Abstract
This article summarizes new knowledge about the contribution of genetic variation to person-to-person differences underlying some sensory aspects of dietary fatty acids. Receptors on the taste cells of the human tongue arise from genes that have marked variation in DNA sequence, which, in some cases, is associated with differences in how these lipids in foods are perceived. These perceptual differences may affect food selection.
Keywords: fatty acids, obesity, taste, lipids, genetics, polymorphism, chemosensory
Introduction
Food is essential to our survival, and although eating is pleasant, it can also be dangerous (1). Our sense of taste developed to identify the pleasure of sweet, the tang of sour, and the brinelike quality of salt. Both the quality (e.g., sweet vs. bitter) and the perceived strength (e.g., strong vs. weak) of sensory experiences help determine whether it is best to swallow or to reject food. Other stimuli contribute to our sense of pleasure or danger from foods, such as carbonation, burn, calcium, and fats (2, 3). Pinguis is a Latin word that translates to “fat taste” (4), and although this is an old concept, only recently has the underlying biology of one of its components, FA perception, been discovered.
Current Status of Knowledge
Anatomy of the taste system
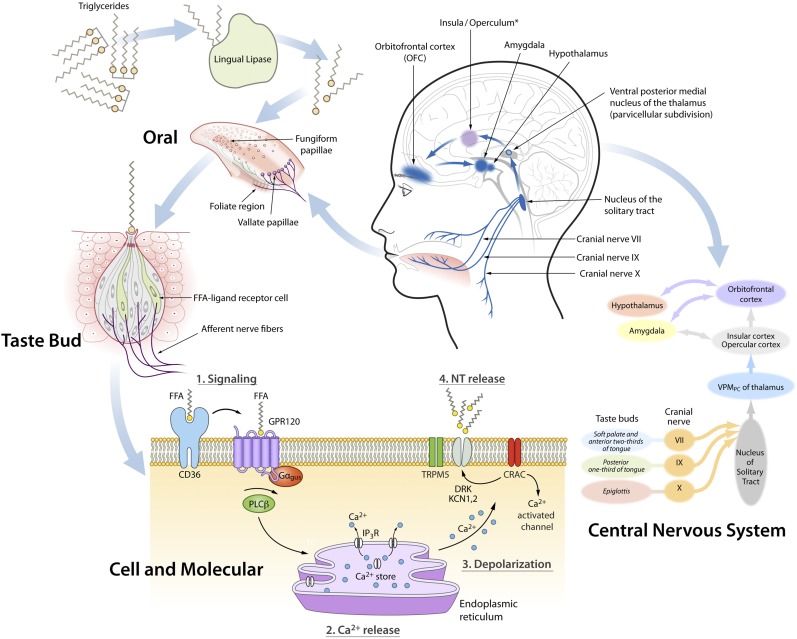
A few general principles about the taste system are useful to understand how and whether the sensations produced by lipids are part of this system (see Figure 1). Taste arises when chemicals stimulate special sensory cells on the human tongue. These cells are contained in a taste bud, a structure that contains dozens of sensory cells arranged like the segments of an orange. The taste buds are contained within taste papillae. These taste papillae, which are visible on the tongue as small pink bumps, comprise 3 types, with names inspired by Latin words that describe their structure. The fungiform papillae are concentrated on the front of the tongue and owe their name to their mushroom-like appearance (from the Latin word fungus). Foliate papillae are concentrated on the sides of the tongue and are similar to the pages or leaves of a book (i.e., folia). Circumvallate papillae are located at the back of the tongue and look like they are encircled with a rampart or entrenchment, hence their name. Early anatomists called circumvallate papillae “calyciform,” which reflects their calyx or podlike appearance.
FIGURE 1.
Process of fat taste perception. The generation of fat taste starts when TGs in the mouth are hydrolyzed into FFAs by one or more lingual lipases (upper left). FAs stimulate taste cells embedded in the fungiform, foliate, and vallate (circumvallate) papillae, which are visible as pink bumps on the human tongue. There are several types of taste cells in the taste bud, and in humans fat is likely to be detected by at least type II cells. At the surface of the cell membrane, the FFA ligand binds to 2 (and possibly more) types of proteins, a GPR120 and CD36. How these 2 molecules interact is currently unknown, and it is also hypothesized that they have nonoverlapping functions. This binding triggers a cascade of signaling events, leading to an increase in intracellular calcium and the ultimate neurotransmitter release that activates an afferent nerve fiber, which transmits the signal via the cranial nerves VII, IX, and X to the brain. *The insula/operculum is lateral to the sagittal plane of the section shown. CRAC, calcium release-activation channel; DRK, delayed rectifying K+ channel; Gαgus, α-gustducin; GPR120, G protein–coupled receptor 120; IP3R, inositol triphosphate 3 receptor; KCN1,2, potassium volate-gated channel, shaker-related subfamily, members 1 and 2; NT, neurotransmitter; PLCβ, phospholipase C β; TRPM5, transient receptor potential cation channel subfamily M, member 5; VPMPC, ventral posterior medial nucleus of the thalamus, parvicellular subdivision.
Taste receptor cells, which are contained within taste buds within papillae, convey signals via 3 sensory nerves: the chorda tympani (cranial nerve VII, one of the facial nerves), the glossopharyngeal nerve (cranial nerve IX), and the vagus nerve (cranial nerve X). These nerves relay sensory input to the nucleus of the solitary tract in the brain stem and from there to the thalamus and forebrain structures.
The anatomy of fat taste
Fat has both taste and texture and only the taste part of perception is discussed here. Fat taste may arise from TGs, but more is known about how FAs elicit sensory signals. The hydrolysis of TGs produces FAs by a series of enzymatic steps that are sensed by cells in the mouth (5–7). The overall idea is that these FAs bind to receptors on distinct types of taste receptor cells. The evidence for this idea comes from several types of experiments. For mice and rats, blocking the ability to convert TGs to FAs in the mouth, either by inhibiting lingual lipase with drugs or by removing the salivary glands, reduces the animal’s preference for fat. Likewise, cutting the nerves between the tongue and brain also reduces fat preference. Humans can sense FAs emulsified in water even when other senses such as sight and trigeminal sensations (e.g., burn) are blocked. However, FAs appear to provide a chemical signal, whereas intact TGs provide the textural signal. Both types of information are distinct, but they both converge to the same brain areas where they contribute to the unique perception of fat. Thus, the chemical signal of fat taste appears not to act alone and instead requires textural cues for its full perceptual embodiment (8).
Brain areas that sense fat taste
Taste cells in the mouth respond to FAs and gustatory nerves relay information about their presence to the brain. Some brain areas respond to the texture of fat, as well as to the texture of other viscous solutions. At least one fMRI study in individuals who tasted vegetable oil showed that the insular taste cortex is activated by fat. Sugar also activates this area (9). Dietary fat is sensed by other brain areas (10). However, these data do not address the thorny problem of what exactly is the stimulus that elicits this activation: it might be TGs or it might be the small amounts of FAs digested orally during the test. Research that parses the effects of FAs from pure TGs would be useful to help understand which signals are important for the pleasant aspects of fat taste.
TGs vs. FAs
To understand fat taste from its chemical perspective, it would be helpful to know just how much TG is hydrolyzed to FAs by chewing food, which presumably mixes saliva and the lingual lipase it contains with TGs to incite the chemical reaction. In a recent study, people chewed high-fat foods (e.g., almonds), and the amount of FFAs liberated was measured. The results from the study allowed the investigators to conclude that chewing released sufficient FFAs to stimulate the receptors on the basis of their known receptive ranges (11). Recently, the lingual lipases most active in humans were identified, which will allow more direct study of the rates of hydrolysis (12).
FAs as pure taste stimuli (e.g., as solutions placed on the tongue) are unpleasant. Perhaps they are meant to warn against the ingestion of oxidized FAs and rancid foods (13, 14). For instance, when airborne FAs are held in the mouth, subjects describe them as “rubbery” or like plastic (15). Even from a distance, people may be able to smell taste stimuli and make judgments about their fat content (16). Thus, FAs have distinct smells as well as distinct and unpleasant tastes.
FAs are associated with rancid food odors, and people working in the food industry strive to reduce the amount of FAs in processed foods because they are off-putting. It is unclear why FAs are unpleasant in these settings, yet are pleasant when released during chewing. It may be that the concentrations of FAs released during chewing are too low to be detected retronasally (17)—that is, the odor of FAs is unpleasant but as taste stimuli in the context of eating they are pleasant. This is one of the gaps in our understanding about fat taste: why, if FAs taste and smell bad, are high-fat foods so popular?
FA chemical transduction cascade
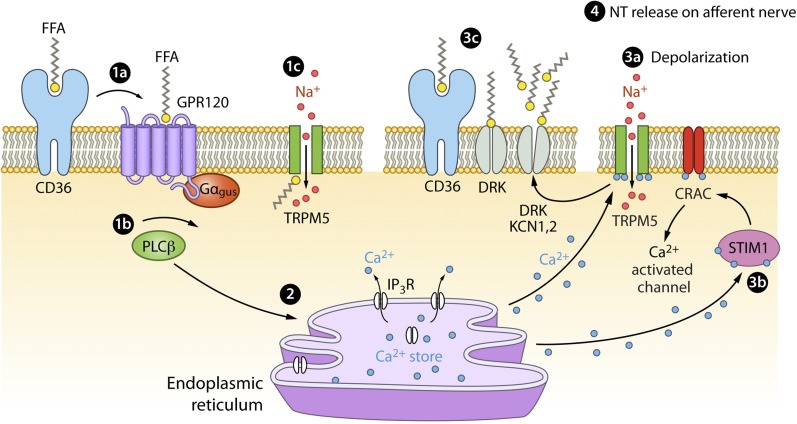
FAs are ligands for receptors found in the specialized taste cells. Each type of papilla—fungiform, foliate, and circumvallate—responds more or less the same to these ligands, and so there is no regional sensitivity to FAs on the tongue (18). Several lines of evidence suggest that FAs bind to at least 2 types of proteins on the cell membranes of taste cells. One type of protein is a member of the G protein–coupled receptor (GPR)4 family (GPR120) and the other type is a molecule called CD36. Stimulation of these proteins generates a signal that travels through a common second-messenger cascade similar to the one generated by bitter and sweet receptors, which couple through heterotrimeric G proteins to initiate intracellular signaling cascades involving effector enzymes, second messengers, and ion channel targets (see Figure 2). The steps in the pathway are thought to be 1) activation of type 3 inositol triphosphate 3 (IP3) receptors and 2) release of Ca2+ from internal stores followed by 3) activation of the calcium-activated cation channel transient receptor potential cation channel subfamily M member 5 (TRPM5). TRPM5 is a key molecule in taste transduction, at least in mice, because taste cells without it fail to depolarize (19). Several other proteins that are now thought to be involved in calcium signaling also contribute to FA taste perception, such as stromal interaction molecule 1 (STIM1) (20). A recent review summarizes these studies (7).
FIGURE 2.
Proposed signaling pathway for fat taste. (1a) CD36 may act as a binding protein for FFAs, such as linoleic acid. It is unclear whether and how CD36, when bound to an FFA, interacts with GPR120 and other membrane proteins. However, evidence in mice indicates that GPR120 and CD36 have different roles in signaling, with CD36 being more reliant on FFAs and GPR120 being activated by a non-FFA mechanism. The presence of CD36 and GPR120 also varies on the basis of BMI, dietary intake, and fasting state. (1b) The binding of FFAs to GPR120 leads to increased signaling in Gαgus and PLC-β. FFAs and CD36 may interact directly with PLC-β. (1c) FFAs (e.g., arachidonic acid) can intracellularly activate TRPM5 channels. Intracellular Ca2+ can also directly interact with TRPM5 channels. (2) Na+ passes through the channel into the intracellular membrane. The result of these various signaling mechanisms is an increase in intracellular Ca2+ (released from IP3 channels on the endoplasmic reticulum). (3a) The Ca2+ release ultimately leads to membrane depolarization, with positive feedback. With the increase in intracellular Ca2+, TRPM5 channels are activated. (3b) STIM1, which detects lowered Ca2+ concentrations in the endoplasmic reticulum, may aid in the activation of store-operated Ca2+ channels such as CRACs. This, in conjunction with other channels, such as TRPM5, directly interacting with Ca2+ allows for continued membrane depolarization. (3c) The membrane potential also interacts with and opens DRK channels, such as KCN5, whereas extracellular FFAs are channel blockers. (4) Depolarization is therefore prolonged with the presence of more FFAs, with CD36 possibly aiding in the interaction between DRK channels and FFAs. The depolarization ultimately causes neurotransmitter release. CRAC, calcium release-activation channel; DRK, delayed rectifying K+ channel; GPR120, G protein–coupled receptor 120; IP3, inositol triphosphate 3; IP3R, inositol triphosphate 3 receptor; KCN5, potassium volate-gated channel, shaker-related subfamily, member 5; NT, neurotransmitter; PLCβ, phospholipase C β; STIM1, stromal interaction molecule; TRPM5, transient receptor potential cation channel subfamily M, member 5.
CD36 is a membrane-bound protein related to fat taste. It is found on the apical surface of taste cells in humans, a location consistent with a role in taste transduction (21). Several other lines of evidence support its role in taste. It colocalizes in taste papillae with known signaling molecules. In CD36-knockout mice, the normal increase in intracellular calcium of taste receptor cells in response to FFAs is blocked, and the activation of brain areas associated with FA stimulation is eliminated. Furthermore, CD36-knockout mice are indifferent to the same fat solutions that mice with an intact CD36 gene prefer.
Most information on fat taste surrounds CD36 because this protein is well studied in other cell types such as those involved in intestinal lipid transport. However, CD36 is a complex molecule that has many alternative forms—in humans, it has many single nucleotide polymorphisms (SNPs), as well as 23 known splice variants (22), 17 of which are protein-coding. However, it is still unclear which variants are expressed for the purpose of fat perception or whether humans express different variants of CD36 depending on other factors or gene interactions upstream. It is also unclear whether some variants are expressed in human taste tissues and other forms are expressed in other tissues. One possibility is that there may be one form of CD36 specific to taste cells, but this hypothesis awaits empirical study.
GPR120 is the other protein that is consistently involved with FA taste signaling. When introduced into a cell-based assay, GPR120 causes the cell to respond to FAs by increasing intracellular calcium, similar to the response seen by other GPRs specific to classic taste stimuli. Much of the evidence on FA transduction in taste cells comes from studies of mice. However, recent evidence supports a similar role for these molecules for human FA taste. Both GPR120 and CD36 are found in human taste receptor cells, and they appear to be found in the same cells (23). The reduction in these receptors in taste cells reduces changes in intracellular calcium, commonly used to measure the vigor of receptor signaling.
However, in human cells, CD36 has higher affinity for its preferred ligand than does GPR120. This observation raises several interesting questions. CD36 may be a more general lipid sensor that can respond to many different types of FAs, even at low concentrations, whereas GPR120 may respond only to specific types of FAs (e.g., grifolic acid, found in a certain type of mushroom) (23) and only at higher concentrations. Another candidate fat receptor was GPR40, but recent studies failed to identify it in human taste cells (24). It may be that unidentified families of FA receptors play a role in fat taste.
Mature taste cells may use dozens or maybe hundreds of proteins to relay FA information to downstream sensory neurons. Currently, investigators are focusing on identifying these unknown molecules. One way to achieve “genomic saturation,” meaning the identification of all relevant genes and their protein products that are crucial in this biology, is to sequence all mRNA that arises from a single taste cell, called single-cell transcriptomics. Comparing mRNA from single taste cells that respond to FAs with RNA from taste cells that are not responsive generates a full or nearly full list of components that may be involved in fat taste. This method offers a way to identify the components of a cell type.
One hypothesis that has not received empirical testing but might be worth exploring is the idea that other taste receptors exist on taste cells that sense TGs themselves, rather than the hydrolyzed FFAs. This idea is appealing because TGs in foods are liked but FAs are not. It could be that FAs and their taste receptors are there to warn about spoiled foods, whereas these hypothetical TG receptors might convey the good taste of fat. Saliva may not only produce FFAs with lipases but could contain substances that might package TGs into lipoproteins to be sensed by receptors on the tongue similar to those that respond to lipoproteins in the gut and liver. The study of saliva focuses on its catabolic properties; however, saliva may also help form complex lipid-protein mixtures that are sensed directly by cells on the tongue.
Cell types in fat taste
There are multiple cell types in taste buds. Type I cells are glia-like and are thought to have a role as supporting cells. Type II cells have bitter and sweet receptors but do not signal directly to the sensory nerve with a direct release of neurotransmitter. Type III cells are mainly responsible for responding to sour and salty tastes and for the release of neurotransmitters that convey information forward through the sensory nerves to the brain. It is not clear which cell types respond to FAs on the basis of direct evidence, but from the importance of the transduction components such as TRPM5 (see Figure 2), which are components of type II cells, it would appear that this cell type is most likely involved.
Identifying cell types in human taste tissue that respond to FAs is difficult. Human taste cells in culture lose some of their characteristic morphology when they are no longer in a tight taste bud formation (25, 26). Studies that use small, interfering RNA or drugs to examine the pharmacology of FA signaling apply these compounds to the whole culture, so it is not possible to distinguish the influential cell types. There is one human taste cell line that can be grown in culture so that at least one cell type can be studied in isolation, and this cell line responds to both FAs and bitter compounds (27). However, we also know that in the fruit fly, FAs and sweet signals arise from the same cell type (28). Perhaps some type II cells coexpress FA and bitter receptors and others express FA and sweet receptors.
FAs in other parts of the body
Another method to look for the components of fat taste is to extrapolate from cell types that respond to FAs in other parts of the body. This concept is not unique to FA receptors. Bitter and sweet taste receptors were identified by their role in taste perception in the mouth but function in other parts of the body as well (29–35). The reverse strategy may work for fat taste: FA signaling. FA detection in other tissues and organs such as the pancreas and liver is better understood than is fat signaling in taste cells and this knowledge can be a useful starting point to study taste.
Human psychophysical studies on FFAs
Before the sensory properties of fat taste can be studied, they must be measured. The field of science devoted to these measurements is called psychophysics. Scientists trained in this field try to understand relations between physical stimuli (e.g., a tastant or odorant) and the psychological responses they elicit (e.g., taste or odor). Thus, an individual’s ability to taste or smell can be determined by using psychophysical testing. For example, a common psychophysical measurement is detection threshold, which is the lowest concentration at which a compound can be detected—subjects often perceive this as only a hint of “something,” just enough to discriminate the stimulus from a blank but not necessarily enough to recognize its type or quality. In contrast, the recognition threshold is the lowest concentration at which a stimulus can be named for its quality (e.g., bitter). Detection thresholds may make more sense as a testing method than recognition thresholds for fats because there is no common word that people use to describe fat taste. To measure the intensity of stimuli, investigators offer them at readily detectible concentrations. Investigators may ask subjects to label the type of sensations they perceive (e.g., sweet, bitter) or to rate the hedonics (e.g., the degree of pleasantness or appeal of the stimuli).
Most studies of FA perception in humans are conducted with precautions to guard against the subjects using sensory qualities other than pure taste. For instance, subjects might be tested with nose clips to reduce the odors arising from the fats, and they might be tested with red ambient illumination to mask the appearance of the stimuli, so they cannot tell the difference between high-fat and low-fat stimuli. Finally, investigators mask the potential sting or burn of FAs by desensitizing the tongue in advance using compounds such as capsaicin. This step helps distinguish taste from trigeminal sensory input.
Measuring the human sensory response to FFAs is not easy for technical reasons. There are dozens of forms of FFAs, which differ in chain length and degree of saturation. It is difficult to standardize stimuli from among the many types of FAs and the many methods of preparation. Investigators have typically focused on FAs found in high concentrations in common foods, but there is much to learn from conducting a broader survey of less-common FAs. SCFAs may be quite different from long-chain or very-long-chain FAs and the degree of saturation may be important, too. FFAs are typically emulsified in water, and small differences in the emulsifying agents, such as the variations that can arise from natural gums or from differences in manufacturing processes, even from the same supplier, may affect results (36). Accurate and consistent reporting about the sensory perception of FAs is also difficult because there is no simple word to describe their quality. In addition, experience and learning affect sensory testing, and subjects tested over time often improve their performance, sometimes markedly (37).
Differences in perception of fats.
The phrase “a matter of taste” indicates that people differ in their patterns of likes and dislikes, and nowhere is this truer than in the realm of taste itself (38–40). One of the most marked human differences that arises from genetic variation is the ability to taste certain types of bitter compounds (41). Even the perception of sweet taste, which is one of the universal likes in human society, indeed in many mammals, is not immune from genetic variation. Genotype in the sweet receptor explains why some people prefer heavily sweetened foods, whereas others do not (42–44). There is diversity among humans in the perception of FAs (36, 45), and it would be logical to speculate that such differences arise from genetic differences in the receptors, as they do for bitter and sweet taste perception.
The results of 2 studies indicated that human differences in fat taste perception may relate to genetic variation in the CD36 gene. As mentioned above, CD36 has many forms due to alternative splicing and SNPs. One SNP in CD36 is associated with the perception of fat (46). In a study, subjects were given samples of Italian salad dressing with varying fat content and asked to rate them for creaminess, fat, and other variables, and their DNA was sequenced for CD36. The A/A genotype at the rs1761667 SNP was associated with all dressings tasting creamier and a higher liking for added fats and oils compared with the G/A genotype. This same SNP may also be associated with the expression of CD36, with the A/A allele being associated with lower CD36 expression and the participants with the G/G allele tasting more oleic acid and having lower oral detection thresholds for oleic acid and triolein (47). Future genetic studies could both help us learn why people differ in the liking for fats and oils and point us to essential yet undiscovered proteins in this transduction pathway.
The heritability of the perception of FAs is unclear. In the genetic studies reviewed above, the stimuli were TGs (salad oil) or an FA (oleic acid), and the presence of the genotype-phenotype relation implies that, for both stimuli, fat liking is heritable. This observation is concordant with the observation that dietary fat intake is heritable (48), but other influences in addition to liking and taste may be important determinants of how much someone eats. We do know that there are large differences between individuals in their taste thresholds (49), but estimates of heritability are needed in genetically informative populations such as twins (50). Thus, it would be possible to conduct genomewide association studies, which have identified genetic variants that influence other types of taste perception (51). Taste strips could be used for rapid testing of FA perception, i.e., with FAs embedded in a dissolvable matrix (52) rather than the use of emulsified FAs that are not easy to use in large-scale testing. Thus far, genomewide association studies have reported loci for dietary fat intake (53) but have not examined fat perception.
Habitual diet, body weight, and fat taste.
In addition to genotype, other factors may affect individual differences in FA perception, with the 2 most salient being habitual diet and BMI. For instance, it could be that people who habitually consume fat are accustomed to its taste and are less sensitive to low concentrations of FAs. To test this idea directly, investigators measured FA thresholds in the same people who were fed both high-fat and low-fat diets. Consuming a low-fat diet for a 2-wk period increased individual sensitivity to fat, whereas consuming a high-fat diet decreased sensitivity—but only in the lean people, presumably because the overweight and obese subjects were already habituated to a high-fat diet (54). Likewise, those individuals whose diets were higher in fat and who were heavier for their height than those who ate less fat were less sensitive to low concentrations of FAs (55).
This insensitivity to FAs also extends beyond the mouth to the gut. Obese men were less sensitive to oral FAs and to infusions of FAs into the gut than were lean men (56). In mice, CD36 expression is reduced in obese compared with lean mice (23), but when CD36 is experimentally manipulated, lean and obese mice showed equal reductions in mRNA involved in the decreased ability to sense fat (57). Studies of rodents also showed that experience is a key determinant of fat intake, and even short periods of access to high-fat foods increase later intake (58). Likewise, rodents fed a diet high in fat drives fat preference even higher (59).
Fat liking and digestion
The fat sensors on the tongue do more than generate taste perception; they are also able to influence digestion even while fats are still in the mouth. Humans who swallowed tasteless capsules of safflower oil and then tasted but did not swallow a high-fat food had higher blood lipid profiles than did subjects who took the capsules but did not taste the high-fat food (14). This result suggests that there is a cephalic phase of digestion, in which the brain sends preparatory signals to the digestive organs in response to sensory cues that are especially potent for fat. Fats are calorically dense and not water soluble, so the body may need extra time to prepare for incoming TGs, for instance by helping the body titer the amount of lipases to secrete in the gastrointestinal tract.
The fat sensors on the tongue are also present in the gut and may affect an animal’s avidity for dietary fat. One study in rodents found that a flavor paired with an infusion of fat directly into the stomach, bypassing the mouth, is preferred over flavors not paired with fat infusions, and that this preference is markedly attenuated in animals that lack a fat receptor gene (60). These results suggest that stimulation of this fat receptor (GPR120; discussed below) may drive fat intake and liking, but the effects may derive from the absorption of fat and its calories rather than from the stimulation of the oral receptors and the pleasure that sensation may bring.
Fat substitutes
Public policy makers and food makers want to reduce fat in food. Noncaloric molecules that mimic the taste of fat would be one method to reduce calories but not fat flavors. Artificial sweeteners—high-potency molecules that provide a strong sweet taste with fewer calories than sugar—provide many examples of the popularity of such substitutes. Whether a similar high-potency fat substitute with the appropriate textural qualities could be developed that becomes as popular as sweeteners such as sucralose is unclear. One way to pursue this idea is to screen small molecule libraries for agonists of GPRs such as GPR120. However, in initial attempts, agonists that alter the signaling cascade through this receptor did not engender a preference in the same way that lipids do (24). Attempts to create products that have a fat texture but are not absorbed, such as olestra, have met with only limited success, in part because of the unpleasant digestive side effects. The failed attempts to find a low-calorie but high-pleasure fat substitute likely reflect our imperfect understanding of the fat taste system.
Conclusions
The study of fat as a taste quality has lagged behind the other more obvious tastes such as sweet or bitter. It is currently unclear whether FAs are the only ligands that stimulate the sense of taste or whether other unknown receptors exist, for instance, for TGs. One puzzle is that FAs taste and smell bad but TGs are well liked. Fat taste may depend on the brain’s integration of multiple inputs, such as texture and odor, more so than do other taste qualities, and thus may be less amenable to the study of fat molecules in isolation. Regardless of the difficulties, it is important to understand why the taste of fat, defined narrowly or broadly, is appealing. High-fat diets contribute to many of the chronic health problems in developed and developing countries, so solving how they are perceived and why they are liked may suggest rational strategies for dietary change. Inborn differences in genotype explain at least some differences between individuals in the perception of FFAs.
Acknowledgments
John E Tordoff assisted with language translation. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRAC, calcium release-activation channel; DRK, delayed rectifying K+ channel; GPR, G protein–coupled receptor; IP3, inositol triphosphate 3; SNP, single nucleotide polymorphism; STIM1, stromal interaction molecule; TRPM5, transient receptor potential cation channel subfamily M, member 5.
References
- 1.Reed DR, Knaapila A. Genetics of taste and smell: poisons and pleasures. Prog Mol Biol Transl Sci 2010;94:213–40. [DOI] [PMC free article] [PubMed]
- 2.Tordoff MG. Some basic psychophysics of calcium salt solutions. Chem Senses 1996;21:417–24. [DOI] [PubMed] [Google Scholar]
- 3.Chalé-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses 2007;32:423–31. [DOI] [PubMed] [Google Scholar]
- 4.Fernelius I. Therapeutices universalis seu medendi rationis, libri septem. [Therapeutics: in theory or in practice.] Frankfurt (Germany): Andream Wechelum; 1581 (in Latin).
- 5.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 2014;53:82–92. [DOI] [PubMed] [Google Scholar]
- 6.Tucker RM, Mattes RD, Running CA. Mechanisms and effects of "fat taste" in humans. Biofactors 2014;40:313–26. [DOI] [PubMed] [Google Scholar]
- 7.Abdoul-Azize S, Selvakumar S, Sadou H, Besnard P, Khan NA. Ca2+ signaling in taste bud cells and spontaneous preference for fat: unresolved roles of CD36 and GPR120. Biochimie 2014;96:8–13. [DOI] [PubMed] [Google Scholar]
- 8.Rolls ET. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc Nutr Soc 2012;71:488–501. [DOI] [PubMed] [Google Scholar]
- 9.De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci 2004;24(12):3086–93. [DOI] [PMC free article] [PubMed]
- 10.Eldeghaidy S, Marciani L, McGlone F, Hollowood T, Hort J, Head K, Taylor AJ, Busch J, Spiller RC, Gowland PA, et al. . The cortical response to the oral perception of fat emulsions and the effect of taster status. J Neurophysiol 2011;105:2572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkarni B, Mattes R. Evidence for presence of nonesterified fatty acids as potential gustatory signaling molecules in humans. Chem Senses 2013;38:119–27. [DOI] [PubMed] [Google Scholar]
- 12.Voigt N, Stein J, Galindo MM, Dunkel A, Raguse JD, Meyerhof W, Hofmann T, Behrens M. The role of lipolysis in human orosensory fat perception. J Lipid Res 2014;55:870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattes RD. Is there a fatty acid taste? Annu Rev Nutr 2009;29:1–23. [DOI] [PMC free article] [PubMed]
- 14.Mattes RD. Effects of linoleic acid on sweet , sour, salty and bitter taste thresholds and intensity ratings of adults. Am J Physiol Gastrointest Liver Physiol 2007;292:G1243–8. [DOI] [PubMed] [Google Scholar]
- 15.Chukir T, Darlington RB, Halpern BP. Shared retronasal identifications of vapor-phase 18-carbon fatty acids. Chem Senses 2013;38:343–53. [DOI] [PubMed] [Google Scholar]
- 16.Boesveldt S, Lundstrom JN. Detecting fat content of food from a distance: olfactory-based fat discrimination in humans. PLoS ONE 2014;9:e85977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalé-Rush A, Burgess JR, Mattes RD. Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol 2007;292:G1206–12. [DOI] [PubMed] [Google Scholar]
- 18.Mattes RD. Oral thresholds and suprathreshold intensity ratings for free fatty acids on 3 tongue sites in humans: implications for transduction mechanisms. Chem Senses 2009;34:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type m5 is essential for fat taste. J Neurosci 2011;31(23):8634–42. [DOI] [PMC free article] [PubMed]
- 20.Dramane G, Abdoul-Azize S, Hichami A, Vogtle T, Akpona S, Chouabe C, Sadou H, Nieswandt B, Besnard P, Khan NA. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J Clin Invest 2012;122(6):2267–82. [DOI] [PMC free article] [PubMed]
- 21.Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem 2011;113:839–43. [DOI] [PubMed] [Google Scholar]
- 22.Sanger Institute trace server. [cited 2015 Feb 21]. Available from: www.ensembl.org.
- 23.Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014;146:995–1005. [DOI] [PMC free article] [PubMed]
- 24.Galindo MM, Voigt N, Stein J, van Lengerich J, Raguse JD, Hofmann T, Meyerhof W, Behrens MG. Protein-coupled receptors in human fat taste perception. Chem Senses 2012;37:123–39. [DOI] [PubMed] [Google Scholar]
- 25.Ozdener MH, Brand JG, Spielman AI, Lischka FW, Teeter JH, Breslin PA, Rawson NE. Characterization of human fungiform papillae cells in culture. Chem Senses 2011;36(7):601–12. [DOI] [PMC free article] [PubMed]
- 26.Ozdener MH, Rawson NE. Culture and maintenance of taste cells in vitro. In Vitro Cell Dev Biol Anim 2011;47:513–4. [DOI] [PubMed] [Google Scholar]
- 27.Hochheimer A, Krohn M, Rudert K, Riedel K, Becker S, Thirion C, Zinke H. Endogenous gustatory responses and gene expression profile of stably proliferating human taste cells isolated from fungiform papillae. Chem Senses 2014;39:359–77. [DOI] [PubMed] [Google Scholar]
- 28.Masek P, Keene AC. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genet 2013;9:e1003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosinger B, Redding KM, Parker MR, Yevshayeva V, Yee KK, Dyomina K, Li Y, Margolskee RF. Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc Natl Acad Sci USA 2013;110:12319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Kokrashvili Z, Mosinger B, Margolskee RF. Gustducin couples fatty acid receptors to GLP-1 release in colon. Am J Physiol Endocrinol Metab 2013;304:E651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swartz TD, Duca FA, de Wouters T, Sakar Y, Covasa M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br J Nutr 2012;107:621–30. [DOI] [PubMed] [Google Scholar]
- 32.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 2012;122(11):4145–59. [DOI] [PMC free article] [PubMed]
- 33.Simon BR, Learman BS, Parlee SD, Scheller EL, Mori H, Cawthorn WP, Ning X, Krishnan V, Ma YL, Tyrberg B, et al. . Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PLoS ONE 2014;9:e86454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, Bravo D. Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr 2014;111(Supp1):S8–15. [DOI] [PubMed] [Google Scholar]
- 35.Wauson EM, Zaganjor E, Lee AY, Guerra ML, Ghosh AB, Bookout AL, Chambers CP, Jivan A, McGlynn K, Hutchison MR, et al. . The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol Cell 2012;47:851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Running CA, Mattes RD, Tucker RM. Fat taste in humans: sources of within- and between-subject variability. Prog Lipid Res 2013;52:438–45. [DOI] [PubMed] [Google Scholar]
- 37.Tucker RM, Mattes RD. Influences of repeated testing on nonesterified fatty acid taste. Chem Senses 2013;38:325–32. [DOI] [PubMed] [Google Scholar]
- 38.Reed DR, Tanaka T, McDaniel AH. Diverse tastes: genetics of sweet and bitter perception. Physiol Behav 2006;88:215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka T, Reed DR, Ordovas J. Taste as the gatekeeper of personalized nutrition. In: Desiere F, Kok F, Bouwman L, editors. Personalized nutrition: principles and applications. Boca Raton (FL): Taylor and Francis, CRC Press; 2008. p. 115–32.
- 40.Newcomb RD, Xia MB, Reed DR. Heritable differences in chemosensory ability among humans. Flavour 2012;1:9. [Google Scholar]
- 41.Fox AL. The relationship between chemical constitution and taste. Proc Natl Acad Sci USA 1932;18:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol 2009;19:1288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mennella JA, Finkbeiner S, Reed DR. The proof is in the pudding: children prefer lower fat but higher sugar than do mothers. Int J Obes (Lond) 2012;36:1285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mennella JA, Finkbeiner S, Lipchock SV, Hwang L-D, Reed DR. Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLoS ONE 2014;9:e92201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr 2010;104:145–52. [DOI] [PubMed] [Google Scholar]
- 46.Keller KL, Liang LC, Sakimura J, May D, van Belle C, Breen C, Driggin E, Tepper BJ, Lanzano PC, Deng L, et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring). 2012 Jan 12 (Epub ahead of print; DOI: 10.1038/oby.2011.374). volume = 20, pages =1066–73. [DOI] [PMC free article] [PubMed]
- 47.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene, CD36, and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res 2012;53(3):561–6. [DOI] [PMC free article] [PubMed]
- 48.Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA. Heritable variation in food preferences and their contribution to obesity. Behav Genet 1997;27:373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattes RD. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem Senses 2009;34:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knaapila A, Hwang LD, Lysenko A, Duke FF, Fesi B, Khoshnevisan A, James RS, Wysocki CJ, Rhyu M, Tordoff MG, et al. . Genetic analysis of chemosensory traits in human twins. Chem Senses 2012;37:869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed DR, Zhu G, Breslin PA, Duke FF, Henders AK, Campbell MJ, Montgomery GW, Medland SE, Martin NG, Wright MJ. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet 2010;19:4278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebba S, Abarintos RA, Kim DG, Tiyouh M, Stull JC, Movalia A, Smutzer G. The examination of fatty acid taste with edible strips. Physiol Behav 2012;106(5):579–86. [DOI] [PMC free article] [PubMed]
- 53.Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, et al. . Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr 2013;97:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart JE, Keast RSJ. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int J Obes (Lond) 2012;36:834–42. [DOI] [PubMed] [Google Scholar]
- 55.Stewart JE, Newman LP, Keast RS. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin Nutr 2011;30:838–44. [DOI] [PubMed]
- 56.Stewart JE, Seimon RV, Otto B, Keast RS, Clifton PM, Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr 2011;93:703–11. [DOI] [PubMed] [Google Scholar]
- 57.Chen CS, Bench EM, Allerton TD, Schreiber AL, Arceneaux KP, III, Primeaux SD. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am J Physiol Regul Integr Comp Physiol 2013;305:R1346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed DR, Friedman MI, Tordoff MG. Experience with a macronutrient source influences subsequent macronutrient selection. Appetite 1992;18:223–32. [DOI] [PubMed] [Google Scholar]
- 59.Reed DR, Friedman MI. Diet composition alters the acceptance of fat by rats. Appetite 1990;14:219–30. [DOI] [PubMed] [Google Scholar]
- 60.Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 2013;305:R1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]