Abstract
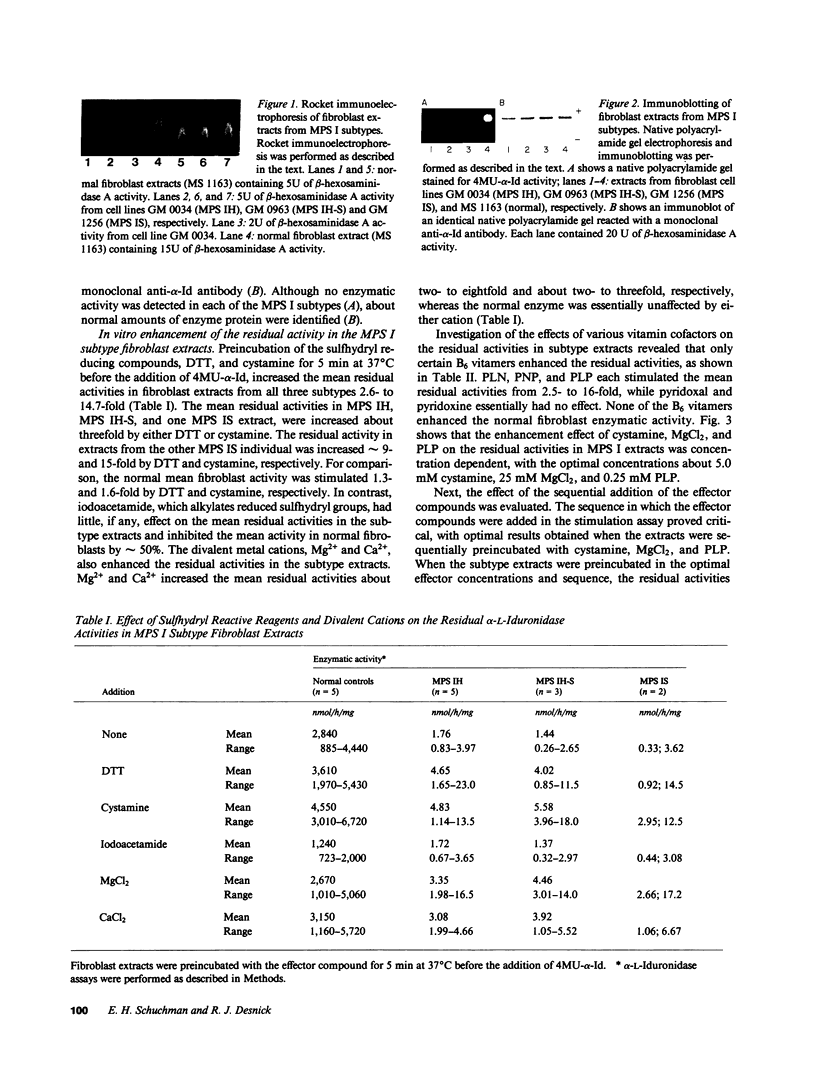
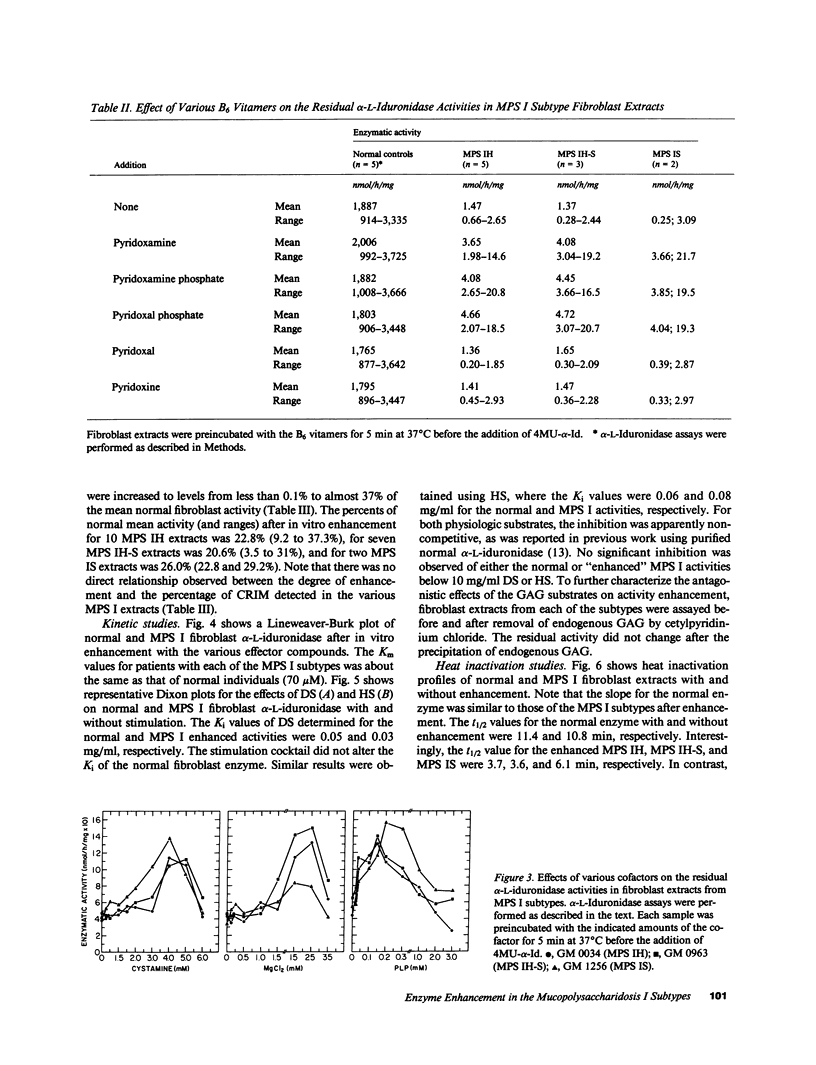
The enzymatic and immunologic properties of the defective residual alpha-L-iduronidase activities were investigated in fibroblast extracts from the three subtypes of mucopolysaccharidosis type I, Hurler (MPS IH), Scheie (MPS IS), and Hurler-Scheie (MPS IH-S) diseases. Using 4-methylumbelliferyl-alpha-L-iduronide (4MU-alpha-Id), the activities in fibroblast extracts from all three subtypes were less than 0.1% of normal. Rocket immunoelectrophoresis with monospecific rabbit anti-human alpha-L-iduronidase polyclonal antibodies, as well as immunoblots using a monoclonal antibody, revealed the presence of cross-reactive immunologic material (CRIM) in extracts prepared from each subtype. When the samples were equalized for beta-hexosaminidase A activity, 38-105% of normal enzyme protein was detected. The sequential addition of cystamine, MgCl2 and pyridoxal phosphate increased the residual 4MU-alpha-Id activities in subtype extracts up to about 35% of normal mean fibroblast activity. Cystamine, MgCl2 or pyridoxal phosphate alone enhanced the residual activities two- to fourfold, whereas the sequential addition of all three compounds was required for maximal effect. Of the six B6 vitamers evaluated, only the negatively charged forms, pyridoxamine (PLN), pyridoxamine phosphate (PNP), and pyridoxal phosphate (PLP), stimulated the residual activities. The addition of dermatan sulfate or heparan sulfate to the subtype extracts, followed by treatment with the effector compounds, similarly inhibited both the normal and enhanced MPS I activities. Heat inactivation experiments confirmed the fact that the mutant iduronidase activity was reconstituted and that the observed increase in enzymatic activity was not an artifact of the fluorogenic assay. These results suggest that the presence of certain thiol reducing agents, divalent cations and negatively charged B6 vitamers can alter the conformation of the mutant alpha-L-iduronidase in vitro such that the hydrolysis of 4MU-alpha-Id is enhanced into the heterozygote range.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J Biol Chem. 1979 Aug 10;254(15):6924–6930. [PubMed] [Google Scholar]
- Bach G., Friedman R., Weissmann B., Neufeld E. F. The defect in the Hurler and Scheie syndromes: deficiency of -L-iduronidase. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2048–2051. doi: 10.1073/pnas.69.8.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach G., Kohn G., Lasch E. E., El Massri M., Ornoy A., Sekeles E., Legum C., Cohen M. M. A new variant of mannosidosis with increased residual enzymatic activity and mild clinical manifestation. Pediatr Res. 1978 Oct;12(10):1010–1015. doi: 10.1203/00006450-197810000-00012. [DOI] [PubMed] [Google Scholar]
- Barnard G. F., Itoh R., Hohberger L. H., Shemin D. Mechanism of porphobilinogen synthase. Possible role of essential thiol groups. J Biol Chem. 1977 Dec 25;252(24):8965–8974. [PubMed] [Google Scholar]
- Beratis N. G., LaBadie G. U., Hirschhorn K. Genetic heterogeneity in acid alpha-glucosidase deficiency. Am J Hum Genet. 1983 Jan;35(1):21–33. [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Desnick R. J. Affinity purification of alpha-galactosidase A from human spleen, placenta, and plasma with elimination of pyrogen contamination. Properties of the purified splenic enzyme compared to other forms. J Biol Chem. 1981 Feb 10;256(3):1307–1316. [PubMed] [Google Scholar]
- Burton B. K., Nadler H. L. Mannosidosis: separation and characterization of two acid alpha-mannosidase forms in mutant fibroblasts. Enzyme. 1978;23(1):29–35. doi: 10.1159/000458546. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., Saccone G. T., Hopwood J. J. Human alpha-L-iduronidase. 1. Purification, monoclonal antibody production, native and subunit molecular mass. Eur J Biochem. 1985 Oct 1;152(1):21–28. doi: 10.1111/j.1432-1033.1985.tb09158.x. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Muller V., Hopwood J. J. Human alpha-L-iduronidase. 2. Catalytic properties. Eur J Biochem. 1985 Oct 1;152(1):29–34. doi: 10.1111/j.1432-1033.1985.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Sharp H. L., Grabowski G. A., Brunning R. D., Quie P. G., Sung J. H., Gorlin R. J., Ikonne J. U. Mannosidosis: clinical, morphologic, immunologic, and biochemical studies. Pediatr Res. 1976 Dec;10(12):985–996. doi: 10.1203/00006450-197612000-00008. [DOI] [PubMed] [Google Scholar]
- Gibbs P. N., Gore M. G., Jordan P. M. Investigation of the effect of metal ions on the reactivity of thiol groups in human 5-aminolaevulinate dehydratase. Biochem J. 1985 Feb 1;225(3):573–580. doi: 10.1042/bj2250573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Walling L., Desnick R. J. Human mannosidosis: in vitro and in vivo studies of cofactor supplementation. Birth Defects Orig Artic Ser. 1980;16(1):319–334. [PubMed] [Google Scholar]
- Haskins M. E., Aguirre G. D., Jezyk P. F., Desnick R. J., Patterson D. F. The pathology of the feline model of mucopolysaccharidosis I. Am J Pathol. 1983 Jul;112(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Shapiro L. J., Neufeld E. F. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun. 1974 Mar 15;57(1):55–61. doi: 10.1016/s0006-291x(74)80356-6. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V. Biochemical discrimination of Hurler and Scheie syndromes. Clin Sci (Lond) 1979 Sep;57(3):265–272. doi: 10.1042/cs0570265. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V., Smithson A., Baggett N. A fluorometric assay using 4-methylumbelliferyl alpha-L-iduronide for the estimation of alpha-L-iduronidase activity and the detection of Hurler and Scheie syndromes. Clin Chim Acta. 1979 Mar 1;92(2):257–265. doi: 10.1016/0009-8981(79)90121-9. [DOI] [PubMed] [Google Scholar]
- Hultberg B., Masson P. K. Activation of residual acidic alpha-mannosidase activity in mannosidosis tissues by metal ions. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1473–1479. doi: 10.1016/0006-291x(75)90192-8. [DOI] [PubMed] [Google Scholar]
- Jaffe E. K., Salowe S. P., Chen N. T., DeHaven P. A. Porphobilinogen synthase modification with methylmethanethiosulfonate. A protocol for the investigation of metalloproteins. J Biol Chem. 1984 Apr 25;259(8):5032–5036. [PubMed] [Google Scholar]
- Kaibara N., Eguchi M., Shibata K., Takagishi K. Hurler-Scheie phenotype: a report of two pairs of inbred sibs. Hum Genet. 1979;53(1):37–41. doi: 10.1007/BF00289448. [DOI] [PubMed] [Google Scholar]
- Karson E. M., Neufeld E. F., Sando G. N. p-Isothiocyanatophenyl 6-phospho-alpha-D-mannopyranoside coupled to albumin. A model compound recognized by the fibroblast lysosomal enzyme uptake system. 2. Biological properties. Biochemistry. 1980 Aug 5;19(16):3856–3860. doi: 10.1021/bi00557a033. [DOI] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Hurler's syndrome, an -L-iduronidase deficiency. Biochem Biophys Res Commun. 1972 May 26;47(4):959–964. doi: 10.1016/0006-291x(72)90586-4. [DOI] [PubMed] [Google Scholar]
- McGovern M. M., Mandell N., Haskins M., Desnick R. J. Animal model studies of allelism: characterization of arylsulfatase B mutations in homoallelic and heteroallelic (genetic compound) homozygotes with feline mucopolysaccharidosis VI. Genetics. 1985 Aug;110(4):733–749. doi: 10.1093/genetics/110.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern M. M., Vine D. T., Haskins M. E., Desnick R. J. Purification and properties of feline and human arylsulfatase B isozymes. Evidence for feline homodimeric and human monomeric structures. J Biol Chem. 1982 Nov 10;257(21):12605–12610. [PubMed] [Google Scholar]
- Muller V. J., Hopwood J. J. alpha-L-Iduronidase deficiency in mucopolysaccharidosis type I against a radiolabelled sulfated disaccharide substrate derived from dermatan sulfate. Clin Genet. 1984 Nov;26(5):414–421. doi: 10.1111/j.1399-0004.1984.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Neufeld E. F. Maturation of alpha-L-iduronidase in cultured human fibroblasts. J Biol Chem. 1981 Mar 25;256(6):3044–3048. [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Miller J. Butanedione treatment reduces receptor binding of a lysosomal enzyme to cells and membranes. Biochem Biophys Res Commun. 1980 Feb 12;92(3):986–993. doi: 10.1016/0006-291x(80)90799-8. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Weissmann B., Neufeld E. F. Direct demonstration of binding of a lysosomal enzyme, alpha-L-iduronidase, to receptors on cultured fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2331–2334. doi: 10.1073/pnas.76.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman E. H., Astrin K. H., Aula P., Desnick R. J. Regional assignment of the structural gene for human alpha-L-iduronidase. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1169–1173. doi: 10.1073/pnas.81.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman E. H., Guzman N. A., Desnick R. J. Human alpha-L-iduronidase. I. Purification and properties of the high uptake (higher molecular weight) and the low uptake (processed) forms. J Biol Chem. 1984 Mar 10;259(5):3132–3140. [PubMed] [Google Scholar]
- Schuchman E. H., Guzman N. A., Takada G., Desnick R. J. Human alpha-L-iduronidase. II. Comparative biochemical and immunologic properties of the purified low and high uptake forms. Enzyme. 1984;31(3):166–175. [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Yoshida A., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. I. Purification and properties. J Biol Chem. 1974 Apr 10;249(7):2043–2048. [PubMed] [Google Scholar]
- Steiner A. W., Rome L. H. Assay and purification of a solubilized membrane receptor that binds the lysosomal enzyme alpha-L-iduronidase. Arch Biochem Biophys. 1982 Apr 1;214(2):681–687. doi: 10.1016/0003-9861(82)90074-1. [DOI] [PubMed] [Google Scholar]
- Vine D. T., McGovern M. M., Schuchman E. H., Haskins M. E., Desnick R. J. Enhancement of residual arylsulfatase B activity in feline mucopolysaccharidosis VI by thiol-induced subunit association. J Clin Invest. 1982 Feb;69(2):294–302. doi: 10.1172/JCI110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann B. Synthetic substrates for alpha-L-iduronidase. Methods Enzymol. 1978;50:141–150. doi: 10.1016/0076-6879(78)50012-8. [DOI] [PubMed] [Google Scholar]





