Abstract
Breastfeeding has been regarded first and foremost as a means of nutrition for infants, providing essential components for their unique growth and developmental requirements. However, breast milk is also rich in immunologic factors, highlighting its importance as a mediator of protection. In accordance with its evolutionary origin, the mammary gland offers via the breastfeeding route continuation of the maternal to infant immunologic support established in utero. At birth, the infant’s immune system is immature, and although it was exposed to the maternal microbial flora during pregnancy, it experiences an abrupt change in its microbial environment during and after birth, which is challenging and renders the infant highly susceptible to infection. Active and passive immunity protects the infant via breast milk, which is rich in immunoglobulins, lactoferrin, lysozyme, cytokines, and numerous other immunologic factors, including maternal leukocytes. Breast milk leukocytes provide active immunity and promote development of immunocompetence in the infant. Additionally, it has been speculated that they play a role in the protection of the mammary gland from infection. Leukocytes are thought to exert these functions via phagocytosis, secretion of antimicrobial factors and/or antigen presentation in both the mammary gland and the gastrointestinal tract of the infant, and also in other infant tissues, where they are transported via the systemic circulation. Recently, it has been demonstrated that breast milk leukocytes respond dynamically to maternal as well as infant infections, and are fewer in nonexclusively compared with exclusively breastfeeding dyads, further emphasizing their importance for both the mother and infant. This review summarizes the current knowledge of human milk leukocytes and factors influencing them, and presents recent novel findings supporting their potential as a diagnostic marker for infections of the lactating breast and of the breastfed infant.
Keywords: immune cells, leukocytes, breast milk, human milk, mammary gland, infant, breastfeeding, protection, infection
Introduction
Breast milk is far more than just a nutrient supply ensuring appropriate growth of the young. It is a complex and dynamic fluid that constitutes the sole source of nutrition for and developmental programming and protection of the neonate. Lactation may continue for from a few weeks to up to several years, with longer lactations tending to be common in women in developing countries (1, 2) or in mammals residing in tropical regions (3). Indeed, breast milk biomolecules, as well as the practice of breastfeeding, have been associated with early life developmental programming, conferring not only short-term effects, such as control of feeding behavior and infant growth (4), but also long-term benefits, including accelerated neurocognitive function (5) and protection against later overweight and obesity, hyperlipidemia, hypertension, type 2 diabetes, and atopic disease (6). Although other animals such as pigeons, sharks, salamanders, and skinks use bodily secretions to nourish their young, the complex and dynamic composition of milk is unique to the class Mammalia, conferring evolutionary conserved functions critical for the survival of the young mammal (3, 7).
Considering the ancestral origin of the mammary gland from sebaceous skin glands, it is not surprising that one of the primary functions of breastfeeding is the immunologic support and protection of the otherwise susceptible young. Oftedal (8) first discussed the origin of the mammary gland from an ancestral apocrine-like gland associated with hair follicles. He suggested that secretion of the milk precursor from these glands was a mechanism favored by natural selection because of its ability to prevent desiccation and microbial attack of the parchment-shelled eggs of the synapsids (3, 8, 9). Shortly after Oftedal, the immunologic importance of mammary gland evolution was revived by Vorbach et al. (10), who reported on the molecular synergy between the innate immune system and milk composition (10). Vorbach et al. supported the origin of the mammary gland and milk from skin glands secreting mucous substances rich in antimicrobial factors in response to infection of damaged skin. Key immunologic factors present in these early secretions, such as xanthine oxidoreductase and lysozyme, formed the biochemical basis for the main nutritional components of milk (11, 12). Based on the innovative work of Oftedal and Vorbach et al., it is now widely accepted that the ancestral mammary gland was protective in its function, and subsequently evolved to also nourish the young (3). Further, the protective components of milk remain highly conserved to date.
At birth, the infant’s immune system is still immature (13) and will be challenged by microbes to which it has not been exposed. Through breastfeeding, the transfer of immune factors from the mother to the infant, which starts in utero, continues postnatally (14, 15). These maternal factors protect the infant from infections and assist in the development of the infant’s intestinal mucosa, gut microflora, and own defenses (16–19). Accumulating evidence demonstrates the substantial impact of breastfeeding on decreasing the risk of many acute and chronic diseases (20–23). Breastfed infants have a lower risk of necrotising enterocolitis and reduced susceptibility to gastrointestinal, respiratory, and other infections than formula-fed infants (6, 14, 16, 23–25). These benefits result in substantial reductions in infant and child mortality, particularly in the developing world (6, 26).
Although the exact mechanisms of these immunomodulatory effects are still not well understood (27, 28), both the biochemical and cellular components of breast milk are thought to mediate its protective functions. These include maternal leukocytes and biomolecules with antimicrobial, anti-inflammatory, antioxidant and prebiotic activities (21, 29–31). Indeed, new proteins with immune functions are continually discovered in breast milk (32, 33). Milk immunoreactive biomolecules, such as secretory IgA, IgM, IgG, lactoferrin, lysozyme, and to a lesser extent cytokines, have been rigorously examined and have been the subject of previous reviews (27, 34). This review will focus on breast milk leukocytes, known differences between the milks of different mammals, factors influencing the content of human milk in leukocytes, and potential pathways through which they confer active immunity to the infant, as well as protect the mammary gland from infection.
The Cellular Nature of Human Milk
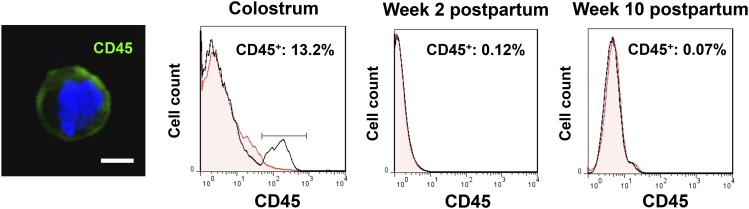
Attempts to classify the maternal cells of breast milk into known categories have been made by many investigators since the 17th century (35–38). However, these were primarily suggestions and not firm conclusions because of the technologic and microscopic limitations of the era. They included the mesenchymal, the immune, and the epithelial subtypes, alone or in combination (39–45). A major hurdle during this period was the lack of knowledge about factors that influence the cellular composition of breast milk, such as the stage of lactation or the health status of the mother and infant, which may have contributed to the classification controversy between early investigators. In the mid-20th century, Smith and Goldman (46, 47) and Holmquist and Papanicolaou (48, 49) revealed that colostrum is rich in leukocytes, but that it also contains epithelial cells. It was then that mammary secretions were used to noninvasively access cells from the mammary epithelium, not only in healthy lactating women, but also in women with breast cancer. This initiative emphasized for the first time the importance of mammary secretions, including breast milk, as a valuable tool for the study of both the normal biology of the breast, particularly during lactation, and breast malignancy and pathology (31), which has been further recognized more recently (7, 31, 50, 51). In the last decade, early observations of a cellular hierarchy in breast milk were confirmed by the discovery and characterization of stem and progenitor cells (31, 52–54). This topic is reviewed by Hassiotou and Hartmann (55). Whereas it is now well accepted that human milk contains epithelial cells and leukocytes, recent advances are revealing how dynamic the cellular content and composition of human milk is, how different it is from other species’ milks, and that it comprises a complex cellular hierarchy including breast-derived and blood-derived cells. Of the latter, the most abundant and most studied cell type is the leukocyte (Figure 1).
FIGURE 1.
Leukocytes in human milk are influenced by the stage of lactation. The immunofluorescence confocal micrograph shows a breast milk leukocyte stained for CD45 (pan-immune surface marker) and DAPI. The flow cytometry histograms demonstrates the decrease in the total population of CD45+ leukocytes (percentage of total cells) from colostrum to transitional milk to mature breast milk when both the mother and infant are healthy. DAPI, 4′,6-diamidino-2-phenylindole. Adapted with permission from (19).
The Breast Milk Leukocyte
Leukocytes migrate to the breast via the lymphatic vessels and systemic circulation (56). From there, they are believed to enter the lumen of alveoli via the paracellular pathway (7). Functional studies have demonstrated differences between breast milk and blood leukocytes, where breast milk T cells and macrophages appear to be more motile than their blood counterparts (57–61), suggesting a selective migration of leukocytes from the maternal circulation into milk (62) and/or selective alterations of leukocyte properties while in the breast. This may be important for their upcoming transfer to, safety, and/or function in the infant. It has been shown recently that colostrum contains a distinct distribution of lymphocytes compared with maternal peripheral blood (62). Colostrum lymphocytes are enriched in subsets with effector functions, as well as in antigen-experienced human leukocyte antigen (HLA)−DR+ and CD 57+ T lymphocytes, indicating transfer of innate immunity from the mother to the infant to ensure a rapid and specific response to pathogens.
In earlier years, investigations of the breast milk leukocyte originated from scientific curiosity of these mysterious cells present in milk. Because of a number of factors mentioned previously that may have contributed to the biased sampling of leukocyte-rich milks, such as human colostrum and bovine milk, the leukocyte soon became the center of attention of milk cell research (19). Its potential importance in providing active immunity to the infant was also likely a driving force. Intensive studies in human milk demonstrated that, similar to blood, leukocytes in milk comprise various subtypes including granulocytes and mononuclear leukocytes such as lymphocytes, monocytes, and macrophages (19, 46, 47, 56, 63, 64). Macrophages are the predominant leukocytes in human colostrum (40–50% of total leukocytes), followed by polymorphonuclear neutrophils (40–50% of total leukocytes) and lymphocytes (5–10% of total leukocytes) (31, 56, 65, 66). T cells constitute the majority of lymphocytes (∼83%) over B cells (4–6%) (31, 56, 66). The proportions of leukocyte subtypes in mature human milk are less well studied, particularly in relation to the health status of the mother–infant dyad. Studies indicate that most leukocytes, are activated in breast milk (thus are positive for CD45RO) (19, 64, 67), whereas infections such as mastitis or infant conditions alter the leukocyte profile of human milk in an infection-specific manner (19, 68). Clearly, this area merits further investigation, showing diagnostic potential. Flow cytometric analyses with the use of the known CD profile of leukocyte subtypes and optimized for milk leukocytes (Table 1) will be crucial in progressing the field.
TABLE 1.
CD profile of main breast milk leukocyte subtypes1
| Cell type | CD profile |
| All leukocytes in milk | CD45 |
| Activated leukocytes | CD45/CD45RO |
| Monocytes and macrophages | CD45/CD14 |
| Granulocytes | CD45/CD15 |
| Antigen-presenting cells | CD45/HLADR |
| T lymphocytes | CD45/CD3 |
| T helper cells | CD45/CD3/CD4 |
| T cytotoxic cells | CD45/CD3/CD8 |
| Natural killer T cells | CD45/CD3/CD56 |
| Other natural killer cells | CD45/CD56/CD3#x2212 |
| B lymphocytes | CD45/CD19 |
HLADR, human leukocyte antigen−DR+.
Although these leukocyte subtypes are typically found in all mammalian milks, both leukocyte content and composition can substantially differ between species. Among different mammals, the milk leukocyte content can be influenced by genetics associated with different mammary anatomy, the evolutionary physiology of lactation and milk secretion, and environmental factors such as systematic milking (31). Boutinaud and Jammes (69) elegantly summarized differences in milk leukocyte and epithelial contents between different mammals, illustrating that bovine, ovine, caprine and porcine milks all contain substantially more leukocytes than human milk, with porcine milk being perhaps the closest of these 4 to human milk. It is important to recognize that, in contrast with women, dairy cows are both selected and milked to produce milk volumes at their highest physiologic capacity, which may partially explain the differing leukocyte contents of human and bovine milk. Within a species, differences also exist in leukocyte content and composition because of a range of factors that require further study.
Factors Influencing the Leukocytic Content of Human Milk
Great variability can be seen in the literature on breast milk leukocyte content in women. This may be partially attributed to differences in the methodologies employed, and the absence of control for factors that could influence the cellular content of milk. It was not until recently that flow cytometry started to replace morphology-based analyses of the cell content of human milk, yielding vastly different results. Ironically, as early as 1953, Engel (43) cautioned the analysis of milk cells, emphasizing that some leukocytes in milk share morphologic characteristics with epithelial cells. Indeed, the early term “foam cells” was used for cells that displayed traits shared between leukocytes and epithelial cells, such as lipid droplets, a large nucleus or multinucleation, and cytoplasmic vacuolation (31). Reportedly, published ranges of breast milk leukocyte content are likely to be overestimated and the epithelial content underestimated in cases in which basic microscopic examinations were used (31). In addition, flow cytometric analysis of milk leukocytes should be applied in an optimal and standardized manner with the use of appropriate milk cell controls instead of blood controls to account for the heterogeneous nature of milk cell content and the known differences between milk and blood leukocytes (7, 19). Therefore, employing accurate and consistent methodology is vital in both illuminating leukocyte content and composition of milk and describing true population variability. To date, research with the use of flow cytometric analysis has revealed 2 key factors that influence leukocyte populations in human milk: the stage of lactation and the health status of the breastfeeding dyad. Earlier studies showed that changes during the menstrual cycle related to ovulation increase the permeability of the mammary epithelium (70), which is consistent with the increase in milk leukocyte content we have observed in this period (F Hassiotou, unpublished data, 2010). Although there has been no study of dietary influences on the leukocyte content of breast milk, this is worthwhile to investigate, particularly in the area of food allergens. Breast milk leukocyte content is known to show a small but significant increase during the systemic allergic response associated with hay fever (71), indicating that a systemic inflammatory response to specific nutrients might be detectable in the breast milk of lactating women. Generally, the diet of women does not adversely affect the composition of breast milk except with respect to micronutrients (72). However, during stages of lactation in which the permeability of the breast is increased, such as at secretory differentiation before secretory activation (7), it is possible that leukocyte numbers might increase due to the opening of the tight junctions between lactocytes (7). These are areas that warrant further investigation.
Stage of lactation.
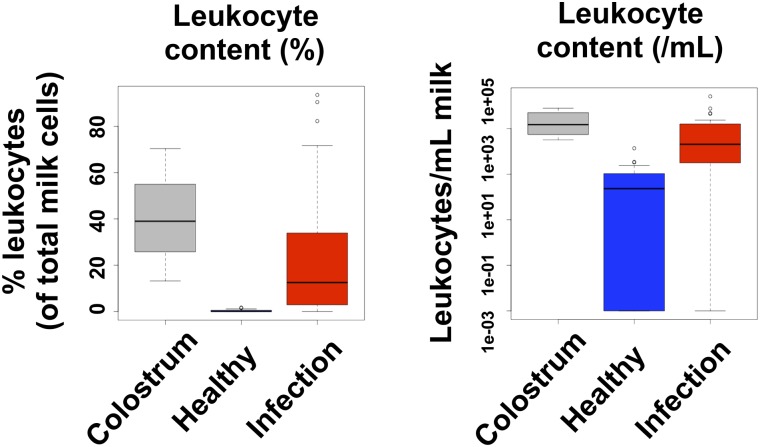
The leukocyte content of colostrum varies in women from ∼13.2% to 70.4% of total cells (19). This translates to ∼32,175–784,080 viable leukocytes per mL of colostrum (19). Remarkably, although colostrum has always been found to contain a considerable number of leukocytes, transitional and mature milk are characterized by very low leukocyte content when both the mother and the infant are healthy (Figure 1) (19, 31). We have previously shown that toward the end of week 1 postpartum, leukocyte numbers rapidly decrease to reach a baseline level in transitional milk (0–1.7% leukocytes of total milk cells; 0–3450 viable leukocytes/mL milk) and mature breast milk (0–1.5% leukocytes of total milk cells; 0–1151 viable leukocytes/mL milk) that is maintained except during periods of infection (19). This low baseline concentration of leukocytes in mature breast milk fluctuates within certain limits (0–2% leukocytes of total milk cells) both between and within individuals, and is observed even at late lactation stages (years 2–4 postpartum) (19, 73). It is of note that in our studies we have consistently measured low leukocyte counts at late lactation stages. Although it has been previously suggested that involution is associated with recruitment of leukocytes into the mammary gland, it is possible that these women are still producing substantial volumes of milk (74). Although the total cell content of breast milk, as well as concentrations of biochemical immune factors, do increase in involution milk, its leukocyte content does not appear to respond in this way. This observation is in agreement with studies demonstrating that cells from the epithelial compartment mediate clearing of apoptotic lactocytes via phagocytosis in the involuting gland (75–77), and requires further investigation.
These data were based on flow cytometric staining with the use of the pan-immune marker CD45, which is known to be expressed only by immune cells. This is a fundamental consideration when selecting panels of markers for flow cytometric analysis of milk cell subpopulations. Importantly, it has been shown that other known immune markers, such as CD14, are also expressed by mammary epithelial cells (31, 78, 79) and must therefore always be used in conjunction with CD45 to accurately identify leukocytes. Moreover, development of specific gating strategies for milk leukocytic populations, as well as exclusion of interference by dead or dying cells and fat globules, can contribute to the correct identification of different subpopulations of cells in breast milk (19, 31) and facilitate the study of factors influencing them.
Infection.
Bryan et al. (80) provided evidence suggesting that the health status of both the mother and the infant may influence breast milk cellular content, revealing an immunologic connection between mothers and their infants during breastfeeding. We have recently shown that in established lactation, breast milk leukocytes are maintained at low concentrations (0–2%), provided both mother and infant are healthy. However, during periods of infection of either the mother or the infant or both, leukocyte concentrations consistently and rapidly increase, returning to baseline concentrations upon recovery (Figure 2) (19, 81). Infections that have been shown to stimulate a leukocytic response in breast milk include bacterial, viral, or fungal infections ranging from maternal conditions such as hay fever, systemic infections (influenza), breast conditions (nipple pain, blocked ducts, or mastitis), other organ infections [respiratory, gastrointestinal tract (GIT), ear, or eye infections] to infant conditions such as influenza, measles, or GIT infections.
FIGURE 2.
Breast milk leukocytes respond to infections of the mother and/or the infant. The box plots show the leukocyte content (as a percentage of total milk cells and as a number of leukocytes per milliliter of milk) of colostrum and mature breast milk in healthy and infected mother–infant dyads (n = 21). Adapted with permission from (19).
Interestingly, statistically significant responses have been observed also when the mother is asymptomatic but the infant has an infection such as a respiratory infection, a GIT infection, or roseola infantum (19). Similar findings were previously reported for the breast milk of mothers whose infants were hospitalized with bronchiolitis, showing greater numbers of viable cells that displayed skewed cytokine response to live respiratory syncytial virus, the major organism responsible for bronchiolitis (80). It cannot be discounted that infants with either respiratory or GIT infections may infect their mother, causing an asymptomatic maternal immune response with subsequent passage of greater numbers of leukocytes into her breast milk (19). However, this is unlikely for infants with localized or bacterial infections. Although the maternal breast response to infant infection is not fully understood, we have proposed that the retrograde ductal flow associated with milk ejection during breastfeeding (82) is a route for the transfer of pathogens from the infant’s oral cavity to the mother’s breast via the nipple, which may locally stimulate an immune response in the breast (19, 31). Clearly, the specific response of breast milk leukocytes to the infant’s infection demonstrates that these cells may play important functions for the offspring.
Function of Breast Milk Leukocytes for the Infant
Arguably, breastfeeding is the optimal and most effective avenue of immunologic protection of the infant (21, 83–85). This protection is both cellular and molecular. Breast milk contains molecules that are both antibacterial and antiviral. Antimicrobial components include Igs (secretory IgA, IgG, and IgM), lactoferrin, lactoferricin B and H, lysozyme, and actoperoxidase (86), which appear to act synergistically with antibodies (87). These proteins have been believed to be relatively resistant to proteolysis in the gut; however, a recent study showed that the 200 peptides present in the breast milk of 3 mothers increased up to 649 in the gastric aspirates of their infants, most of which were shown to be bioactive (88). This suggests that the by-products of these proteins may be just as important to the infant as the parent protein. With regard to antiviral components of milk, some substances, such as lactoferrin, have antiviral effects in addition to their antibacterial properties. Indeed, lactoferrin (89) and tenascin-C (90) have been shown to inactivate the HIV-1 virus. Similarly, higher concentrations of human milk oligosaccharides are associated with reduced transmission of HIV to the human infant (91). Human milk has also been shown to reduce transmission of other maternal viruses, such as hepatitis C (92), and to protect the infant from viruses such as reovirus (93). Moreover, although the antimicrobial and antiviral components of milk benefit the infant enormously, it is likely that the mammary gland is also afforded a degree of protection.
It has been proposed that biochemical immunologic components may act synergistically with breast milk leukocytes to directly or indirectly increase infant immunity (e.g., modifying the microenvironment of the infant gut) (19, 31). Notably, in the majority of freshly expressed breast milk samples, >90% of total milk cells are viable. Of these, up to 2% are leukocytes when the breastfeeding dyad is healthy. Given the known cellular content of human milk of 10,000–13 million cells/mL (31) and the normal daily breast milk consumption range of 470–1350 mL (94), it can be estimated that human milk contains ∼200–260,000 leukocytes/mL, and that normally breastfed infants receive ∼94,000–351 million leukocytes from breast milk on a daily basis, of which >90% are viable and can exert immunomodulatory functions. During periods of infection of either the mother or the infant, the number of leukocytes ingested daily by breastfed infants can reach the billions. Mechanisms that prevent the infant’s immune system from attacking maternal breast milk cells and, conversely, the mother’s breast milk leukocytes from attacking infant tissues have likely evolved over time, but very little is known about this, warranting further research in this area.
Studies so far indicate that maternal breast milk–derived leukocytes provide active immunity to the infant, both assisting the development of its own immune system and fighting pathogens directly. These leukocytic functions are exerted via phagocytosis, secretion of antimicrobial factors such as cytokines and Igs, or antigen presentation (21–23, 34, 64, 95), and are performed not only inside the gastrointestinal tract of the infant, but also in distant tissues to which leukocytes are transferred via the systemic circulation. Indeed, breast milk leukocytes have been shown to be activated, motile, and interactive (19, 46, 47, 64). In vivo studies in animal models have elegantly demonstrated active breast milk leukocyte transfer through the intestinal mucosa into the blood circulation of the young, and movement to and engraftment in different organs, including the mesenteric nodes, liver, and spleen (15, 30, 57, 95, 96).
It will be of interest to examine whether the immune system and health of infants fed formula, which does not include any viable cells, are in any way compromised specifically by the lack of maternal breast milk leukocytic support in the short- and/or long term compared with infants fed fresh breast milk. Previous studies have investigated the immune function of breastfed vs. formula-fed infants and have evidently shown higher infection rates in infants fed formula than in those fed breast milk (6, 14, 16, 20, 23–25, 97). Although the long-term health of breastfed infants is also better than that of formula fed infants, this has been in part attributed to early life programming during pregnancy and lactation (4, 21–23). Education and maturation of the infant’s immune system via breast milk likely plays a major role in this process. However, it is still unclear to what extent these effects are mediated by leukocytes or other immune factors, such as secretory IgA, lactoferrin, and others (13, 16, 20–25) that are contained in breast milk but are absent in artificial formulas. In this respect, it is of note that mothers of exclusively breastfed infants have higher baseline leukocyte counts in their breast milk than those who are not exclusively feeding breast milk (19).
A New Diagnostic Tool
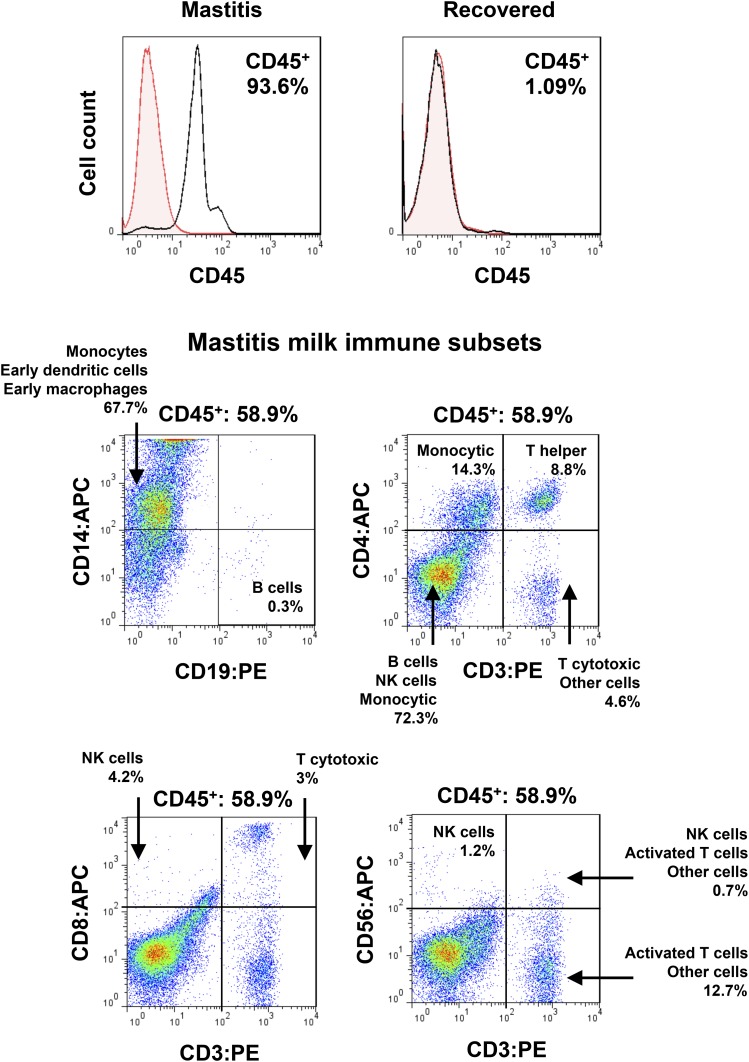
Breast milk leukocytes not only function in the young, but they also protect the mammary gland from infection during lactation (98). In this period, inadequate emptying of the breast, blockage of ducts, microbial invasion, or trauma can cause inflammation in the mammary gland, often resulting in severe mastitis, abscess, and septicemia (98, 99). These conditions cause an increased influx of leukocytes to mammary infection sites; many of these cells pass into milk and are protective (64, 98). Even milder breast conditions, such as nipple pain or sore breast, can stimulate recruitment of leukocytes in the breast. This is rapid and can be seen at its maximum during episodes of mastitis, at which time up to 95% or more of total milk cells are leukocytes (19). These include predominantly monocytes and macrophages, but also dendritic cells, T helper cells, cytotoxic T cells, natural killer cells, and a small population of B lymphocytes (Figure 3). They contain activated cell subsets that respond to viral antigens and leucoagglutinin PHA-L (PHA) in culture, showing an increased proliferation rate and altered expression of IL-6, IL-17A, IFN-γ, and TNF-α (19). Although biochemical immune factors (e.g., secretory IgA, IgG, IgM, and lactoferrin) in breast milk generally respond to breast infections, the most consistent and rapid response is that of the leukocytes, suggesting that they could be used diagnostically.
FIGURE 3.
Effect of mastitis on breast milk immune cell content. The flow cytometry histograms show the leukocyte content of human milk collected from a mastitic breast and its return to baseline concentrations upon recovery. The dot plots demonstrate the breast milk leukocyte subsets in breast milk collected from a woman with mastitis, including monocytes, dendritic cells, macrophages, few B cells, T helper and T cytotoxic cells, NK cells, and activated T lymphocytes. APC, allophycocyanin; NK, natural killer; PE, phycoerythrin. Adapted with permission from (19).
When treatment of mastitis is delayed, we have observed a corresponding delay in the reduction of leukocyte concentrations, suggesting that the health status of the breast is directly mirrored in the breast milk leukocyte content. Interestingly, milder breast conditions such as sore nipple or breast and blocked ducts showed less dramatic responses, further supporting the notion that the maternal response is protective of further infection of the breast. More importantly, the marked difference in the leukocyte response between mild conditions and mastitis highlights the potential for a new diagnostic test for the lactating breast (19, 68).
In women, no clinical test exists to assess the health status of the lactating breast, despite the fact that it is a metabolically important organ in the body requiring >25% of the caloric energy consumed daily (100). The leukocyte status of human breast milk provides a novel tool to assess the health status of the lactating breast, given that it rapidly and specifically responds to different infections, with an equally rapid return to its normal baseline concentration upon recovery. It even provides a simple means through which to test the efficacy of medication, allowing for timely adjustment and management of breast infections. Because of multibillion dollar losses it incurs annually from mastitis of the dairy cow, the dairy industry routinely has been using similar tests to assess the quality of cow milk and the presence of intramammary infection, e.g., the California Mastitis Test (101, 102). This test measures the somatic cell count, which corresponds to the total number of milk cells. In the dairy cow, this accurately reflects an increase in leukocyte numbers, because 1) the majority of bovine milk cells are leukocytes, and 2) contrary to most other infections we have examined in human breastfeeding dyads, mastitis is characterized by substantially higher total as well as leukocyte cell contents (19).
There has been an increasing number of anecdotal reports of diminished and unrecoverable milk production as a result of severe mastitis (103). In an Australian study, 21% of women who stopped breastfeeding by 6 wk after birth did so due to mastitis (104). Further, a US study showed that women were 6 times more likely to wean if they had mastitis in the first 3 wk postpartum (105). Clearly, premature cessation of breastfeeding has major implications for both the mother and the infant, particularly if it is unexpected. A diagnostic tool such as measurement of the leukocyte content of breast milk that reliably, consistently, and rapidly assesses breast infection will be of immense value for lactating women, their infants, and associated health professionals. Toward this, ongoing studies are concentrating on further delineating the pathogenesis of mastitis via examinations of the leukocytic and biochemical composition of human milk and associations with clinical symptoms and the severity of mastitis. Given that breast milk cells also include prokaryotes, recent studies have started to evaluate mastitis-driven alterations in the breast milk microbiome, which, together with breast milk leukocytic responses, may lead to a better understanding of the causes of mastitis.
Outlook
Human milk–mediated protection of the infant is long known and has been intensively studied for decades. More recent research is revealing that breast milk leukocytes play a major role in affording maternal and infant protection from infection. Further, they are distinct from their blood counterparts, specifically adjusted for their transfer, integration, and function in the infant. They not only provide ongoing immunologic support to the infant, but also respond to infections, aiding recovery. This further supports the important protective role of human milk, particularly in areas where mothers and infants do not have ready access to medicine, such as in developing countries. In these situations, breastfeeding-mediated protection, which is dynamic and adjusts to the infant’s needs, is often a determining factor for infant recovery and survival. The knowledge of breast milk leukocytes summarized in this review supports public policy on early infant nutrition highlighting the important role of breastfeeding in maximizing immunologic development and protection of infants against infections. Moreover, new information is now available to examine the mechanisms behind the very low rates of symptomatic cytomegalovirus and HIV disease observed in infants when they are exclusively breastfed by infected mothers, despite their exposure to virus-infected breast milk leukocytes and in contrast with infants who are mixed-fed (106, 107). At the same time, new tools with the use of breast milk leukocytes are starting to be used to diagnose infections of the lactating breast, which may substantially aid effective management of lactation pathologies such as mastitis and enable the continuation of breastfeeding for longer periods, providing maximal health benefits to both the mother and the infant.
Acknowledgments
Both authors read and approved the final manuscript.
References
- 1.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002;360:187–95. [DOI] [PubMed] [Google Scholar]
- 2.Imdad A, Yakoob MY, Bhutta ZA. Effect of breastfeeding promotion interventions on breastfeeding rates, with special focus on developing countries. BMC Public Health 2011;11: Suppl 3:S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClellan HL, Miller SJ, Hartmann PE. Evolution of lactation: nutrition v. protection with special reference to five mammalian species. Nutr Res Rev 2008;21:97–116. [DOI] [PubMed] [Google Scholar]
- 4.Hassiotou F, Geddes DT. Programming of appetite control during breastfeeding as a preventative strategy against the obesity epidemic. J Hum Lact 2014;30(2):136–42. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr 1999;70:525–35. [DOI] [PubMed] [Google Scholar]
- 6.Kramer MS. "Breast is best": The evidence. Early Hum Dev 2010;86:729–32. [DOI] [PubMed] [Google Scholar]
- 7.Hassiotou F, Geddes D. Anatomy of the human mammary gland: Current status of knowledge. Clin Anat 2013;26:29–48. [DOI] [PubMed] [Google Scholar]
- 8.Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia 2002;7:225–52. [DOI] [PubMed] [Google Scholar]
- 9.Oftedal OT. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia 2002;7:253–66. [DOI] [PubMed] [Google Scholar]
- 10.Vorbach C, Capecchi MR, Penninger JM. Evolution of the mammary gland from the innate immune system? Bioessays 2006;28(6):606–16. [DOI] [PubMed] [Google Scholar]
- 11.Vorbach C, Scriven A, Capecchi MR. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev 2002;16:3223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vorbach C, Harrison R, Capecchi MR. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol 2003;24:512–7. [DOI] [PubMed] [Google Scholar]
- 13.Chirico G, Marzollo R, Cortinovis S, Fonte C, Gasparoni A. Antiinfective properties of human milk. J Nutr 2008;138:1801S–6S. [DOI] [PubMed] [Google Scholar]
- 14.Perez PF, Dore J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 2007;119:e724–32. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, Shimamura M. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology 2000;101:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 2010;23:23–36. [DOI] [PubMed] [Google Scholar]
- 17.Slade HB, Schwartz SA. Mucosal immunity: the immunology of breast milk. J Allergy Clin Immunol 1987;80:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker WA. The dynamic effects of breastfeeding on intestinal development and host defense. Adv Exp Med Biol 2004;554:155–70. [DOI] [PubMed] [Google Scholar]
- 19.Hassiotou F, Hepworth AR, Metzger P, Lai C-T, Trengove N, Hartmann PE, Filgueira L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin Transl Immunology 2013;2:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr 1995;126:696–702. [DOI] [PubMed] [Google Scholar]
- 21.Hanson LA. Breastfeeding stimulates the infant immune system. Sci Med 1997;4:12–21. [Google Scholar]
- 22.Hanson LA. The mother-offspring dyad and the immune system. Acta Paediatr 2000;89:252–8. [PubMed] [Google Scholar]
- 23.Hanson LA, Winberg J. Breast milk and defence against infection in the newborn. Arch Dis Child 1972;47:845–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.César JA, Victora CG, Barros FC, Santos IS, Flores JA. Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: nested case-control study. BMJ 1999;318:1316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. Protective effect of breast feeding against infection. BMJ 1990;300:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maternal and Child Nutrition Study Group. Maternal and child nutrition: building momentum for impact. Lancet 2013;382:372–5. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal S, Karmaus W, Davis S, Gangur V.. Immune markers in breast milk and fetal and maternal body fluids: a systematic review of perinatal concentrations. J Hum Lact 2011;27(2):171–86. [DOI] [PubMed] [Google Scholar]
- 28.França EL, Nicomedes TR, Calderon IMP, , Honorio-França AC. Time-dependent alterations of soluble and cellular components in human milk. Biol Rhythm Res 2010;41:333–47. [Google Scholar]
- 29.Kmetz M, Dunne HW, Schultz RD. Leukocytes as carriers in the transmission of bovine leukemia: invasion of the digestive tract of the newborn by ingested, cultured, leukocytes. Am J Vet Res 1970;31:637–41. [PubMed] [Google Scholar]
- 30.Weiler IJ, Hickler W, Sprenger R. Demonstration that milk cells invade the suckling neonatal mouse. Am J Reprod Immunol 1983;4(2):95–8. [DOI] [PubMed] [Google Scholar]
- 31.Hassiotou F, Geddes DT, Hartmann PE. Cells in human milk: State of the science. J Human Lact 2013;29(2):171–82. [DOI] [PubMed] [Google Scholar]
- 32.Gao X, McMahon RJ, Woo JG, Davidson BS, Morrow AL, Zhang Q. Temporal changes in milk proteomes reveal developing milk functions. J Proteome Res 2012;11:3897–907. [DOI] [PubMed] [Google Scholar]
- 33.Molinari CE, Casadio YS, Hartmann BT, Livk A, Bringans S, Arthur PG, Hartmann PE. Proteome mapping of human skim milk proteins in term and preterm milk. J Proteome Res 2012;11:1696–714. [DOI] [PubMed] [Google Scholar]
- 34.Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr 2003;77:1537S–43S. [DOI] [PubMed] [Google Scholar]
- 35.Van Leeuwenhoek A. Epistola 106. Arcana naturae detecta delphis batavorum. Apud Henricum a Krooneveld 1695.
- 36.Donné A. Du lait et en particulier de celui de nourrices, considéré sous le rapport de ses bonnes et de ses mavaises qualités nitritives et de ses altérations. Paris, Rue de Condé 1837:no 15; Les Libraires de Médecine, Chevalier, Palais Royal, no 163.
- 37.Henle J. Allgem. Anatomie. Germany: 2 Aufl. L. Voss. Leipzig, 1841.
- 38.Mayer G, Klein M. Histology and cytology of the mammary gland. New York: Academic Press, 1961.
- 39.Bizzozero G, Vassale G. Über die erganzung und die physiologische regeneration der drüsenzellen bei den säugetieren. Virchows Arch Pathol Anat Physiol 1887;111:155–214. [Google Scholar]
- 40.Czerny A. Über das colostrum. Prager Med Woch 1890;15:401–2. [Google Scholar]
- 41.Gruber GB. Beiträge zur histologie und pathologie der mamma. Virchows Arch Pathol Anat Physiol 1924;248:397–426. [Google Scholar]
- 42.Gregoire C. Nature épithéliale de certains corpuscules de colostrum. C R Soc Biol 1930;104:1308–10. [Google Scholar]
- 43.Engel S. An investigation of the origin of the colostrum cells. J Anat 1953;87:362–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Wallich V, Levaditi C. Sur la nature des éléments cellulaires du colostrum et du lait chez la femme. Ann Inst Pasteur (Paris) 1905;19:321–34. [Google Scholar]
- 45.Varrier-Jones PC. The cellular content of milk. Lancet Oncol 1924;2:537–42. [Google Scholar]
- 46.Smith CW, Goldman AS. The cells of human colostrum. I. In vitro studies of morphology and functions. Pediatr Res 1968;2:103–9. [DOI] [PubMed] [Google Scholar]
- 47.Smith CW, Goldman AS. Interactions of lymphocytes and macrophages from human colostrum: characteristics of the interacting lymphocyte. J Reticuloendothel Soc 1970;8:91–104. [PubMed] [Google Scholar]
- 48.Holmquist DG, Papanicolaou GN. The exfoliative cytology of the mammary gland during pregnancy and lactation. Ann N Y Acad Sci 1956;63:1422–35. [DOI] [PubMed] [Google Scholar]
- 49.Papanicolaou GN, Bader GM, Holmquist DG, Falk EA. Cytologic evaluation of breast secretions. Ann N Y Acad Sci 1956;63:1409–21. [DOI] [PubMed] [Google Scholar]
- 50.Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, Michels KB, Palmer JR, Schedin P, Stuebe AM, et al. . Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst 2013;105:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassiotou F, Hepworth AR, Beltran AS, Mathews MM, Stuebe AM, Hartmann PE, Filgueira L, Blancafort P. Expression of the pluripotency transcription factor OCT4 in the normal and aberrant mammary gland. Front Oncol 2013;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, Mitoulas LR, Piper KM, Choolani MA, Chong YS, et al. . Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res 2007;329:129–36. [DOI] [PubMed] [Google Scholar]
- 53.Thomas E, Zeps N, Cregan M, Hartmann P, Martin T. 14–3-3sigma (sigma) regulates proliferation and differentiation of multipotent p63-positive cells isolated from human breastmilk. Cell Cycle 2011;10:278–84. [DOI] [PubMed] [Google Scholar]
- 54.Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, Trengove N, Lai CT, Filgueira L, Blancafort P, et al. . Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012;30:2164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassiotou F, Hartmann PE. At the dawn of a new discovery: The potential of breastmilk stem cells. Adv Nutr 2014;5:770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldman AS, Goldblum RM. Transfer of maternal leukocytes to the infant by human milk. Curr Top Microbiol Immunol 1997;222:205–13. [DOI] [PubMed] [Google Scholar]
- 57.Michie CA. The long term effects of breastfeeding: a role for the cells in breast milk? J Trop Pediatr 1998;44:2–3. [DOI] [PubMed] [Google Scholar]
- 58.Sabbaj S, Ghosh MK, Edwards BH, Leeth R, Decker WD, Goepfert PA, Aldrovandi GM. Breast milk-derived antigen-specific CD8+ T cells: an extralymphoid effector memory cell population in humans. J Immunol 2005;174:2951–6. [DOI] [PubMed] [Google Scholar]
- 59.Tuaillon E, Valea D, Becquart P, Al Tabaa Y, Meda N, Bollore K, Van de Perre P, Vendrell JP. Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J Immunol 2009;182:7155–62. [DOI] [PubMed] [Google Scholar]
- 60.Ozkaragöz F, Rudloff HB, Rajaraman S, Mushtaha AA, Schmalstieg FC, Goldman AS. The motility of human milk macrophages in collagen gels. Pediatr Res 1988;23:449–52. [DOI] [PubMed] [Google Scholar]
- 61.Dickey WD, Rudloff HB, Goldman AS, Schmalstieg FC. Human uropod bearing lymphocytes: isolation of a factor from human milk that abrogates the uropod inhibitory protein from human serum. Biochem Biophys Res Commun 1981;100:138–45. [DOI] [PubMed] [Google Scholar]
- 62.Peroni DG, Chirumbolo S, Veneri D, Piacentini GL, Tenero L, Vella A, Ortolani R, Raffaelli R, Boner AL. Colostrum-derived B and T cells as an extra-lymphoid compartment of effector cell populations in humans. J Matern Fetal Neonatal Med 2013;26:137–42. [DOI] [PubMed] [Google Scholar]
- 63.Goldman AS, Garza C, Nichols BL, Goldblum RM. Immunologic factors in human milk during the first year of lactation. J Pediatr 1982;100:563–7. [DOI] [PubMed] [Google Scholar]
- 64.Wirt DP, Adkins LT, Palkowetz KH, Schmalstieg FC, Goldman AS. Activated and memory T lymphocytes in human milk. Cytometry 1992;13:282–90. [DOI] [PubMed] [Google Scholar]
- 65.Brooker BE. The epithelial cells and cell fragments in human milk. Cell Tissue Res 1980;210:321–32. [DOI] [PubMed] [Google Scholar]
- 66.Xanthou M. Immune protection of human milk. Biol Neonate 1998;74:121–33. [DOI] [PubMed] [Google Scholar]
- 67.Keeney SE, Schmalstieg FC, Palkowetz KH, Rudloff HE, Le BM, Goldman AS. Activated neutrophils and neutrophil activators in human milk: increased expression of CD11b and decreased expression of L-selectin. J Leukoc Biol 1993;54:97–104. [DOI] [PubMed] [Google Scholar]
- 68.Geddes D, Hassiotou F, Underhill J, Hartmann PE. Immune cells in breastmilk. Experimental Biology. San Diego, USA, 26–30 Apr 2014.
- 69.Boutinaud M, Jammes H. Potential uses of milk epithelial cells: a review. Reprod Nutr Dev 2002;42:133–47. [DOI] [PubMed] [Google Scholar]
- 70.Hartmann PE, Prosser CG. Acute changes in the composition of milk during the ovulatory menstrual cycle in lactating women. J Physiol 1982;324:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bode L, McGuire M, Rodriguez JM, Geddes DT, Hassiotou F, Hartmann PE, McGuire MK. It's alive: microbes and cells in human milk and their potential benefits to mother and infant. Adv Nutr 2014;5:571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kent JC, Christen L, Hassiotou F, Hartmann PE. Role of breast milk. In: Patole S, editor. Nutrition for the preterm neonate. The Netherlands: Springer, 2013:311–35.
- 73.Jin YY, Wei Z, Cao RM, Xi W, Wu SM, Chen TX. Characterization of immunocompetent cells in human milk of Han Chinese. J Hum Lact 2011;27(2):155–62. [DOI] [PubMed] [Google Scholar]
- 74.Kent JC, Mitoulas L, Cox DB, Owens RA, Hartmann PE. Breast volume and milk production during extended lactation in women. Exp Physiol 1999;84:435–47. [PubMed] [Google Scholar]
- 75.Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol Neoplasia 2002;7:163–76. [DOI] [PubMed] [Google Scholar]
- 76.Monks J, Henson PM. Differentiation of the mammary epithelial cell during involution: implications for breast cancer. J Mammary Gland Biol Neoplasia 2009;14:159–70. [DOI] [PubMed] [Google Scholar]
- 77.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod 2008;78:586–94. [DOI] [PubMed] [Google Scholar]
- 78.Vidal K, Labeta MO, Schiffrin EJ, Donnet-Hughes A. Soluble CD14 in human breast milk and its role in innate immune responses. Acta Odontol Scand 2001;59:330–4. [DOI] [PubMed] [Google Scholar]
- 79.Funda DP, Tuckova L, Farre MA, Iwase T, Moro I, Tlaskalova-Hogenova H. CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect Immun 2001;69:3772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bryan DL, Hart PH, Forsyth KD, Gibson RA. Immunomodulatory constituents of human milk change in response to infant bronchiolitis. Pediatr Allergy Immunol 2007;18(6):495–502. [DOI] [PubMed] [Google Scholar]
- 81.Riskin A, Almog M, Peri R, Halasz K, Srugo I, Kessel A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatr Res 2012;71:220–5. [DOI] [PubMed] [Google Scholar]
- 82.Ramsay DT, Kent JC, Owens RA, Hartmann PE. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics 2004;113:361–7. [DOI] [PubMed] [Google Scholar]
- 83.Newburg DS. Innate immunity and human milk. J Nutr 2005;135:1308–12. [DOI] [PubMed] [Google Scholar]
- 84.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 2007;61:2–8. [DOI] [PubMed] [Google Scholar]
- 85.Goldman AS. The immune system in human milk and the developing infant. Breastfeed Med 2007;2(4):195–204. [DOI] [PubMed] [Google Scholar]
- 86.Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr 2005;135:1–4. [DOI] [PubMed] [Google Scholar]
- 87.Florisa R, Recio I, Berkhout B, Visser S. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr Pharm Des 2003;9:1257–75. [DOI] [PubMed] [Google Scholar]
- 88.Dallas DC, Guerrero A, Khaldi N, Borghese R, Bhandari A, Underwood MA, Lebrilla CB, German JB, Barile D. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J Nutr 2014;144:815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Groot F, Geijtenbeek TB, Sanders RW, Baldwin CE, Sanchez-Hernandez M, Floris R, van Kooyk Y, de Jong EC, Berkhout B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN–gp120 interaction. J Virol 2005;79:3009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fouda GG, Jaeger FH, Amos JD, Ho C, Kunz EL, Anasti K, Stamper LW, Liebl BE, Barbas KH, Ohashi T, et al. . Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proc Natl Acad Sci USA 2013;110:18220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bode L, Kuhn L, Kim HY, Hsiao L, Nissan C, Sinkala M, Kankasa C, Mwiya M, Thea DM, Aldrovandi GM. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr 2012;96:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pfaender S, Heyden J, Friesland M, Ciesek S, Ejaz A, Steinmann J, Steinmann J, Malarski A, Stoiber H, Tsiavaliaris G, et al. . Inactivation of hepatitis C virus infectivity by human breast milk. J Infect Dis 2013;208:1943–52. [DOI] [PubMed] [Google Scholar]
- 93.Iskarpatyoti JA, Morse EA, McClung RP, Ikizler M, Wetzel JD, Contractor N, Dermody TS. Serotype-specific differences in inhibition of reovirus infectivity by human-milk glycans are determined by viral attachment protein sigma1. Virology 2012;433:489–97. [DOI] [PubMed] [Google Scholar]
- 94.Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006;117:e387–95. [DOI] [PubMed] [Google Scholar]
- 95.Jain L, Vidyasagar D, Xanthou M, Ghai V, Shimada S, Blend M.. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch Dis Child 1989;64(7 Spec No):930–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schnorr KL, Pearson LD. Intestinal absorption of maternal leucocytes by newborn lambs. J Reprod Immunol 1984;6:329–37. [DOI] [PubMed] [Google Scholar]
- 97.Jalil F, Karlberg J, Hanson LA, Lindblad BS. Growth disturbance in an urban area of Lahore, Pakistan related to feeding patterns, infections and age, sex, socio-economic factors and seasons. Acta Paediatr Scand Suppl 1989;350:44–54. [DOI] [PubMed] [Google Scholar]
- 98.Fetherston CM, Lee CS, Hartmann PE. Mammary gland defense: the role of colostrum, milk and involution secretion. Adv Nutr Res 2001;10:167–98. [DOI] [PubMed] [Google Scholar]
- 99.Amir LH, Forster DA, Lumley J, McLachlan H. A descriptive study of mastitis in Australian breastfeeding women: incidence and determinants. BMC Public Health 2007;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hartmann PE. The lactating breast: an overview from down under. Breastfeed Med 2007;2(1):3–9. [DOI] [PubMed] [Google Scholar]
- 101.Sargeant JM, Leslie KE, Shirley JE, Pulkrabek BJ, Lim GH. Sensitivity and specificity of somatic cell count and California Mastitis Test for identifying intramammary infection in early lactation. J Dairy Sci 2001;84:2018–24. [DOI] [PubMed] [Google Scholar]
- 102.Sharma N. Relationship of somatic cell count and mastitis: An overview. Asian-Aust J Anim Sci 2011;24:429–38. [Google Scholar]
- 103.Fetherston C. Mastitis in lactating women: physiology or pathology?. Breastfeed Rev 2001;9:5–12. [PubMed] [Google Scholar]
- 104.Fetherston C. Risk factors for lactation mastitis. J Hum Lact 1998;14(2):101–9. [DOI] [PubMed] [Google Scholar]
- 105.Kinlay JR, O'Connell DL, Kinlay S. Incidence of mastitis in breastfeeding women during the six months after delivery: a prospective cohort study. Med J Aust 1998;169:310–2. [DOI] [PubMed] [Google Scholar]
- 106.Kourtis AP, Ibegbu CC, Theiler R, Xu YX, Bansil P, Jamieson DJ, Lindsay M, Butera S, Duerr A. Breast milk CD4+ T cells express high levels of C chemokine receptor 5 and CXC chemokine receptor 4 and are preserved in HIV-infected mothers receiving highly active antiretroviral therapy. J Infect Dis 2007;195:965–72. [DOI] [PubMed] [Google Scholar]
- 107.Kurath S, Halwachs-Baumann G, Muller W, Resch B.. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect 2010;16(8):1172–8. [DOI] [PubMed] [Google Scholar]