Abstract
Objective
To estimate trends in prepregnancy obesity prevalence among women who delivered live births in the US during 2003–2009, by state, age, and race–ethnicity.
Methods
We used Pregnancy Risk Assessment Monitoring System (PRAMS) data from 2003, 2006, and 2009 to measure prepregnancy obesity (body mass index [BMI] ≥ 30 kg/m2) trends in 20 states. Trend analysis included 90,774 records from 20 US states with data for all 3 study years. We used a chi-square test for trend to determine the significance of actual and standardized trends, standardized to the age and race–ethnicity distribution of the 2003 sample.
Results
Prepregnancy obesity prevalence increased by an average of 0.5 percentage points per year, from 17.6% in 2003 to 20.5% in 2009 (P < 0.001). Obesity increased among women aged 20–24 (P < 0.001), 30–34 (P = 0.001) and 35 years or older (P = 0.003), and among non-Hispanic white (P < .001), non-Hispanic black (P = 0.02), Hispanic (P = 0.01), and other women (P = 0.03).
Conclusion
Overall, prepregnancy obesity prevalence continues to increase and varies by race–ethnicity and maternal age. These findings highlight the need to address obesity as a key component of preconception care, particularly among high-risk groups.
Keywords: PRAMS, Pregnancy, Body mass index, Obesity
Introduction
Prepregnancy obesity (body mass index [BMI] ≥ 30 kg/m2) (World Health Organization, 2000) is a well-documented risk factor for obstetric complications, including gestational diabetes mellitus, hypertension, cesarean delivery, miscarriage, stillbirth, fetal macrosomia, preterm birth, and select birth defects (Cedergren, 2004; Chu et al., 2007a,b,c; Gilboa et al., 2010; Metwally et al., 2008; O’Brien et al., 2003; Rasmussen et al., 2008; Stothard et al., 2009; Torloni et al., 2009). However data about obesity trends among pregnant women in the US are limited. Recent evidence among non-pregnant women ages 20–39 years suggests that obesity prevalence has plateaued, but we do not know whether this is true among pregnant women (Flegal et al., 2010).
Two studies show an increasing trend in prepregnancy obesity (Hinkle et al., 2011; Kim et al., 2007); however, one only examined nine states during 1993–2003 (Kim et al., 2007), and the other was restricted to low-income women (Hinkle et al., 2011). We estimate recent trends in prepregnancy obesity prevalence among women who delivered live births in 20 states during 2003–2009.
Materials and methods
Study population
We analyzed data from the Pregnancy Risk Assessment Monitoring System (PRAMS), an ongoing, state-based, population-based surveillance system collecting information about maternal behaviors before, during, and after pregnancies resulting in live births. Each month in each participating jurisdiction, PRAMS uses birth certificates to draw a stratified sample of 100–300 live births delivered within the previous 2–6 months. PRAMS uses stratified sampling to oversample certain high-risk populations. Self-administered questionnaires are mailed to the mothers’ homes, with telephone follow-up for nonresponders. Each questionnaire is linked to the respondent’s child’s birth certificate. Data are weighted for each participating state to account for the sample design, nonresponse, and noncoverage. More detail on PRAMS methodology is available at http://www.cdc.gov/prams/methodology.
We used 2003, 2006, and 2009 data from states that met the PRAMS response rate threshold of ≥70% response in 2003 or 2006, or ≥65% response in 2009. Thirty-six states and New York City met these criteria in at least 1 of the 3 study years, and 20 states met these criteria in all 3 study years. We excluded records (6.0%; n = 7323) with missing BMI or with biologically implausible height (48 > inches > 78), weight (75 > pounds > 500), or BMI (12.55 > kg/m2 > 77.79, based on the data’s upper and lower 0.01 percentile). After exclusions, 114,899 records remained; among the 20 consistently reporting states, 90,744 weighted records were available for trend analysis, representing more than 3.2 million births.
For women 20 years or older, we calculated BMI as (weight in kilograms)/(height in meters)2, using self-reported height and weight from PRAMS questionnaires. We categorized adult women as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (>30 kg/m2). For subanalyses, we assessed class I (30–34.9 kg/m2), class II (35–39.9 kg/m2), and class III (>40 kg/m2) obesity (World Health Organization, 2000).
For women younger than 20 years, we used the 2000 CDC Growth Charts to calculate BMI-for-age percentile scores (Ogden et al., 2002). To estimate maternal birth date we used maternal birth year from PRAMS and set maternal birth day to July 1. We used infant birth month and year from the birth certificate and set infant birth day to 15 to estimate maternal age (in months) at delivery. We categorized adolescent women as underweight (<5th BMI-for-age percentile), normal weight (5th–84.9th BMI-for-age percentile), overweight (85th–94.9th BMI-for-age percentile), and obese (>95th BMI-for-age percentile) (Barlow and Expert, 2007). For subanalyses, we assessed trends among adolescents in the 95th–96.9th BMI-for-age percentile and in the 97th or higher BMI-for-age percentile (Ogden et al., 2012).
We used birth certificate data to categorize maternal race–ethnicity as: non-Hispanic white, non-Hispanic black, Hispanic, American Indian/Alaskan Native, Asian/Pacific Islander, and other. We categorized Chinese, Japanese, Filipino, Hawaiian, and “Other Asian” as Asian/Pacific Islander; “other” includes those who reported “mixed race” or any race–ethnicity other than those described above. On the 2003 birth certificate, respondents may select Hispanic ethnicity and a separate race category. We categorized anyone who reported Hispanic ethnicity as Hispanic, regardless of any secondary race classification.
We used Medicaid and Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) enrollment as dichotomous proxy indicators of socioeconomic status. We recorded women as enrolled in Medicaid if they reported using Medicaid before pregnancy, for prenatal care, or for delivery care. We defined WIC enrollment as having received WIC assistance during pregnancy. We categorized women as having smoked before pregnancy if they reported on the PRAMS questionnaire that they smoked more than zero cigarettes per day in the 3 months before pregnancy.
Statistical analysis
We calculated the prevalence and standard error of each BMI category for each state contributing to each study year. We restricted trend analyses to the 20 states with PRAMS data for all 3 study years: 2003, 2006, and 2009. Previous studies indicate that prepregnancy obesity prevalence is associated with maternal age and race–ethnicity, and that the distribution of these demographics of pregnant women in the US is changing (Chu et al., 2009; Hinkle et al., 2011; Kim et al., 2007). We directly standardized the overall prevalence of each BMI category for each study year and the overall obesity trend to the 2003 age and race–ethnicity distribution among the 20 consistently reporting states. To estimate the trajectory of trends over time, we calculated mean annual percentage point change in obesity prevalence by comparing 2003 to 2006, 2006 to 2009, and 2003 to 2009. We used a Cochran-Mantel-Haenszel chi-square test to determine the significance of the trend in obesity prevalence. We considered a P-value < 0.05 statistically significant.
We calculated the prevalence of each prepregnancy obesity severity category by state, stratified by adults and adolescents. We calculated the state-specific trends by obesity severity and the crude and standardized overall trends by obesity severity among the 20 consistently reporting states. Finally, we estimated the 2009 prevalence of each BMI group, overall and by maternal age and race–ethnicity, using data from all states (n = 29) with 2009 data.
We conducted all analyses with SAS 9.2 (Cary, NC, USA) and SUDAAN 10.0.1 (Research Triangle Park, NC, USA) to account for PRAMS’ complex survey design.
Results
Across the study period, respondents were predominantly non-Hispanic white, married, post-high school, not enrolled in WIC or Medicaid, and nonsmokers before pregnancy (Table 1).
Table 1.
Maternal characteristics among consistently reporting US states (20 states), 2003, 2006 and 2009a.
| Characteristic | 2003 | 2006 | 2009 | P-value |
|---|---|---|---|---|
| Maternal age (y) | <0.001 | |||
| <20 | 9.2 (0.3) | 9.1 (0.3) | 9.0 (0.3) | |
| 20–24 | 25.5 (0.4) | 23.7 (0.4) | 22.8 (0.4) | |
| 25–29 | 27.3 (0.4) | 28.9 (0.4) | 30.1 (0.4) | |
| 30–34 | 24.7 (0.4) | 23.9 (0.4) | 24.4 (0.4) | |
| ≥35 | 13.4 (0.3) | 14.4 (0.3) | 13.8 (0.3) | |
| Maternal race–ethnicity | <0.001 | |||
| Non-Hispanic White | 69.1 (0.3) | 66.9 (0.4) | 64.9 (0.4) | |
| Non-Hispanic Black | 13.1 (0.3) | 13.1 (0.3) | 13.6 (0.2) | |
| Hispanic | 11.3 (0.3) | 12.6 (0.3) | 13.1 (0.3) | |
| American Indian/Alaskan Native | 1.2 (0.1) | 1.3 (0.1) | 1.3 (0.1) | |
| Asian/Pacific Islander | 4.9 (0.1) | 5.1 (0.2) | 5.0 (0.1) | |
| Other | 0.4 (0.1) | 1.1 (0.1) | 2.1 (0.1) | |
| Parity | 0.16 | |||
| 0 | 41.2 (0.5) | 40.8 (0.5) | 40.7 (0.5) | |
| 1 | 32.1 (0.4) | 31.9 (0.4) | 33.2 (0.4) | |
| ≥2 | 26.7 (0.4) | 27.2 (0.4) | 26.1 (0.4) | |
| Maternal education (y) | <0.001 | |||
| <12 | 15.6 (0.4) | 15.2 (0.3) | 14.3 (0.3) | |
| 12 | 31.9 (0.4) | 29.1 (0.4) | 26.7 (0.4) | |
| ≥13 | 52.5 (0.5) | 55.8 (0.4) | 59.0 (0.5) | |
| Married | 67.2 (0.4) | 65.8 (0.4) | 62.5 (0.4) | <0.001 |
| WIC enrolled | 39.0 (0.4) | 40.3 (0.4) | 44.5 (0.5) | <0.001 |
| Medicaid enrolled | 40.1 (0.4) | 43.0 (0.4) | 46.8 (0.5) | <0.001 |
| Smoking before pregnancy | 24.5 (0.4) | 24.5 (0.4) | 26.9 (0.4) | <0.001 |
Abbreviations: WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Values are weighted percentages (standard error).
Overall, the standardized prepregnancy obesity prevalence increased during 2003–2009 (P-trend < 0.001), from 17.6% in 2003 to 20.5% in 2009 (Fig. 1). The standardized trend was similar to the crude trend (Supplementary Table 1). The rate of increase slowed over time, from a mean of 0.6 percentage points per year during 2003–2006 (P = 0.003), to 0.4 percentage points per year during 2006–2009 (P = 0.02). Prepregnancy overweight prevalence also increased, from 23.0% to 24.3% (P-trend = 0.04), whereas the proportion of normal-weight women entering pregnancy decreased from 54.5% to 51.5% (P-trend < 0.001).
Fig. 1.
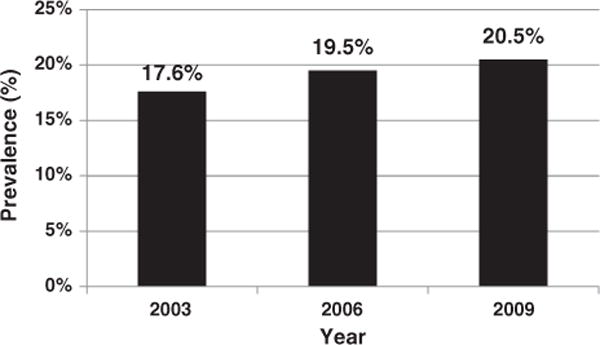
Prepregnancy obesity (BMI ≥ 30 kg/m2) prevalence among 20 US States, 2003, 2006, and 2009. P-trend < 0.001. Prevalence estimate is standardized to the sample’s 2003 race–ethnicity and age distribution. Standard errors are: 2003–0.4, 2006–0.4, 2009–0.4.
Obesity increased significantly among women aged 20–24, 30–34, and 35 years or older (P-trend < 0.001, P-trend = 0.001, P-trend = 0.003, respectively), but not among other age groups (Supplementary Table 1). Obesity also increased among women categorized as non-Hispanic white, non-Hispanic black, Hispanic, and other (P-trend < 0.001, P-trend = 0.02, P-trend = 0.01, P-trend = 0.03, respectively).
Obesity prevalence increased significantly in eight states during 2003–2009: Arkansas, Maryland, Michigan, Mississippi, Nebraska, New Jersey, Oklahoma, and Washington (Supplementary Table 1). The average annual rate of increase in prepregnancy obesity prevalence ranged from 0.6 percentage points per year in Michigan (P-trend = 0.04) to 1.2 percentage points per year in Oklahoma (P-trend = 0.001).
Prevalence estimates from states with data available for any of the 3 study years suggest that the proportion of states with prepregnancy obesity prevalence 20% or higher increased over time (Fig. 2). In 2003, 26% (7/27) of states had prepregnancy obesity prevalence 20% or higher; in 2009, 66% (19/29) of states had prepregnancy obesity prevalence 20% or higher.
Fig. 2.
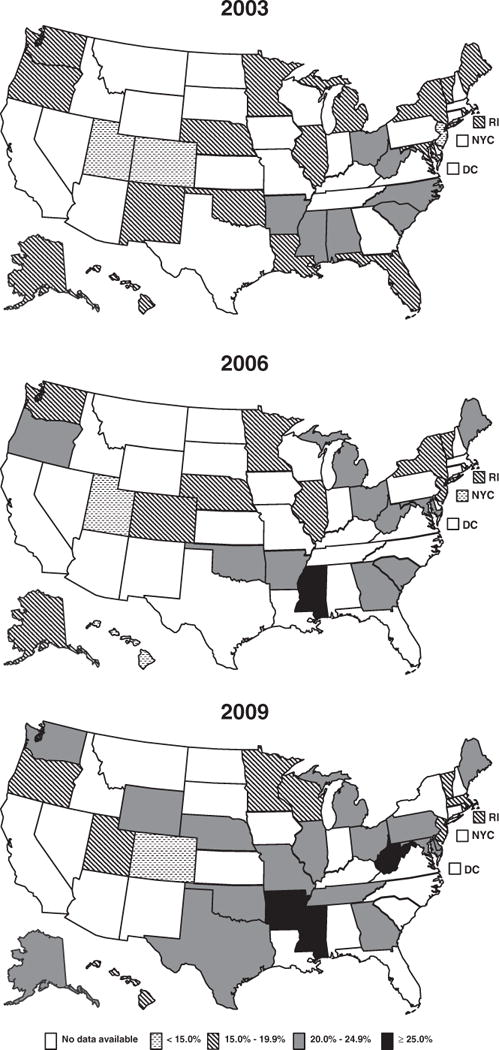
Prepregnancy obesity prevalence by US state, 2003, 2006, and 2009 (obesity ≥ 30 kg/m2).
Among adults, the standardized prevalence of all three obesity classes increased over time (Table 2). Class I obesity prevalence increased from 9.7% to 10.7% (P-trend = 0.009), class II obesity prevalence increased from 4.3% to 5.2% (P-trend = 0.001), and class III obesity prevalence increased from 2.8% to 3.6% (P-trend b 0.001). The standardized proportion of adolescent women at or above the 97th BMI-for-age percentile increased from 3.9% to 6.3% (P-trend = 0.02) (Table 3); the proportion in the 95th–96.9th percentile did not change.
Table 2.
Prepregnancy obesity prevalence among women aged 20 years or older by obesity severity and by US state, 2003, 2006, and 2009a.
| Obese class I
|
Obese class II
|
Obese class III
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2003 | 2006 | 2009 | P-trend | 2003 | 2006 | 2009 | P-trend | 2003 | 2006 | 2009 | P-trend | |
| Overallb | 10.7 (0.3) | 11.8 (0.3) | 11.9 (0.3) | 0.004 | 4.7 (0.2) | 5.2 (0.2) | 5.7 (0.2) | 0.001 | 3.1 (0.2) | 3.4 (0.2) | 4.0 (0.2) | <0.001 |
| Overallc | 9.7 (0.3) | 10.7 (0.3) | 10.7 (0.3) | 0.009 | 4.3 (0.2) | 4.7 (0.2) | 5.2 (0.2) | 0.001 | 2.8 (0.2) | 3.1 (0.2) | 3.6 (0.2) | <0.001 |
| State | ||||||||||||
| Alabama | 13.0 (1.2) | – | – | – | 6.8 (0.9) | – | – | – | 3.2 (0.6) | – | – | – |
| Alaska | 10.8 (1.0) | 11.6 (1.1) | 12.4 (1.2) | 0.29 | 5.6 (0.7) | 5.9 (0.8) | 6.6 (0.9) | 0.37 | 3.8 (0.6) | 3.7 (0.7) | 3.8 (0.7) | 0.99 |
| Arkansas | 12.4 (1.2) | 11.8 (1.1) | 15.4 (1.6) | 0.11 | 6.0 (0.8) | 6.8 (0.8) | 8.5 (1.2) | 0.10 | 4.6 (0.7) | 5.3 (0.7) | 4.1 (0.8) | 0.59 |
| Colorado | 7.3 (0.8) | 11.0 (1.1) | 7.7 (0.9) | 0.72 | 2.9 (0.5) | 4.4 (0.8) | 4.2 (0.6) | 0.09 | 2.0 (0.4) | 3.8 (0.8) | 2.7 (0.6) | 0.33 |
| Delaware | – | – | 12.4 (1.1) | – | – | – | 6.3 (0.8) | – | – | – | 5.8 (0.8) | – |
| Florida | 14.0 (1.3) | – | – | – | 3.6 (0.6) | – | – | – | 2.4 (0.5) | – | – | – |
| Georgia | – | 11.1 (1.1) | 10.6 (1.6) | – | – | 7.2 (0.9) | 7.2 (1.5) | – | – | 4.5 (0.7) | 3.1 (0.9) | – |
| Hawaii | 9.8 (0.8) | 9.8 (0.8) | 11.7 (1.1) | 0.18 | 3.9 (0.6) | 3.8 (0.5) | 4.2 (0.7) | 0.71 | 2.2 (0.4) | 1.8 (0.4) | 2.2 (0.5) | 0.97 |
| Illinois | 10.9 (1.0) | 12.7 (1.0) | 12.6 (1.0) | 0.23 | 4.5 (0.6) | 4.6 (0.6) | 5.2 (0.7) | 0.43 | 3.3 (0.6) | 2.7 (0.5) | 3.6 (0.6) | 0.80 |
| Louisiana | 9.5 (0.9) | – | – | – | 6.0 (0.7) | – | – | – | 3.9 (0.6) | – | – | – |
| Maine | 10.6 (1.1) | 12.8 (1.2) | 11.8 (1.2) | 0.48 | 6.1 (0.8) | 6.0 (0.8) | 4.9 (0.8) | 0.32 | 3.4 (0.6) | 3.2 (0.6) | 5.8 (0.9) | 0.03 |
| Maryland | 8.3 (1.1) | 11.3 (1.3) | 13.1 (1.4) | 0.007 | 5.3 (0.9) | 6.9 (1.1) | 5.3 (0.9) | 0.99 | 4.3 (0.9) | 2.6 (0.6) | 4.0 (0.8) | 0.78 |
| Massachusetts | – | – | 9.7 (1.1) | – | – | – | 6.8 (1.0) | – | – | – | 1.7 (0.5) | – |
| Michigan | 11.2 (1.0) | 12.3 (1.2) | 12.2 (1.0) | 0.44 | 4.7 (0.7) | 6.1 (0.8) | 6.1 (0.7) | 0.15 | 3.3 (0.6) | 4.5 (0.8) | 5.1 (0.7) | 0.05 |
| Minnesota | 10.4 (1.0) | 10.7 (0.8) | 11.9 (1.0) | 0.28 | 4.4 (0.7) | 5.2 (0.6) | 4.0 (0.6) | 0.67 | 3.0 (0.5) | 2.3 (0.4) | 2.6 (0.5) | 0.58 |
| Mississippi | 13.1 (1.2) | 12.7 (1.5) | 16.7 (1.4) | 0.05 | 6.9 (0.9) | 8.9 (1.3) | 7.4 (1.0) | 0.69 | 3.4 (0.6) | 7.2 (1.1) | 7.0 (0.9) | 0.002 |
| Missouri | – | – | 12.6 (1.2) | – | – | – | 6.3 (0.9) | – | – | – | 5.4 (0.8) | – |
| Nebraska | 10.2 (0.9) | 12.8 (1.1) | 12.6 (1.0) | 0.08 | 4.7 (0.6) | 5.1 (0.8) | 5.6 (0.7) | 0.31 | 1.9 (0.4) | 2.6 (0.5) | 3.6 (0.6) | 0.01 |
| New Jersey | 8.6 (0.7) | 9.8 (0.8) | 11.5 (1.0) | 0.02 | 3.0 (0.5) | 4.6 (0.6) | 3.6 (0.6) | 0.38 | 1.8 (0.3) | 2.0 (0.4) | 3.0 (0.5) | 0.06 |
| New Mexico | 14.6 (1.2) | – | – | – | 4.7 (0.7) | – | – | – | 2.5 (0.5) | – | – | – |
| New York | 12.9 (1.3) | 13.0 (1.9) | – | – | 3.5 (0.7) | 3.4 (1.0) | – | – | 2.3 (0.6) | 3.2 (1.0) | – | – |
| New York City | – | 9.2 (1.0) | – | – | – | 3.2 (0.6) | – | – | – | 2.0 (0.5) | – | – |
| North Carolina | 12.2 (1.2) | – | – | – | 5.3 (0.8) | – | – | – | 5.2 (0.8) | – | – | – |
| Ohio | 13.9 (1.3) | 12.6 (1.2) | 11.3 (1.2) | 0.13 | 6.5 (0.9) | 5.1 (0.8) | 8.0 (1.1) | 0.30 | 4.6 (0.8) | 3.9 (0.7) | 5.5 (0.9) | 0.49 |
| Oklahoma | 11.6 (1.3) | 12.8 (1.3) | 14.2 (1.4) | 0.17 | 4.4 (0.8) | 6.1 (0.9) | 6.5 (1.0) | 0.11 | 2.8 (0.6) | 5.3 (0.9) | 4.5 (0.8) | 0.11 |
| Oregon | 11.1 (1.3) | 12.4 (1.3) | 11.6 (1.3) | 0.76 | 5.0 (1.0) | 6.7 (1.0) | 4.8 (0.8) | 0.83 | 2.4 (0.7) | 4.4 (0.9) | 4.1 (0.8) | 0.10 |
| Pennsylvania | – | – | 13.3 (1.3) | – | – | – | 6.4 (0.9) | – | – | – | 3.8 (0.7) | – |
| Rhode Island | 10.6 (1.0) | 11.2 (1.1) | 11.7 (1.1) | 0.47 | 4.3 (0.7) | 5.2 (0.8) | 3.7 (0.7) | 0.57 | 2.5 (0.5) | 3.0 (0.6) | 3.5 (0.7) | 0.24 |
| South Carolina | 11.5 (1.5) | 14.3 (2.3) | – | – | 7.4 (1.2) | 5.3 (1.4) | – | – | 4.3 (0.9) | 4.1 (1.2) | – | – |
| Tennessee | – | – | 10.4 (1.6) | – | – | – | 7.4 (1.4) | – | – | – | 5.5 (1.2) | – |
| Texas | – | – | 15.2 (1.3) | – | – | – | 7.0 (0.9) | – | – | – | 4.0 (0.7) | – |
| Utah | 10.3 (1.0) | 9.5 (0.8) | 9.0 (0.9) | 0.34 | 2.9 (0.6) | 2.8 (0.4) | 5.3 (0.7) | 0.006 | 1.7 (0.4) | 2.0 (0.4) | 2.4 (0.5) | 0.31 |
| Vermont | 12.1 (1.0) | 13.1 (1.1) | 10.1 (1.0) | 0.16 | 4.2 (0.6) | 4.0 (0.6) | 6.1 (0.8) | 0.06 | 3.1 (0.5) | 3.1 (0.6) | 4.0 (0.7) | 0.30 |
| Washington | 9.1 (1.1) | 12.1 (1.2) | 10.8 (1.1) | 0.29 | 4.9 (0.9) | 3.9 (0.8) | 6.4 (1.0) | 0.21 | 2.3 (0.5) | 3.2 (0.7) | 3.5 (0.7) | 0.18 |
| West Virginia | 12.9 (1.4) | 14.0 (1.4) | 14.4 (1.2) | 0.41 | 6.1 (0.9) | 6.4 (1.0) | 7.0 (0.9) | 0.50 | 4.4 (0.8) | 5.8 (0.9) | 6.9 (0.9) | 0.03 |
| Wisconsin | – | – | 11.4 (1.3) | – | – | – | 5.0 (0.9) | – | – | – | 4.3 (0.8) | – |
| Wyoming | – | – | 13.4 (1.4) | – | – | – | 5.1 (1.1) | – | – | – | 3.7 (0.7) | – |
Values are weighted percent (standard error).
Includes only states with data for all 3 years (20 states).
Includes only states with data for all 3 years (20 states), standardized by age and race–ethnicity.
Table 3.
Prepregnancy obesity prevalence among women aged less than 20 years by obesity severity and by US state, 2003, 2006, and 2009a.
| 95th–96.9th BMI-for-age percentile
|
≥97th BMI-for-age percentile
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 2003 | 2006 | 2009 | P-trend | 2003 | 2006 | 2009 | P-trend | |
| Overallb | 4.8 (0.8) | 5.3 (0.8) | 4.6 (0.6) | 0.79 | 3.9 (0.6) | 5.3 (0.7) | 6.1 (0.8) | 0.03 |
| Overallc | 4.9 (0.7) | 5.4 (0.8) | 4.6 (0.7) | 0.81 | 3.9 (0.6) | 5.2 (0.7) | 6.3 (0.8) | 0.02 |
| State | ||||||||
| Alabama | 2.3 (1.1) | – | – | – | 8.1 (2.4) | – | – | – |
| Alaska | 5.6 (2.3) | 1.1 (0.6) | 4.3 (2.6) | 0.76 | 2.6 (0.8) | 5.2 (2.2) | 5.5 (2.5) | 0.31 |
| Arkansas | 1.8 (0.9) | 4.9 (1.8) | 1.8 (1.0) | 0.95 | 6.2 (2.0) | 7.4 (2.1) | 7.5 (3.1) | 0.72 |
| Colorado | 3.0 (1.9) | 5.7 (3.2) | 1.7 (0.7) | 0.53 | 1.3 (0.9) | 5.9 (2.8) | 3.2 (1.4) | 0.28 |
| Delaware | – | – | 4.4 (2.3) | – | – | – | 9.7 (3.3) | – |
| Florida | 3.6 (1.5) | – | – | – | 5.2 (1.7) | – | – | – |
| Georgia | – | 7.2 (2.8) | 8.1 (3.8) | – | – | 3.8 (1.6) | 8.5 (3.8) | – |
| Hawaii | 4.4 (1.7) | 0 (0) | 3.8 (1.9) | 0.89 | 4.1 (1.8) | 0 (0) | 4.4 (2.4) | 0.86 |
| Illinois | 5.0 (2.0) | 3.9 (1.8) | 4.7 (2.0) | 0.92 | 3.0 (1.7) | 8.5 (2.7) | 5.6 (2.1) | 0.33 |
| Louisiana | 2.3 (1.1) | – | – | – | 2.2 (1.2) | – | – | – |
| Maine | 6.2 (3.3) | 6.7 (4.4) | 6.0 (3.3) | 0.96 | 9.7 (4.0) | 4.9 (2.9) | 2.8 (2.1) | 0.13 |
| Maryland | 2.9 (1.8) | 9.0 (3.9) | 7.5 (4.9) | 0.29 | 2.8 (2.2) | 4.8 (3.2) | 7.5 (4.9) | 0.36 |
| Massachusetts | – | – | 6.5 (4.3) | – | – | – | 16.5 (6.8) | – |
| Michigan | 5.9 (2.6) | 6.8 (2.5) | 6.5 (2.5) | 0.87 | 5.1 (2.1) | 7.0 (3.0) | 1.7 (0.7) | 0.11 |
| Minnesota | .7 (0.4) | 3.8 (2.1) | 2.2 (1.2) | 0.15 | 1.7 (0.7) | 1.1 (1.1) | 1.4 (0.8) | 0.73 |
| Mississippi | 7.0 (2.2) | 5.8 (2.5) | 4.4 (1.8) | 0.36 | 6.7 (2.3) | 5.2 (2.5) | 4.6 (1.7) | 0.45 |
| Missouri | – | – | 6.2 (2.9) | – | – | – | 2.9 (1.2) | – |
| Nebraska | 1.9 (0.7) | 1.2 (0.7) | 4.0 (1.9) | 0.31 | 5.0 (2.3) | 2.4 (1.1) | 5.3 (2.4) | 0.98 |
| New Jersey | 5.2 (2.4) | 2.0 (1.3) | 8.5 (3.9) | 0.50 | 1.6 (1.1) | 4.7 (2.5) | 8.7 (3.8) | 0.07 |
| New Mexico | 1.4 (0.7) | – | – | – | 4.2 (1.4) | – | – | – |
| New York | 2.8 (2.8) | .5 (0.5) | – | – | 5.6 (3.8) | 10.4 (7.0) | – | – |
| New York City | – | 4.2 (2.6) | – | – | – | 7.0 (3.7) | – | – |
| North Carolina | 3.5 (2.1) | – | – | – | 6.2 (2.7) | – | – | – |
| Ohio | 8.3 (3.5) | 6.7 (2.8) | 2.1 (1.0) | 0.11 | 2.8 (2.1) | 2.9 (0.9) | 9.4 (3.8) | 0.13 |
| Oklahoma | 2.1 (1.3) | 8.2 (3.6) | 2.5 (1.6) | 0.87 | 2.7 (1.5) | 3.9 (2.2) | 11.6 (3.6) | 0.03 |
| Oregon | 8.1 (4.4) | 3.2 (1.0) | 7.0 (4.0) | 0.85 | 5.1 (2.6) | 9.2 (4.0) | 4.7 (3.0) | 0.93 |
| Pennsylvania | – | – | 4.5 (2.7) | – | – | – | 1.9 (1.9) | – |
| Rhode Island | 3.0 (1.7) | 2.0 (1.6) | 5.9 (2.9) | 0.39 | 6.4 (2.6) | 3.7 (2.2) | 7.4 (3.1) | 0.81 |
| South Carolina | 6.3 (3.0) | 2.3 (2.3) | – | – | 7.2 (3.4) | 13.2 (5.8) | – | – |
| Tennessee | – | – | 7.0 (3.2) | – | – | – | 6.2 (3.3) | – |
| Texas | – | – | 6.5 (2.4) | – | – | – | 5.1 (1.8) | – |
| Utah | 3.0 (2.7) | 2.4 (1.0) | 5.5 (2.6) | 0.49 | 0.1 (0.1) | 2.4 (1.4) | 4.5 (1.9) | 0.03 |
| Vermont | 3.4 (2.6) | 5.6 (2.8) | 2.5 (2.3) | 0.73 | 1.0 (0.4) | 5.6 (2.8) | 10.9 (4.4) | 0.04 |
| Washington | 3.8 (1.9) | 6.4 (3.2) | 8.0 (3.7) | 0.34 | 10.0 (4.4) | 6.1 (3.1) | 7.2 (3.3) | 0.63 |
| West Virginia | 3.1 (0.7) | 4.3 (0.8) | 3.0 (1.3) | 0.89 | 3.7 (0.8) | 5.1 (0.9) | 6.5 (2.0) | 0.20 |
| Wisconsin | – | – | 4.4 (2.8) | – | – | – | 5.1 (3.0) | – |
| Wyoming | – | – | 5.2 (2.6) | – | – | – | 1.8 (1.1) | – |
Values are weighted percent (standard error).
Includes only states with data for all 3 years (20 states).
Includes only states with data for all 3 years (20 states), standardized by age and race–ethnicity.
Among all states that contributed data in 2009 (n = 29), the prevalence (SE) of prepregnancy underweight, normal weight, overweight, and obesity were: 3.9% (0.2), 50.2% (0.5), 24.5% (0.4), and 21.4% (0.4), respectively (data not shown). Non-Hispanic black women and American Indian/Alaskan Native women had the highest prepregnancy obesity prevalence (29.2% [1.0] and 28.9% [2.7], respectively). Asian/Pacific Islander women had the lowest prepregnancy obesity prevalence (7.2%, [0.8]). Non-Hispanic white and Hispanic women had 20% (0.4) and 23.2% (1.3) prevalence, respectively. Women aged 35 years or older had the highest prepregnancy obesity prevalence (24.0% [1.1]); women aged less than 20 years had the lowest (11.4% [1.0]).
Discussion
These data show that the proportion of US women who are obese upon entering pregnancy continues to increase. The overall trend remained significant after standardizing to account for changing maternal age and race–ethnicity distributions over time. Overall, the rate of increase appears to be slowing; however, this varies by state, maternal age, and race–ethnicity. Nevertheless, prepregnancy obesity remains high; in 2009, more than one in five pregnant women were obese across almost every age and racial–ethnic group. This represents 221,165 obese pregnant women in 2009, 30,655 more than in 2003.
This study provides the only current population-based evidence of prepregnancy obesity trends in the US. Our findings are consistent with earlier studies that found increasing trends in prepregnancy obesity, and expand the population to which these results can be generalized. An earlier analysis used PRAMS 1993–2003 to analyze prepregnancy obesity trends, but was limited to nine states, used now outdated cutpoints for obesity (BMI > 29.0), and only differentiated race–ethnicity as white, black, and other (Kim et al., 2007). A more recent study of prepregnancy obesity trends was limited to adult women enrolled in WIC during 1999–2008 (Hinkle et al., 2011). To our knowledge, ours is the first study to assess trends by obesity severity among adolescent pregnant women. With expanded geographic coverage, differentiation among six race–ethnicity categories, and broader criteria to include women regardless of socioeconomic status, our study provides more representative data about prepregnancy obesity trends in the US.
Our results are consistent with previous findings that prepregnancy obesity varies among states (Chu et al., 2009). We speculate this is partly due to varying racial–ethnic and maternal age distributions (Kim et al., 2007; Martin et al., 2011). Additional research is needed to identify the specific drivers of racial–ethnic differences in obesity. Socioeconomic status may vary by race–ethnicity and determine access to healthy food and physical activity resources (Nicholson and Browning, 2012). Broader contextual factors that vary by state, such as neighborhood safety, urban planning policies, and zoning regulations for supermarkets, may also affect obesity (Khan et al., 2009).
Our prepregnancy obesity trend differs from recent data on obesity among women of reproductive age. NHANES data do not indicate an increase in obesity among women aged 20–39 years during 1999–2008 (Flegal et al., 2010). These differences could indicate that pregnant women and women of reproductive age are two distinct populations that should be analyzed separately when examining obesity. The latter includes women regardless of pregnancy status, with a large proportion (18%) who will never give birth (Dye, 2008). PRAMS and NHANES are methodologically different; PRAMS has a much larger sample than NHANES, providing greater power to detect prevalence changes. Additionally, unlike NHANES, PRAMS is not nationally representative, limiting our ability to compare the two estimates.
Evidence of increasing prepregnancy obesity is particularly concerning given the known dose–response relationship between increasing prepregnancy BMI and increased risk of obstetric complications (Yogev and Catalano, 2009). We show that extreme obesity prevalence is increasing among pregnant women, suggesting a growing burden of complications on mothers, their offspring, and the health care system. Costs for prenatal care may be up to five times higher for obese than normal weight women, with additional delivery and post-partum costs associated with longer hospital stays, more procedures to address complications, and increased infections (Chu et al., 2008; Galtier-Dereure et al., 2000; Heslehurst et al., 2008). Prepregnancy obesity has also been linked to obesity and overweight among offspring, thus perpetuating an obesity cycle (Whitaker, 2004). We found that 46% of US women entered pregnancy at above normal weight in 2009, making high prepregnancy BMI an extremely common risk factor for adverse obstetric outcomes.
Given the known health implications and high prevalence of prepregnancy obesity, obesity should be addressed as a key component of preconception care among all women, regardless of pregnancy intentions. Half of US pregnancies are unintended, so many women do not have the opportunity to lose weight in preparation for pregnancy (Finer and Zolna, 2011). Emphasis should be placed on ensuring access to weight management counseling and treatment as a standard component of routine preconception care, particularly among high-risk groups. Both the US Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists recommend preconception care, including obesity screening (American College of Obstetricians and Gynecologists, 2005; Johnson et al., 2006). Counseling about nutrition and physical activity, as well as appropriate contraceptive use, can help women achieve a healthy weight before pregnancy. However, lack of providers offering this kind of preconception care, public awareness to seek preconception care services, and insurance coverage of those services represent significant barriers to access (Cogswell et al., 2010; Johnson et al., 2006). The trend shown here indicates that current efforts to provide these services may be insufficient.
Our analysis is limited to those states that contributed data for the three study years and may not be representative of the entire US. However, with population-based data from 20 states, our study is considerably more representative than the previous nine-state analysis (Kim et al., 2007).
Additionally, the respondents included in this study may differ from those who were excluded. PRAMS systematically excludes women who had stillbirths or fetal deaths, both of which are associated with prepregnancy obesity (Chu et al., 2007b; Cnattingius et al., 1998). Records excluded because of missing data were disproportionately young, Hispanic, had two or more previous live births, had completed fewer than 12 years of education, were unmarried, nonsmokers, and enrolled in WIC and Medicaid (P < 0.001). Current evidence suggests that obesity is more prevalent among non-Hispanic black and other minority women, women with less education, and women enrolled in WIC (Kim et al., 2007; Wang and Beydoun, 2007). Furthermore, BMI data from PRAMS is based on maternal self-report, which is known to underestimate BMI (Gorber et al., 2007). However we do not expect the amount of this bias to have changed over time (Merrill and Richardson, 2009). Finally, our estimate of maternal age to calculate BMI-for-age percentiles among adolescent women is based on age at delivery, plus or minus 6.5 months. This yields a 2.5–15.5 month overestimate of maternal age at conception, resulting in a slight underestimate of maternal BMI-for-age percentile. Based on these limitations, we infer that our study presents a conservative estimate of prepregnancy obesity.
Conclusion
In conclusion, our results indicate that, overall, prepregnancy obesity prevalence is high and continues to increase in the US, with potentially substantial negative public and clinical health implications. The US Department of Health and Human Services has identified increasing the proportion of women who enter pregnancy at a healthy weight as a priority in its Healthy People 2020 initiative (US Department of Health and Human Services). Yet our data indicate that this trend is moving in the opposite direction. Regular national surveillance is needed to better understand the health needs of this population and to guide targeted and effective interventions to reduce obesity among pregnant women.
Supplementary Material
Acknowledgments
All data included in this study were collected at the state level by the following state collaborators and their staff: Alabama–Izza Afgan, MPH; Alaska–Kathy Perham-Hester, MS, MPH; Arkansas–Mary McGehee, PhD; Colorado–Alyson Shupe, PhD; Delaware–George Yocher, MS; Florida–Cynthia Ulysee, MPH; Georgia–Yan Li, MD, MPH; Hawaii–Emily Roberson, MA; Illinois–Theresa Sandidge, MA; Louisiana–Amy Zapata, MPH; Maine–Tom Patenaude, MPH; Maryland–Diana Cheng, MD; Massachusetts–Emily Lu, MPH; Michigan–Cristin Larder, MS; Minnesota–Judy Punyko, PhD, MPH; Mississippi–Brenda Hughes, MPPA; Missouri–Venkata Garikapaty, MSc, MS, PhD, MPH; Nebraska–Brenda Coufal; New Jersey–Lakota Kruse, MD; New Mexico–Eirian Coronado, MPH; New York State–Anne Radigan-Garcia; New York City–Candace Mulready-Ward, MPH; North Carolina–Kathleen Jones-Vessey, MS; Ohio–Connie Geidenberger PhD; Oklahoma–Alicia Lincoln, MSW, MSPH; Oregon–Kenneth Rosenberg, MD, MPH; Pennsylvania–Tony Norwood; Rhode Island–Sam Viner-Brown, PhD; South Carolina–Mike Smith, MSPH; Texas–Rochelle Kingsley, MPH; Tennessee–David Law, PhD; Utah–Laurie Baksh; Vermont–Peggy Brozicevic; Washington–Linda Lohdefinck; West Virginia–Melissa Baker, MA; Wisconsin–Katherine Kvale, PhD; Wyoming–Angi Crotsenberg; and CDC PRAMS Team, Applied Sciences Branch, Division of Reproductive Health. The Centers for Disease Control and Prevention reviewed and approved this report before submission for publication.
Funding/support: There was no external funding support for this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ypmed.2013.02.015.
Conflict of interest statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion number 313, September 2005. The importance of preconception care in the continuum of women’s health care. Obstet Gynecol. 2005;106:665–666. doi: 10.1097/00006250-200509000-00052. [DOI] [PubMed] [Google Scholar]
- Barlow SE, Expert, C Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007a;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Lau J, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007b;197:223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Schmid CH, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007c;8:385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- Chu SY, Bachman DJ, Callaghan WM, et al. Association between obesity during pregnancy and increased use of health care. N Engl J Med. 2008;358:1444–1453. doi: 10.1056/NEJMoa0706786. [DOI] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern Child Health J. 2009;13:614–620. doi: 10.1007/s10995-008-0388-3. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Power ML, Sharma AJ, Schulkin J. Prevention and management of obesity in nonpregnant women and adolescents: beliefs and practices of U.S. obstetricians and gynecologists. J Women’s Health. 2010;19:1625–1634. doi: 10.1089/jwh.2009.1838. [DOI] [PubMed] [Google Scholar]
- Dye JL. Fertility of American Women: June 2008, Current Population Reports. U.S. Census Bureau; Washington, DC: 2008. pp. 20–563. [Google Scholar]
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478–485. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71:1242S–1248S. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- Gilboa SM, Correa A, Botto LD, et al. Association between prepregnancy body mass index and congenital heart defects. Am J Obstet Gynecol. 2010;202:51 e51–51 e10. doi: 10.1016/j.ajog.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Simpson H, Ells LJ, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev. 2008;9:635–683. doi: 10.1111/j.1467-789X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- Hinkle SN, Sharma AJ, Kim SY, et al. Prepregnancy obesity trends among low-income women, United States, 1999–2008. Matern Child Health J. 2011:1339–1348. doi: 10.1007/s10995-011-0898-2. [DOI] [PubMed] [Google Scholar]
- Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care–United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- Khan LK, Sobush K, Keener D, Goodman K, Lowry A, Kakietek J, Zaro S, Centers for Disease, C., Prevention Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm Rep. 2009;58:1–26. [PubMed] [Google Scholar]
- Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in prepregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Kirmeyer S, Matthews TJ, Wilson EC. Births: final data for 2009. Natl Vital Stat Rep. 2011;60 [PubMed] [Google Scholar]
- Merrill RM, Richardson JS. Validity of self-reported height, weight, and body mass index: findings from the National Health and Nutrition Examination Survey, 2001–2006. Prev Chronic Dis. 2009;6:A121. [PMC free article] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- Nicholson LM, Browning CR. Racial and ethnic disparities in obesity during the transition to adulthood: the contingent and nonlinear impact of neighborhood disadvantage. J Youth Adolesc. 2012;41:53–66. doi: 10.1007/s10964-011-9685-z. [DOI] [PubMed] [Google Scholar]
- O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009 Feb 11;301(6):636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Torloni MR, Betran AP, Daher S, et al. Maternal BMI and preterm birth: a systematic review of the literature with meta-analysis. J Matern Fetal Neonatal Med. 2009;22:957–970. doi: 10.3109/14767050903042561. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion, a. Healthy People 2020. Washington, DC: Available at http://healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicId=26 (Accessed Jan 13 2012) [Google Scholar]
- Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity: preventing and managing the global epidemic. (World Health Organization technical report series).Report of a WHO consultation. 2000;894:1–253. i–xii. [PubMed] [Google Scholar]
- Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gynecol Clin North Am. 2009;36:285–300. doi: 10.1016/j.ogc.2009.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


