Costell E. Perception of taste: physicochemical and psychophysical aspects. Food Sci Technol Int 1999;5:299–309. [Google Scholar]
- 48.Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol 2000;10:519–27. [DOI] [PubMed] [Google Scholar]
- 49.Smith DV, Margolskee RF. Making sense of taste. Sci Am 2001;284:32–9. [DOI] [PubMed] [Google Scholar]
- 50.Larsson M, Larsson K. Neglected aspects of food flavor perception. Colloids Surf A Physicochem Eng Asp 1997;123:651–5. [Google Scholar]
- 51.Taylor AJ, Linforth RST. Flavor release in the mouth. Trends Food Sci Technol 1996;33:444–8. [Google Scholar]
- 52.Stewart JE, Feinle-Bisset C, Keast RSJ. Fatty acid detection during food consumption and digestion: associations with ingestive behavior and obesity. Prog Lipid Res 2011;50:225–33. [DOI] [PubMed] [Google Scholar]
- 53.de Roos KB. How lipids influence flavor perception. In: Shahidi S, Weenen H, editors. Food lipids: chemistry, flavor, and texture. Washington (DC): American Chemical Society; 2006. p. 145–58.
- 54.Frank DC, Eyres GT, Piyasiri U, Delahunty CM. Effect of food matrix structure and composition on aroma release during oral processing using in vivo monitoring. Flavour Fragrance J 2012;27:433–44. [Google Scholar]
- 55.van Aken GA, Hoog EHA. Oral processing and perception of food emulsions: relevance for fat reduction in food. In: McClements DJ, editor. Designing Functional Foods: Boca Raton (FL); CRC Press; 2009, p. 481–501.
- 56.Malone ME, Appelqvist IAM, Goff TC, Homan JE, Wilkins JPG. A novel approach to the selective control of lipophilic flavor release in low fat foods. In: Roberts DD, Taylor AJ, editors. Flavor release. Washington (DC): American Chemical Society; 2000. p. 212–27.
- 57.Mela DJ, Langley KR, Martin N. Sensory assessment of fat content: effect of emulsion and subject characteristics. Appetite 1994;22:67–81. [DOI] [PubMed] [Google Scholar]
- 58.Malone ME, Appelqvist IAM, Norton IT. Oral behavior of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations. Food Hydrocoll 2003;17:763–73. [Google Scholar]
- 59.Malone ME, Appelqvist IAM, Norton IT. Oral behavior of food hydrocolloids and emulsions. Part 2. Taste and aroma release. Food Hydrocoll 2003;17:775–84. [Google Scholar]
- 60.Guinard JX, Mazzucchelli R. The sensory perception of texture and mouthfeel. Trends Food Sci Technol 1996;7:213–9. [Google Scholar]
- 61.Prakash S, Tan DDY, Chen J. Applications of tribology in studying food oral processing and texture perception. Food Res Int 2013;54:1627–635. [Google Scholar]
- 62.van Aken GA. Coalescene mechanisms in protein-stabilized emulsion. In: Friberg S, Larsson K, Sjoblom J, editors. Food emulsions. New York: Marcel Dekker; 2004.
- 63.Roland AM, Phillips LG, Boor KJ. Effects of fat content on the sensory properties, melting, color, and hardness of ice cream. J Dairy Sci 1999;82:32–8. [Google Scholar]
- 64.Frost MB, Dijksterhuis G, Martens M. Sensory perception of fat in milk. Food Qual Prefer 2001;12:327–36. [Google Scholar]
- 65.Kilcast D, Clegg S. Sensory perception of creaminess and its relationship with food structure. Food Qual Prefer 2002;13:609–23. [Google Scholar]
- 66.van Aken GA. Modelling texture perception by soft epithelial surfaces. Soft Matter 2010;6:826–34. [Google Scholar]
- 67.Doyen K, Carey M, Linforth RST, Taylor AJ. Volatile release from an emulsion: headspace and in-mouth studies. J Agric Food Chem 2001;49:804–10. [DOI] [PubMed] [Google Scholar]
- 68.Prinz JF, de Wijk RA, Huntjens LA, Engelen L, Polet IA. Is fat perception a thermal effect? Percept Mot Skills 2007;104:381–6. [DOI] [PubMed] [Google Scholar]
- 69.Weenen H, van Gemert LJ, van Doorn JM, Duksterhuis GB, de Wijk RA. Texture and mouthfeel of semisolid foods: commercial mayonnaises, dressings, custard desserts and warm sauces. J Texture Stud 2003;34:159–79. [Google Scholar]
- 70.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002;288:2569–78. [DOI] [PubMed] [Google Scholar]
- 71.Erkkilä A, de Mello VDF, Riserus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res 2008;47:172–87. [DOI] [PubMed] [Google Scholar]
- 72.Aller EE, Abete I, Astrup A, Martinez JA, van Baak MA. Starches, sugars and obesity. Nutrients 2011;3:341–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brennan CS. Dietary fibre, glycaemic response, and diabetes. Mol Nutr Food Res 2005;49:560–70. [DOI] [PubMed] [Google Scholar]
- 74.McClements DJ, Decker EA, Park Y. Controlling lipid bioavailability through physicochemical and structural approaches. Crit Rev Food Sci Nutr 2009;49:48–67. [DOI] [PubMed] [Google Scholar]
- 75.Singh H, Ye A, Horne D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog Lipid Res 2009;48:92–100. [DOI] [PubMed] [Google Scholar]
- 76.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr 2004;80:396–403. [DOI] [PubMed] [Google Scholar]
- 77.Rao J, Decker EA, Xiao H, McClements DJ. Nutraceutical nanoemulsions: influence of carrier oil composition (digestible versus indigestible oil) on beta-carotene bioavailability. J Sci Food Agric 2013;93:3175–83. [DOI] [PubMed] [Google Scholar]
- 78.Goncalves A, Gleize B, Roi S, Nowicki M, Dhaussy A, Huertas A, Amiot MJ, Reboul E. Fatty acids affect micellar properties and modulate vitamin D uptake and basolateral efflux in Caco-2 cells. J Nutr Biochem 2013;24:1751–7. [DOI] [PubMed] [Google Scholar]
- 79.Reboul E, Richelle M, Perrot E, Desmoulins-Malezet C, Pirisi V, Borel P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J Agric Food Chem 2006;54:8749–55. [DOI] [PubMed] [Google Scholar]
- 80.Humberstone AJ, Porter CJH, Charman WN. A physicochemical basis for the effect of food on the absolute oral bioavailability of halofantrine. J Pharm Sci 1996;85:525–9. [DOI] [PubMed] [Google Scholar]
- 81.Porter CJH, Charman WN. In vitro assessment of oral lipid based formulations. Adv Drug Deliv Rev 2001;50:S127–47. [DOI] [PubMed] [Google Scholar]
- 82.Charman WN, Porter CJH, Mithani S, Dressman JB. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J Pharm Sci 1997;86:269–82. [DOI] [PubMed] [Google Scholar]
- 83.Blundell JE, Goodson S, Halford JCG. Regulation of appetite: role of leptin in signalling systems for drive and satiety. Int J Obes Relat Metab Disord 2001;25:S29–34. [DOI] [PubMed] [Google Scholar]
- 84.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–71. [DOI] [PubMed] [Google Scholar]
- 85. Del Prete A, Iadevaia M, Loguercio, C. The role of gut hormones in controlling the food intake. What is their role in emerging diseases? Endocrinol Nutr 2012;59(3):197–206. [DOI] [PubMed]
- 86.Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept 2008;149:70–8. [DOI] [PubMed] [Google Scholar]
- 87.Kim KH, Park Y. Food components with anti-obesity effect. Ann Rev Food Sci Technol 2011;2:237–57. [DOI] [PubMed] [Google Scholar]
- 88.Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell 2007;129:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arora S, Anubhuti. Role of neuropeptides in appetite regulation and obesity—a review. Neuropeptides 2006;40:375–401. [DOI] [PubMed]
- 90.Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am J Clin Nutr 2007;86:531–41. [DOI] [PubMed] [Google Scholar]
- 91.Halford JCG, Harrold JA. Satiety-enhancing products for appetite control: science and regulation of functional foods for weight management. Proc Nutr Soc 2012;71:350–62. [DOI] [PubMed] [Google Scholar]
- 92.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Salah S, Schuring E, van der Knaap H, Westerterp M. Appetite control: methodological aspects of the evaluation of foods. Obes Rev 2010;11:251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rego Costa AC, Rosado EL, Soares-Mota M. Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: a systematic review. Nutr Hosp 2012;27:103–8. [DOI] [PubMed] [Google Scholar]
- 94.Fiszman S, Varela P. The satiating mechanisms of major food constituents—an aid to rational food design. Trends Food Sci Technol 2013;32:43–50. [Google Scholar]
- 95.Shin HS, Ingram JR, McGill AT, Poppitt SD. Lipids, CHOs, proteins: can all macronutrients put a ‘brake’ on eating? Physiology Behavior 2013;120:114–23. [DOI] [PubMed] [Google Scholar]
- 96.Westerterp KR. Specific fatty acids and structured lipids for weight control. In: Williams C, Buttriss J, editors. Improving the fat content of foods. Boca Raton (FL): CRC Press; 2006. p. 162–81.
- 97.Lustig RH, Schmidt LA, Brindis CD. The toxic truth about sugar. Nature 2012;482:27–9. [DOI] [PubMed] [Google Scholar]
- 98.Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012;108:S105–12. [DOI] [PubMed] [Google Scholar]
- 99.Anderson GH, Luhovyy B, Akhavan T, Panahi S. Milk proteins in the regulation of body weight, satiety, food intake and glycemia. In: Clemens RA, Hernell O, Michaelsen KM, editors. Milk and milk products in human nutrition. Basel, Switzerland; S. Karger AG; 2011. p. 147–59. [DOI] [PubMed]
- 100.Seyler JE, Layman DK. Effect of food protein on blood glucose regulation, body composition and weight loss. Agro Food Industry Hi-Tech 2012;23:35–7. [Google Scholar]
- 101.Devkota S, Layman DK. Protein metabolic roles in treatment of obesity. Curr Opin Clin Nutr Metab Care 2010;13:403–7. [DOI] [PubMed] [Google Scholar]
- 102.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 2002;13:51–9. [DOI] [PubMed] [Google Scholar]
- 103.Hetherington MM, Cunningham K, Dye L, Gibson EL, Gregersen NT, Halford JCG, Lawton CL, Lluch A, Mela DJ, Van Trijp HCM, Potential benefits of satiety to the consumer: scientific considerations. Nutr Res Rev 2013;26:22–38. [DOI] [PubMed] [Google Scholar]
- 104.Lundin L, Golding M, Wooster TJ. Understanding food structure and function in developing food for appetite control. Nutr Diet 2008;65:S79–85. [Google Scholar]
- 105.van Aken GA. Relating food emulsion structure and composition to the way it is processed in the gastrointestinal tract and physiological responses: what are the opportunities? Food Biophys 2010;5:258–83. [Google Scholar]
- 106.Li Y, Hu M, Du YM, Xiao H, McClements DJ. Control of lipase digestibility of emulsified lipids by encapsulation within calcium alginate beads. Food Hydrocoll 2011;25:122–30. [Google Scholar]
- 107.McClements DJ. Design of nano-laminated coatings to control bioavailability of lipophilic food components. J Food Sci 2010;75:R30–42. [DOI] [PubMed] [Google Scholar]
- 108.McClements DJ, Li Y. Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv Colloid Interface Sci 2010;159:213–28. [DOI] [PubMed] [Google Scholar]
- 109.Chu BS, Rich GT, Ridout MJ, Faulks RM, Wickham MSJ, Wilde PJ. Modulating pancreatic lipase activity with galactolipids: effects of emulsion interfacial composition. Langmuir 2009;25:9352–60. [DOI] [PubMed] [Google Scholar]
- 110.Wilde PJ, Chu BS. Interfacial & colloidal aspects of lipid digestion. Adv Colloid Interface Sci 2011;165:14–22. [DOI] [PubMed] [Google Scholar]
- 111.Maljaars PWJ, Peters HPF, Mela DJ, Masclee AAM. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav 2008;95:271–81. [DOI] [PubMed] [Google Scholar]
- 112.Haenni A, Sundberg B, Yazdanpanah N, Viberg A, Olsson J. Effect of fat emulsion (Fabuless) on orocecal transit time in healthy men. Scand J Gastroenterol 2009;44:1186–90. [DOI] [PubMed] [Google Scholar]
- 113.Smit HJ, Keenan E, Kovacs EMR, Wiseman SA, Mela DJ, Rogers PJ. No appetite efficacy of a commercial structured lipid emulsion in minimally processed drinks. Int J Obes (Lond) 2012;36:1222–8. [DOI] [PubMed] [Google Scholar]
- 114.Smit HJ, Keenan E, Kovacs EMR, Wiseman SA, Peters HPF, Mela DJ, Rogers PJ. No efficacy of processed Fabuless (Olibra) in suppressing appetite or food intake. Eur J Clin Nutr 2011;65:81–6. [DOI] [PubMed] [Google Scholar]
- 115.Marciani L, Faulks R, Wickham MSJ, Bush D, Pick B, Wright J, Cox EF, Fillery-Travis A, Gowland PA, Spiller RC. Effect of intragastric acid stability of fat emulsions on gastric emptying, plasma lipid profile and postprandial satiety. Br J Nutr 2009;101:919–28. [DOI] [PubMed] [Google Scholar]
- 116. Marciani L, Wickham M, Singh G, Bush D, Pick B, Cox E, Fillery-Travis A, Faulks R, Marsden C, Gowland PA, et al. Delaying gastric emptying and enhancing cholecystokinin release and satiety by using acid stable fat emulsions. Gastroenterology 2006;130(4):A227.
- 117.Marciani L, Wickham M, Singh G, Bush D, Pick B, Cox E, Fillery-Travis A, Faulks R, Marsden C, Gowland PA, et al. Enhancement of intragastric acid stability of a fat emulsion meal delays gastric emptying and increases cholecystokinin release and gallbladder contraction. Am J Physiol Gastrointest Liver Physiol 2007;292:G1607–13. [DOI] [PubMed] [Google Scholar]
- 118.Marciani L, Wickham MSJ, Bush D, Faulks R, Wright J, Fillery-Travis AJ, Spiller RC, Gowland PA. Magnetic resonance imaging of the behaviour of oil-in-water emulsions in the gastric lumen of man. Br J Nutr 2006;95:331–9. [DOI] [PubMed] [Google Scholar]
- 119.Aranceta J, Moreno B, Moya M, Anadon A. Prevention of overweight and obesity from a public health perspective. Nutr Rev 2009;67:S83–8. [DOI] [PubMed] [Google Scholar]
- 120.Grunert KG. How changes in consumer behavior and retailing affects competence requirements for food producers and processors. EcoEconomia Agraria y Recursos Naturales 2006;6:3–22. [Google Scholar]
- 121.Hoefkens C, Verbeke W, Van Camp J. Europeans consumers’ perceived importance of qualifying and disqualifying nutrients in food choices. Food Qual Prefer 2011;22:550–8. [Google Scholar]
- 122.Liu H, Xu XM, Guo SD. Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. LWT -. Food Sci Technol (Campinas) 2007;40:946–54. [Google Scholar]
- 123.Mun S, Kim YL, Kang CG, Park KH, Shim JY, Kim YR. Development of reduced-fat mayonnaise using 4α Gtase-modified rice starch and xanthan gum. Int J Biol Macromol 2009;44:400–7. [DOI] [PubMed] [Google Scholar]
- 124.Bimal C, Zhang G. Olestra: a solution to food fat? Food Rev Int 2006;22:245–58. [Google Scholar]
- 125.Fouad FM, Mamer OA, Sauriol F, Shahidi F, Ruhenstroth-Bauer G. Olestra versus natural lipids: a critical review. In: Spanier AM, Shahidi F, Parliment TH, Mussinan C, Ho CT, Contis ET, editors. Food flavors and chemistry: advances of the new millennium. Cambridge (United Kingdom): Royal Soc Chemistry; 2001. p. 17–29.
- 126.Prince DM, Welschenbach MA. Olestra: a new food additive. J Am Diet Assoc 1998;98:565–9. [DOI] [PubMed] [Google Scholar]
- 127.Finley JW, Leveille GA, Dixon RM, Walchak CG, Sourby JC, Smith RE, Francis KD, Otterburn MS. Clinical-assessment of salatrim, a reduced-calorie triacylglycerol. J Agric Food Chem 1994;42:581–96. [Google Scholar]
- 128.Softly BJ, Huang AS, Finley JW, Petersheim M, Yarger RG, Chrysam MM, Wieczorek RL, Otterburn MS, Manz A, Templeman GJ. Composition of representative SALATRIM fat preparations. J Agric Food Chem 1994;42:461–7. [Google Scholar]
- 129.Smith RE, Finley JW, Leveille GA. Overview of SALATRIM, a family of low-calorie fats. J Agric Food Chem 1994;42:432–4. [Google Scholar]
- 130.Sørensen LB, Cueto HT, Andersen MT, Bitz C, Holst JJ, Rehfeld JF, Astrup A. The effect of salatrim, a low-calorie modified triacylglycerol, on appetite and energy intake. Am J Clin Nutr 2008;87:1163–9. [DOI] [PubMed] [Google Scholar]
- 131.French S, Robinson T. Fats and food intake. Curr Opin Clin Nutr Metab Care 2003;6:629–34. [DOI] [PubMed] [Google Scholar]
- 132.Small DM. The effect of glyceride structure on absorption and metabolism. Annu Rev Nutr 1991;11:413–34. [DOI] [PubMed] [Google Scholar]
- 133.Flint A, Helt B, Raben A, Toubro S, Astrup A. Effects of different dietary fat types on postprandial appetite and energy expenditure. Obes Res 2003;11:1449–55. [DOI] [PubMed] [Google Scholar]
- 134.Kamphuis MMUW, Mela DJ, Westerterp-Plantenga MS. Diacylglycerols affect substrate oxidation and appetite in humans. Am J Clin Nutr 2003;77:1133–9. [DOI] [PubMed] [Google Scholar]
- 135.St-Onge M-P, Ross R, Parsons WD, Jones PJH. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res 2003;11:395–402. [DOI] [PubMed] [Google Scholar]
- 136.Van Wymelbeke V, Himaya A, Louis-Sylvestre J, Fantino M. Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr 1998;68:226–34. [DOI] [PubMed] [Google Scholar]
- 137.Kahler A, Zimmerman M, Langhans W. Suppression of hepatic fatty acid oxidation and food intake in men. Nutrition 1999;15:819–28. [DOI] [PubMed] [Google Scholar]
- 138.Bremer J. Carnitine: metabolism and functions. Physiol Rev 1983;63:1420–80. [DOI] [PubMed] [Google Scholar]
- 139.French SJ, Conlon CA, Mutuma ST, Arnold M, Read NW, Meijer G, Francis J. The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology 2000;119:943–8. [DOI] [PubMed] [Google Scholar]
- 140.Risérus U, Berglund L, Vessby B. Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in obese middle-aged men with signs of the metabolic syndrome: a randomised controlled trial. Int J Obes Relat Metab Disord 2001;25:1129–35. [DOI] [PubMed] [Google Scholar]
- 141.Dilzer A, Park Y. Implication of conjugated linoleic acid (CLA) in human health. Crit Rev Food Sci Nutr 2012;52:488–513. [DOI] [PubMed] [Google Scholar]
- 142.Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, Ernst E. The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials. Eur J Nutr 2012;51:127–34. [DOI] [PubMed] [Google Scholar]
- 143.Cui SW. Food carbohydrates: chemistry, physical properties and applications. Boca Raton (FL): Taylor and Francis; 2005. [Google Scholar]
- 144.Stephen AM, Phillips GO, Williams PA. Food polysaccharides and their applications. 2nd ed Boca Raton (FL): CRC Press; 2006. [Google Scholar]
- 145.Williams PA. Gelling agents. In: Williams PA, editor. Handbook of industrial water soluble polymers. Oxford (United Kingdom): Blackwell Publishing; 2007. p. 73–97.
- 146.McClements DJ. Comments on viscosity enhancement and depletion flocculation by polysaccharides. Food Hydrocoll 2000;14:173–7. [Google Scholar]
- 147.Cheftel JC, Dumay E. Microcoagulation of proteins for development of creaminess. Food Rev Int 1993;9:473–502. [Google Scholar]
- 148.Aykan V, Sezgin E, Guzel-Seydim ZB. Use of fat replacers in the production of reduced-calorie vanilla ice cream. Eur J Lipid Sci Technol 2008;110:516–20. [Google Scholar]
- 149.Karaca OB, Guven M, Yasar K, Kaya S, Kahyaoglu T. The functional, rheological and sensory characteristics of ice creams with various fat replacers. Int J Dairy Technol 2009;62:93–9. [Google Scholar]
- 150.Sahan N, Yasar K, Hayaloglu AA, Karaca OB, Kaya A. Influence of fat replacers on chemical composition, proteolysis, texture profiles, meltability and sensory properties of low-fat Kashar cheese. J Dairy Res 2008;75:1–7. [DOI] [PubMed] [Google Scholar]
- 151.Yilsay TO, Yilmaz L, Bayizit AA. The effect of using a whey protein fat replacer on textural and sensory characteristics of low-fat vanilla ice cream. Eur Food Res Technol 2006;222:171–5. [Google Scholar]
- 152.Chung C, Degner B, McClements DJ. Physicochemical characteristics of mixed colloidal dispersions: models for foods containing fat and starch. Food Hydrocoll 2013;30:281–91. [Google Scholar]
- 153.Chung C, Olson K, Degner B, McClements DJ. Textural properties of model food sauces: correlation between simulated mastication and sensory evaluation methods. Food Res Int 2013;51:310–20. [Google Scholar]
- 154.Sikora M, Badrie N, Deisingh AK, Kowalski S. Sauces and dressings: a review of properties and applications. Crit Rev Food Sci Nutr 2008;48:50–77. [DOI] [PubMed] [Google Scholar]
- 155.Parada J, Aguilera JM. Review: starch matrices and the glycemic response. Food Sci Technol Int 2011;17:187–204. [DOI] [PubMed] [Google Scholar]
- 156.Peng BL, Dhar N, Liu HL, Tam KC. Chemistry and applications of nanocrystalline cellulose and its derivatives: a nanotechnology perspective. Can J Chem Eng 2011;89:1191–206. [Google Scholar]
- 157.Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 2012;46:2242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chung C, Degner B, Decker EA, McClements DJ. Oil-filled hydrogel particles for reduced-fat food applications: fabrication, characterization, and properties. Innov Food Sci Emerg Technol 2013;20:324–34. [Google Scholar]
- 159.Jones OG, McClements DJ. Functional biopolymer particles: design, fabrication, and applications. Comprehensive Reviews in Food Science and Food Safety 2010;9:374–97. [DOI] [PubMed] [Google Scholar]
- 160.Norton IT, Frith WJ, Ablett S. Fluid gels, mixed fluid gels and satiety. Food Hydrocoll 2006;20:229–39. [Google Scholar]
- 161.Spyropoulos F, Norton AB, Norton IT. Self-structuring foods based on acid-sensitive mixed biopolymer to impact on satiety. 11th International Congress on Engineering and Food (Icef11). Procedia Food Science 2011;1:1487–93.
- 162.Golding M, Wooster TJ, Day L, Xu M, Lundin L, Keogh J, Clifton P. Impact of gastric structuring on the lipolysis of emulsified lipids. Soft Matter 2011;7:3513–23. [Google Scholar]
- 163.Tchuenbou-Magaia FL, Norton IT, Cox PW. Hydrophobins stabilized air-filled emulsions for the food industry. Food Hydrocoll 2009;23:1877–85. [Google Scholar]
- 164.Tchuenbou-Magaia FL, Cox PW. Tribological study of suspensions of cysteine-rich protein stabilized microbubbles and subsequent triphasic A/O/W Emulsions. J Texture Stud 2011;42:185–96. [Google Scholar]
- 165.Tchuenbou-Magaia FL, Al-Rifai N, Mohamed-Ishak NE, Norton IT, Cox PW. Suspensions of air cells with cysteine-rich protein coats: air-filled emulsions. J Cell Plastics 2011;47:217–32. [Google Scholar]
- 166.Golding M, Wooster TJ. The influence of emulsion structure and stability on lipid digestion. Curr Opin Colloid Interface Sci 2010;15:90–101. [Google Scholar]
- 167.McClements DJ, Decker EA, Park Y, Weiss J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit Rev Food Sci Nutr 2009;49:577–606. [DOI] [PubMed] [Google Scholar]