Abstract
Synthetic ghrelin agonists, predominantly small molecules, are being developed as prokinetic agents that may prove useful in the treatment of gastrointestinal motility disorders. Relamorelin (RM-131) is a pentapeptide synthetic ghrelin analog that activates the growth hormone secretagogue (GHS)-1a (also called the ghrelin) receptor with approximately 6-fold greater potency than natural ghrelin. The ability of relamorelin to stimulate growth hormone (GH) release is comparable to that of native ghrelin. Relamorelin has enhanced efficacy and plasma stability compared to native ghrelin. In this review, we discuss the pharmacokinetics, pharmacodynamics and potential indications for relamorelin. Relamorelin is administered subcutaneously, dosed daily or twice daily. Relamorelin is being studied for the treatment of patients with gastrointestinal motility disorders. Phase IIA pharmacodynamic studies have demonstrated acceleration of gastric emptying in patients with type 1 diabetes mellitus (T1DM) and type 2 DM (T2DM) and upper gastrointestinal symptoms. In a phase IIA study in patients with diabetic gastroparesis, relamorelin accelerated gastric emptying and significantly improved vomiting frequency compared to placebo and improved other symptoms of gastroparesis in a pre-specified subgroup of patients with vomiting at baseline. In patients with chronic idiopathic constipation with defined transit profile at baseline, relamorelin relieved constipation and accelerated colonic transit compared to placebo. These characteristics suggest that this new ghrelin analog shows great promise to relieve patients with upper or lower gastrointestinal motility disorders.
Keywords: RM-131, gastroparesis, constipation
BACKGROUND
Currently Approved Medications for GI Motility Disorders
Disorders of gastrointestinal (GI) motility such as gastroparesis, post-operative ileus (POI), and chronic constipation are associated with significant morbidity. Development of effective pharmacologic therapies for such disorders is desirable, particularly for gastroparesis which represents a significant unmet medical need.
The only currently marketed medication for gastroparesis in the United States is metoclopramide (1), and its use is limited by a black box warning from the Food and Drug Administration (FDA) recommending use for less than three months for a chronic disease that may last several years. In other countries, domperidone is approved; its efficacy appears similar to that of metoclopramide (2) and, until relatively recently, it was considered safer than metoclopramide. However, a recent communication from the European Medicine Agency alerted prescribers and the public to the potential cardiac adverse effects (including prolongation of the QTc interval and cardiac arrhythmias) and, even, mortality (www.ema.europa.eu, accessed May 30, 2014) (3).
There is only one drug approved for POI; that is alvimopan which is administered at a dose of 12mg, 30 minutes to 5 hours prior to surgery, followed by 12mg twice daily, beginning the day after surgery until the patient is discharged from hospital, for a maximum of 7 days. However, it also has a black box warning because of the potential risk of myocardial infarction (www.fda.gov, accessed May 30, 2014) (4). Alvimopan is available only through the Entereg Access Support and Education (E.A.S.E.) Program that restricts use to enrolled hospitals. This program was required by the FDA because of the potential risk of myocardial infarction with long-term use of alvimopan, 0.5mg b.i.d, and duration of at least one month.
There are several medications available for the treatment of chronic idiopathic constipation (5) including medications available over the counter that accelerate colonic transit such as bisacodyl (6) and prescribed drugs such as the chloride secretagogues, linaclotide and lubiprostone (in the United States), and the 5-HT4 receptor agonist, prucalopride (in virtually all countries other than the United States). None of these medications has been approved for the treatment of constipation in patients with neurological diseases such as paraplegia or Parkinsonism or in more severe forms of idiopathic constipation such as slow transit constipation or colonic inertia.
GHRELIN
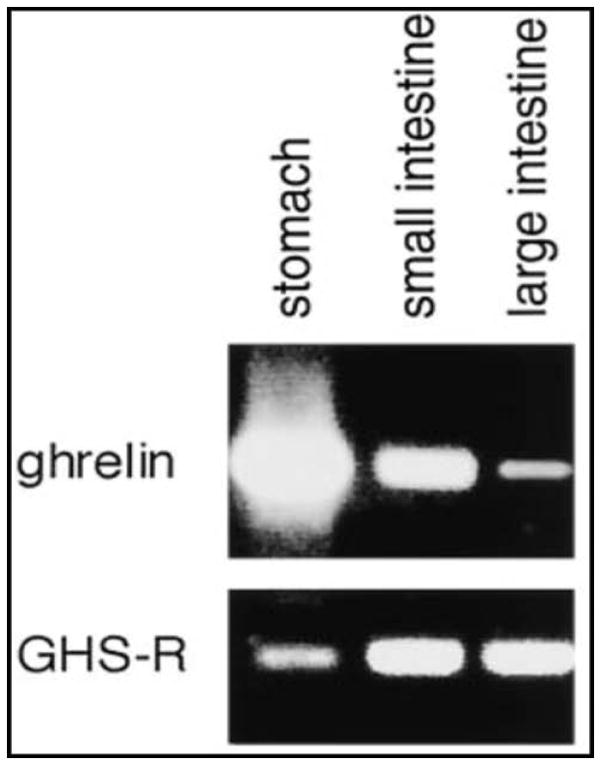
Ghrelin is a 28-amino acid peptide which was first isolated from rat stomach. Ghrelin is identified in most gastrointestinal organs, predominantly in the stomach (Table 1 and Figure 1, upper panel). It is the natural ligand for the ghrelin or growth hormone secretagogue (GHS-1a receptor) and a potential target for treatment of clinical conditions associated with impaired gastric motility and energy balance (7). Administration of ghrelin promotes gastric motility in mice, rats, dogs and humans (7–9). However, the short half-life and plasma instability of native ghrelin limit its utility as a potential treatment for GI motility disorders. Synthetic ghrelin agonists, predominantly small molecules, are being developed as prokinetic agents that may prove useful in the treatment of GI motility disorders such as POI and gastroparesis (10). Actions and therapeutic pathways of ghrelin for upper gastrointestinal disorders have been reviewed elsewhere (11). In addition to ghrelin receptor expression in the central nervous system (CNS), there is growing evidence for ghrelin receptor expression throughout the gastrointestinal tract. Thus, whereas the protein expression of ghrelin is far greater in the stomach than the small intestine and colon (12), the expression of ghrelin receptors is higher in the intestines than in the stomach, and these data are confirmed by the demonstration of ghrelin receptors in mucosa and myenteric plexus neurons of rodent colon (9) (Figure 1, lower panel and Figure 2). These observations support the potential for effects of ghrelin receptor agonists on colonic motor function. However, the reliability of the ghrelin receptor anti-GHSR1a antibodies has been recently questioned (13) and further studies are needed to characterize the presence and role of the ghrelin receptor in the colon.
Table 1. Tissue Content of Immunoreactive Ghrelin.
(reproduced from Date Y, et al. Endocrinology 2000)
| Tissue | RIA for C-terminus (pmol/g wet wt) | RIA for N-terminus (pmol/g wet wt) |
|---|---|---|
| Stomach | ||
| Fundus | 4633.5 ± 440.1 | 1845.6 ± 290.0 |
| Pylorus | 120.7 ± 19.4 | 63.6 ± 21.1 |
| Duodenum | 262.5 ± 13.3 | 50.9 ± 7.2 |
| Jejunum | 102.3 ± 13.2 | 44.6 ± 8.4 |
| Ileum | 27.7 ± 1.1 | 1.5 ± 0.1 |
| Colon | 73.4 ± 19.2 | 11.7 ± 4.2 |
Values are the mean ± SEM (n = 6).
Figure 1.
Ghrelin and ghrelin receptor expression in gastrointestinal tract. Representative electrophoretic analysis patterns of the RT-PCR products of ghrelin and GHS-R mRNAs in rats. Upper panel: Ghrelin transcripts in the stomach and small and large intestines. Lower panel: GHS-R transcripts in those same organs. Reproduced with permission from Date, Kojima, et al. 2000.
Figure 2.
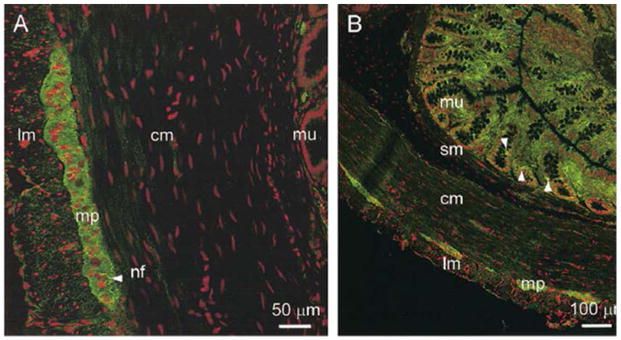
GHS-R-immunofluorescence (green) in transverse frozen sections of the rat distal colon observed in (A) neuronal cell bodies and nerve fibres of the myenteric plexus, and (B) cells scattered in the mucosa, exhibiting an entero-endocrine and/or mast cell-like distribution, size and density. Reproduced with permission from Dass NB, et al. Neuroscience 2003.
THE GHRELIN AGONIST, RELAMORELIN
Chemistry
Relamorelin is a pentapeptide synthetic ghrelin analog with a molecular weight of 791 daltons (free base) and similar characteristics to native ghrelin. However, it has improved stability and longer plasma circulating half-life. The structure of relamorelin is not in the public domain; the holder of rights to relamorelin, Rhythm Pharmaceuticals has revealed that it is a synthetic pentapeptide.
Preclinical Pharmacology
In vitro
Relamorelin binds to the growth hormone secretagogue (GHS)-1a (or ghrelin) receptor with approximately 3-fold greater potency than natural ghrelin (Ki of relamorelin 0.42±0.06nM; Ki of h-ghrelin 1.22±0.17nM) studied in CHO-K1 cells, expressing the human recombinant GHS1a receptor. Relamorelin is also ~ 6-fold more potent than human ghrelin (h-ghrelin) in activating the ghrelin receptor and inducing downstream intracellular mediators, specifically intracellular calcium (EC50 of relamorelin 0.71±0.09nM; EC50 of h-ghrelin 4.2±1.2nM) determined in CHO-K1 cells, expressing the hGHS1a (14).
In vivo studies in animal models
Growth hormone release
The ability of relamorelin to stimulate growth hormone (GH) release in conscious freely moving rats is comparable to that of native ghrelin, with average peak GH concentrations of ~3000ng/mL in response to i.v. administration of relamorelin and h-ghrelin at the same dose of 50nmol/kg (data on file, Rhythm Pharmaceuticals). Relamorelin effects were noted in isolated smooth muscle from the human stomach and in circular smooth muscle from mouse and human colon. Hence, while GHSR1a has not been molecularly identified in the GI tract (15, 16), there is pharmacological evidence for expression of the GHSR1a in the GI tract (17).
In vivo studies in rats and dogs confirmed the in vitro studies that relamorelin increases GH levels similar to native h-ghrelin, while repeated dosing attenuated the effect on GH release (18). This attenuation is a potential advantage in long-term administration of the agent, as it would be expected to reduce any risks associated with chronically elevated GH levels.
Postoperative ileus
Relamorelin reversed laparotomy-induced POI in rats and was 15-fold more potent than native ghrelin. In normal Sprague-Dawley rats with more severe ileus induced by the combined effects of laparotomy and morphine administration, relamorelin was potentially up to 300-fold more potent than natural ghrelin in reversing POI, and significantly reversed the delay in intestinal transit time (18).
Morphine-induced gastroparesis and small intestine ileus
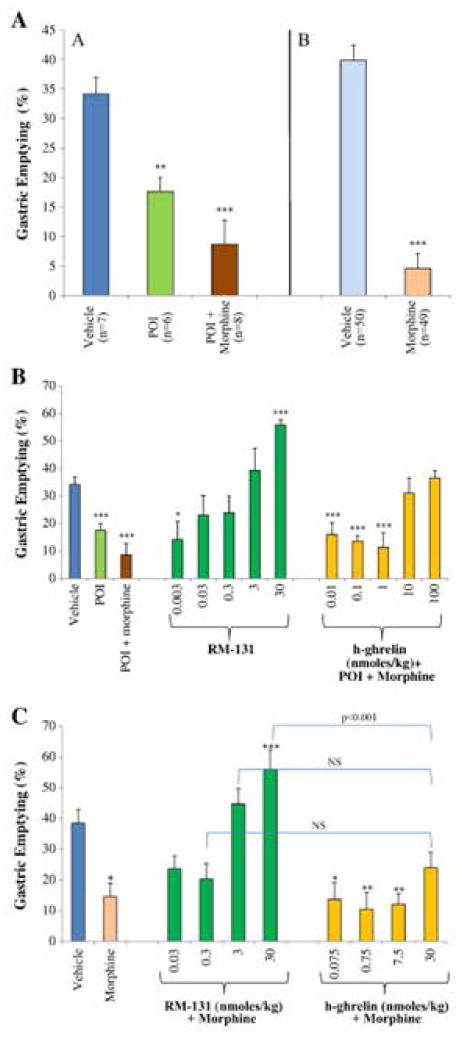
Relamorelin reversed morphine-induced gastroparesis in normal Sprague-Dawley rats. Relamorelin was about 100-fold more potent than h-ghrelin in the morphine-induced delayed gastric emptying (Figure 3) (18).
Figure 3.
Percent of gastric emptying (mean±SEM) at baseline and in different rat gastric dysmotility models: POI, POI plus morphine, or morphine-only model (Panel A), and the morphine-only model of delayed gastric emptying (Panels B and C). Animals received the phenol red-marked meal by esophageal gavage. Vehicle is 0.9% NaCl. Data show mean ± standard error of the mean (SEM). Percentage of phenol-red gastric emptying rate (% GE) determined. Groups of rats were treated each as indicated and dosed with RM-131 or h-ghrelin. Reproduced with permission from Van der Ploeg, Laken, et al. 2014.
Food intake and weight gain
Relamorelin increased food intake and induced weight gain in normal C57Bl6/J mice and Sprague-Dawley rats in a dose-dependent manner, and it induced a balanced increase in both fat and lean mass (19, 20). In multiple independent studies in normal rats, relamorelin increased body weight significantly beyond levels achieved with native ghrelin (20). Additionally, relamorelin increased food intake and body weight in animal models of cachexia (21–23), and similarly in Beagle dogs, it induced a significant, dose-dependent increase of body weight and food consumption, with no compromise in glycemic control (data on file, Rhythm Pharmaceuticals).
Anti-inflammatory activity
Relamorelin inhibited endotoxin-induced tumor necrosis factor (TNF)-α release in vivo as well as TNF-α-induced lipolysis in vitro. In cachexia models, relamorelin reduced the elevated levels of interleukin-1, interleukin-6, interleukin-12, and TNF-α to control levels (21). In a mouse model of inflammatory bowel disease induced by intra-rectal administration of trinitrobenzene sulfonic acid (TNBS), relamorelin improved survival, reduced signs of macroscopic colonic inflammation, and reversed weight loss when compared to controls (18).
Comparison of relamorelin to ghrelin and other ghrelin agonists
Relamorelin was compared to other investigational ghrelin agonists, anamorelin, ibutamoren and ipamorelin, in the rat POI plus morphine model. The results showed relamorelin had a great effect on gastric emptying when compared to each of the other agents. In this assay, relamorelin was ~100-fold more potent than native ghrelin. In the rat morphine model of gastric dysmotility, relamorelin was 600- to 1800-fold more potent than TZP-101 and TZP-102, two other ghrelin receptor agonists (18).
Preclinical Toxicology
Toxicological profiles of relamorelin have been evaluated in studies using repeated dose subcutaneous (s.c.) injection of up to 13 weeks in rats and monkeys. Overall, the 13-week repeat-dose toxicological studies provide very large safety margins for clinical investigation. There are margins of >100-fold based on dose (mg/m2; assuming a 70kg human), > 330-fold based on Cmax exposures, and >760-fold based on AUC (data on file, Rhythm Pharmaceuticals).
Pharmacokinetics and Metabolism
Relamorelin is administered subcutaneously and is considered to be metabolically stable, as no metabolites have been detected in vitro in any of the species tested. The mean percentage binding in vitro of 14C-relamorelin to serum proteins ranged from 83% to 96% with no substantial differences in rat, dog, or human sera.
In humans, across all dosing groups studied as well as after single or multiple administrations for 10 days, the median time to peak plasma concentration (Tmax) was 0.74 hour (minimum and maximum 0.27 and 1.02 hour, respectively). The relamorelin Cmax increased proportionately with dose across all doses, with Cmax of 74.8±15.3ng/mL at the highest dose (2400μg) tested. The plasma relamorelin AUC was proportional to dose across all doses. In a single-ascending dose study, relamorelin was rapidly absorbed and appeared to exhibit two-compartment pharmacokinetics. Across all dose groups, the average relamorelin half-life was ~4.5 hours in the single ascending dose study, but measurements were limited at later time points; a relatively long mean terminal half-life of ~19 hours was observed at the 2400μg dose, which was the only dose 24 hours post-administration that was associated with blood levels above the lower limit of quantification (LLOQ; 0.1 ng/mL). There was no apparent accumulation of relamorelin after repeated administration for 10 days.
The pharmacokinetics profile of RM-131 was similar after morning and evening administration of 5 and 10μg, b.i.d, with no detectable drug levels observed in the morning following evening administration of 10μg.
The pharmacokinetics of relamorelin appears similar in healthy volunteers and patients with diabetes mellitus. Across all dose groups, no metabolism has been detected in human microsomes and hepatocytes, and approximately 8% of the dose was excreted in urine.
Safety and Tolerability in Humans
Relamorelin has been evaluated in a single-ascending dose clinical study, over a dose range of 3 to 2400μg relamorelin or placebo in 36 healthy male volunteers, of whom 27 received relamorelin and 9 received placebo. Assessments of laboratory parameters, ECGs, adverse event occurrences, vital signs and injection site evaluations suggest that relamorelin was well tolerated at all dose levels. There were no study discontinuations due to adverse events, and there were no serious adverse events or deaths.
Relamorelin has also been evaluated in a 14-day, multiple-ascending dose study in 40 healthy male and female adults, of whom 32 received relamorelin and 8 received placebo. Each participant received a constant total daily dose of relamorelin (subcutaneous doses of 10, 20, 80, or 300μg). Relamorelin was generally well tolerated at doses up to 300μg per day by evaluation of laboratory parameters, ECGs, adverse event occurrences, vital signs and injection sites.
To date, over 240 persons have been exposed to relamorelin at doses ranging from 3 to 2400μg in seven clinical studies. Of these, 59 were healthy volunteers; 27 received a single relamorelin dose and 32 received repeated doses over 14 days. In addition, approximately 210 patients with type 1 or type 2 diabetes mellitus and gastroparesis, and 24 with functional constipation have received multiple doses of relamorelin. In these different groups, relamorelin was consistently well-tolerated.
Drug Interactions
The IC50s for inhibition of CYP3A4/5, CYP2A6, CYP2C8, CYP2C19, and CYP2D6 were all >15μM. Therefore, the potential for relamorelin to inhibit the metabolism of concomitantly administered drugs is weak, considering that the highest dose administered to humans (300μg/day, Study RM-131-002) only achieved a mean Cmax of 13ng/mL (approximately 0.02μM).
Pharmacodynamic Studies in Humans
Pharmacodynamic evaluations of relamorelin demonstrated evidence for robust biological activity, including increased GH levels and acceleration of gastric emptying by gastric emptying breath test following administration of RM-131. In a double-blind, placebo-controlled, single-ascending dose study, doses of 3 to 2400μg relamorelin were administered by subcutaneous injection once daily in nine separate cohorts of four healthy volunteers (3 active/1 placebo) and gastric emptying was assessed by breath test. In addition, relamorelin was also evaluated in a multiple-ascending dose study at 10 to 300μg subcutaneously once or twice daily for 14 days in four separate cohorts (8 active/2 placebo). Accelerated gastric emptying was demonstrated after single and multiple doses of ≥ 10μg relamorelin (35 to 55% change from baseline; p<0.05). Gastric emptying results on days 1 and 7 were similar, with a comparable change in gastric emptying following daily doses of 10, 20, 80 and 300μg relamorelin (24)].
EFFECTS OF RELAMORELIN ON GASTRIC EMPTYING IN GASTROPARESIS
Phase IB Studies in Patients with Diabetes Mellitus and Prior Documentation of Delayed Gastric Emptying
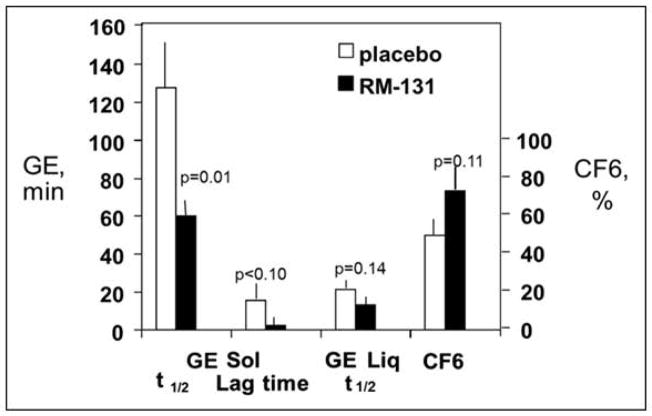
Results from two small, phase IB, placebo-controlled, single-dose (100μg subcutaneously), 2-period, cross-over studies in patients with type 2 (25) and type 1 (26) diabetes mellitus and prior documentation of delayed gastric emptying indicated that relamorelin was generally well tolerated. Relamorelin significantly decreased gastric retention of solids at 1 and 2 hours; the solid gastric emptying T1/2 was faster in patients with type 1 diabetes mellitus, with a median difference of −33.9 minutes (equivalent to −54.7% decrease) (26). Analysis of primary efficacy data showed relamorelin was associated with significant acceleration of T1/2 of gastric emptying of solids assessed by scintigraphy (Figure 4) (25). In patients with type 1I diabetes mellitus, there were also significant differences in the symptoms of gastroparesis during treatment with relamorelin compared to placebo. Thus, the total gastroparesis cardinal symptom index-daily diary (GCSI-DD) scores (27, 28) were 0.79 (IQR, 0.75, 2.08) on placebo and 0.17 (IQR, 0.00, 0.67; P=0.026) on relamorelin; similarly, a composite of nausea, vomiting, fullness and epigastric pain scores were lower on relamorelin (P=0.041) compared to placebo (26).
Figure 4.
Effects of RM-131 in type 2 diabetic gastroparesis patients with delayed gastric emptying. Reproduced with permission from Shin, Camilleri, et al. Clin Gastroenterol Hepatol 2013.
Phase 2 Clinical Trial of RM-131 in Diabetic Gastroparesis
A randomized, double-blind, placebo-controlled, adaptive design, parallel-group, 28-day study was conducted in patients with diabetic gastroparesis. The study design consisted of an initial 1-week, single-blind, placebo run-in, followed by randomization in double-blind fashion to s.c. placebo b.i.d. or relamorelin, 10μg s.c. b.i.d. (before breakfast and evening meal), or relamorelin, 10μg s.c. q.d. (placebo before breakfast and relamorelin before evening meal). For eligibility, all eligible patients had: baseline gastric emptying T1/2 >79 minutes by 13-C spirulina breath test, Gastroparesis Cardinal Symptom Index (GCSI) score ≥ 2.6, and a history of nausea and/or vomiting ≥ 1/week during the 2 weeks prior to screening. Compared to baseline, relamorelin, 10μg b.i.d., resulted in significant acceleration of gastric emptying (p<0.03). There were also significant improvements in vomiting endpoints on relamorelin treatment compared to placebo; these effects were most impressive in the ~60% patients who had vomiting during the baseline period. In the subgroup of patients with vomiting at baseline, other symptoms of gastroparesis were also significantly improved. Overall, there were no safety concerns, few adverse events, and no significant effects on body weight (29).
EFFECTS OF RELAMORELIN ON COLONIC TRANSIT IN CHRONIC CONSTIPATION
Ghrelin receptors are identified throughout the gastrointestinal tract, including the colon (12); as indicated above, the expression of ghrelin receptors in rodent intestine is actually higher in small and large intestine than in the stomach. Relamorelin hyperpolarized resting membrane potential of human colon circular smooth muscle cells in vitro (17), suggesting that it has also functional effects on colonic motility. In an in vivo model in conscious mice, with a miniaturized pressure transducer catheter introduced into the colon ~2.5cm proximal to the anus, i.p. relamorelin reduced phasic contractile activity in the colon. Others have also shown that i.t. ghrelin induced strong propulsive contractions in the distal colon. These effects are thought to be mainly mediated by activating the defecation center in the L1-S3 lumbo-sacral region (30, 31). Thus, the site of stimulation of colonic transit appears to be peripheral. Shimizu et al. showed that acyl-ghrelin stimulated rat colonic contractility when given intravenously or intrathecally, but not when given into the 4th ventricle. Additionally, similar delivery methods had no effect on small bowel motility, which would be a manifestation of vagal stimulation in the brainstem. They concluded that ghrelin activates pelvic nerves at the sacral defecation center, innervating the enteric neurons in the distal colon (31). On the contrary, other authors have shown that ghrelin has no effect on contractility in the rat stomach, distal colon, human ascending and human sigmoid colon (32, 33). It has been suggested, from rat studies, that GHSR1a agonists affect colonic motility through actions in the CNS (34). However, in preclinical studies in rats, relamorelin had a low penetration in the CNS, showing that availability of relamorelin in the brain of rats is 0.015% at 5mg/kg (data on file, Rhythm Pharmaceuticals). It is therefore possible that relamorelin acts at a different receptor to those receptors (including GHSR1a) that are activated by ghrelin. Hence, further studies are needed to understand the mechanism and site of action of relamorelin.
A preliminary report documented the propulsive effects of relamorelin in humans with chronic constipation. In a single-center, randomized, double-blind, placebo-controlled, parallel-group study of a single daily dose of 100μg, s.c. relamorelin for 14 days, there was significant improvement in bowel functions, relieving constipation and significant acceleration of gastric emptying T1/2, colonic filling at 6 hours (surrogate for small bowel transit), and overall colonic transit at 32 and 48 hours. Hunger or increased appetite were reported more frequently in patients who received relamorelin compared to placebo (p=0.012) (35).
Safety Profile in Humans
The pilot studies in patients with type 1 and type 2 diabetes mellitus indicated that 100μg relamorelin was well tolerated with no serious adverse events, deaths, or adverse events leading to withdrawal. The most common adverse events reported in these single-dose, pilot studies were hyperhidrosis and dizziness; fatigue and abdominal pain/cramping; and decreased blood pressure, hunger, feeling cold, and muscular weakness (25).
In the larger study in over 200 patients with diabetic gastroparesis, the safety and tolerability profiles of relamorelin were generally good, the number of treatment-emergent adverse effects were low, and there were no imbalances or concerns identified by physical examinations, ECGs, vital signs, and safety laboratory tests including liver function tests. In addition, there were no clinically relevant injection site reactions or changes in weight or fasting blood glucose (29). In a crossover, placebo-controlled study in 6 healthy volunteers, a ghrelin agonist produced a decrease in insulin sensitivity (which might theoretically represent a diabetogenic effect) and stimulated lipolysis (36).
The long-term adverse effects and safety of relamorelin are under evaluation. A clinical review of ghrelin administration for multiple indications other than gastroparesis or constipation showed that the main potential side effect of ghrelin is increased appetite (37). Ghrelin plays a key role in stimulation of appetite in healthy individuals by stimulating the appetite centers in the hypothalamus, including the agouti-related peptide and neuropeptide Y receptors in the arcuate nucleus of the hypothalamus (reviewed in (38). Additionally, ghrelin stimulates the secretion of orexins, growth hormone, insulin-growth factor 1, and insulin, which have orexigenic properties (11). Conversely, increased appetite may be a desirable effect in certain populations such as cachexia due to cancer (39), chronic obstructive pulmonary disease (40) or advanced heart disease (41) There is a theoretical risk that ghrelin may be involved in autocrine and paracrine processes resulting in cancer progression, including cell proliferation, cell migration and apoptosis (42). Currently, it is unclear whether ghrelin induces cell proliferation or inhibits cell proliferation, apoptosis and angiogenesis (43). It is also conceivable that the anti-inflammatory properties of ghrelin may have a protective role for some cancers (44). In the published human studies to date, there is no association of exogenous ghrelin or ghrelin agonist administration with cancer progression, and this includes patients who received these medications for cancer-induced cachexia (37). The potential effects attributable to stimulation of ghrelin receptors with relamorelin require more thorough evaluation with long-term administration.
CONCLUSIONS
Relamorelin is a pentapeptide synthetic ghrelin analog that is well tolerated and accelerates gastric emptying, small bowel transit and colonic transit in humans with gastroparesis and chronic constipation. These promising data and the safety profile of relamorelin suggest that further studies and development of this medication are warranted. In addition, given the pharmacodynamics data observed to date, relamorelin should be explored for the treatment of organic, neurological constipation, in addition to its effects in functional or chronic idiopathic constipation.
Acknowledgments
We are grateful to Rhythm Pharmaceuticals who own Relamorelin for allowing us to refer to their in-house data as Data on file, Rhythm Pharmaceuticals.
Funding: Dr. Camilleri is supported by grants R01-DK92179 and R01-DK67071 from National Institutes of Health.
Footnotes
Disclosures: The authors have conducted research funded by Rhythm Pharmaceuticals; Dr. Camilleri serves on the advisory board of Rhythm Pharmaceuticals with payment for his services to his employer, Mayo Clinic.
References
- 1.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L American College of G. Clinical guideline: management of gastroparesis. The American journal of gastroenterology. 2012;108:18. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson D, Abell T, Rothstein R, Koch K, Barnett J. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. The American journal of gastroenterology. 1999;94:1230–1234. doi: 10.1111/j.1572-0241.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 3.European Medicine Agency. 2014 [Google Scholar]
- 4.FDA. 2014 [Google Scholar]
- 5.Camilleri M. Pharmacology of the new treatments for lower gastrointestinal motility disorders and irritable bowel syndrome. Clinical pharmacology and therapeutics. 2011;91:44–59. doi: 10.1038/clpt.2011.261. [DOI] [PubMed] [Google Scholar]
- 6.Manabe N, Cremonini F, Camilleri M, Sandborn WJ, Burton DD. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Alimentary pharmacology & therapeutics. 2009;30:930–936. doi: 10.1111/j.1365-2036.2009.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine reviews. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 8.Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. American journal of physiology. Gastrointestinal and liver physiology. 2002;282:G948–952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 9.Dass NB, Munonyara M, Bassil AK, Hervieu GJ, Osbourne S, Corcoran S, Morgan M, Sanger GJ. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120 doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Alimentary pharmacology & therapeutics. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nature reviews. Gastroenterology & hepatology. 2009;6:343–352. doi: 10.1038/nrgastro.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal M, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 13.Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perello M, Andrews ZB, Zigman JM. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. The Journal of comparative neurology. 2014;522:3644–3666. doi: 10.1002/cne.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snider RM, Forray C, Pfenning M, Richelson E. Neurotensin stimulates inositol phospholipid metabolism and calcium mobilization in murine neuroblastoma clone N1E-115. Journal of neurochemistry. 1986;47:1214–1218. doi: 10.1111/j.1471-4159.1986.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 15.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. The Journal of clinical endocrinology and metabolism. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 16.Ueberberg B, Unger N, Saeger W, Mann K, Petersenn S. Expression of ghrelin and its receptor in human tissues. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2009;41:814–821. doi: 10.1055/s-0029-1233462. [DOI] [PubMed] [Google Scholar]
- 17.Sha L, Farrugia G, Van der Ploeg LHT, JHS Effect of RM-131 on circular smooth muscle cells in human and mouse colon and on colonic intraluminal pressure in conscious mice. Gastroenterology. 2014;146(Suppl 1):S363. [Google Scholar]
- 18.Van der Ploeg L, Laken H, Sharma S, Datta R, Halem H, Dong J, Touvay C, Teillot M, Noonan P, Tartaglia L, et al. Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131. Life Sciences. 2014;109:20–29. doi: 10.1016/j.lfs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Hassouna R, Labarthe A, Zizzari P, Videau C, Culler M, Epelbaum J, Tolle V. Actions of Agonists and Antagonists of the ghrelin/GHS-R Pathway on GH Secretion, Appetite, and cFos Activity. Frontiers in endocrinology. 2012;4:25. doi: 10.3389/fendo.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strassburg S, Anker SD, Castaneda TR, Burget L, Perez-Tilve D, Pfluger PT, Nogueiras R, Halem H, Dong JZ, Culler MD, et al. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. American journal of physiology. Endocrinology and metabolism. 2008;295:84. doi: 10.1152/ajpendo.00040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deboer MD, Zhu X, Levasseur PR, Inui A, Hu Z, Han G, Mitch WE, Taylor JE, Halem HA, Dong JZ, et al. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149:827–835. doi: 10.1210/en.2007-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palus S, von Haehling S, Doehner W, Datta R, Zhang J, Dong JZ, Culler MD, Anker SD, Springer J. Effect of application route of the ghrelin analog BIM-28131 (RM-131) on body weight and body composition in a rat heart failure model. International journal of cardiology. 2013;168:2369–2374. doi: 10.1016/j.ijcard.2013.01.263. [DOI] [PubMed] [Google Scholar]
- 23.DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148:3004–3012. doi: 10.1210/en.2007-0016. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan L, White J, Spence S, Kennedy S, Stoner E, Noonan P, Laken H, KG RM-131, a Novel Ghrelin Analog, Demonstrates Potent Prokinetic Activity in Phase 1 Single- and Multiple-dose Studies in Healthy Volunteers. Am J Gastro. 2012;107:S706–7. [Google Scholar]
- 25.Shin A, Camilleri M, Busciglio I, Burton D, Stoner E, Noonan P, Gottesdiener K, Smith SA, Vella A, Zinsmeister AR. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin A, Camilleri M, Busciglio I, Burton D, Smith SA, Vella A, Ryks M, Rhoten D, Zinsmeister AR. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:1453–1459. e1454. doi: 10.1016/j.cgh.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revicki DA, Camilleri M, Kuo B, Norton NJ, Murray L, Palsgrove A, Parkman HP. Development and content validity of a gastroparesis cardinal symptom index daily diary. Alimentary pharmacology & therapeutics. 2009;30:670–680. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 28.Revicki DA, Camilleri M, Kuo B, Szarka LA, McCormack J, Parkman HP. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:456–463. e215–456. doi: 10.1111/j.1365-2982.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 29.Lembo A, Camilleri M, McCallum R, Sastre R, Breton C, Spence S, White J, Gottesdiener K, Stoner E. A phase 2, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of RM-131 in patients with diabetic gastroparesis. Gastroenterology. 2014;146(Suppl 1) [Google Scholar]
- 30.Avau B, Carbone F, Tack J, Depoortere I. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25:720–732. doi: 10.1111/nmo.12193. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu Y, Chang E, Shafton A, Ferens D, Sanger G, Witherington J, Furness J. Evidence that stimulation of ghrelin receptors in the spinal cord initiates propulsive activity in the colon of the rat. The Journal of physiology. 2006;576:329–338. doi: 10.1113/jphysiol.2006.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dass NB, Munonyara M, Bassil AK, Hervieu GJ, Osbourne S, Corcoran S, Morgan M, Sanger GJ. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–453. doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- 33.Broad J, Goralczyk A, Mannur K, Dukes GE, Sanger GJ. Drugs acting at 5-HT4, D2, motilin, and ghrelin receptors differ markedly in how they affect neuromuscular functions in human isolated stomach. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:851–861. doi: 10.1111/nmo.12338. [DOI] [PubMed] [Google Scholar]
- 34.Pustovit RV, Callaghan B, Kosari S, Rivera LR, Thomas H, Brock JA, Furness JB. The mechanism of enhanced defecation caused by the ghrelin receptor agonist, ulimorelin. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:264–271. doi: 10.1111/nmo.12259. [DOI] [PubMed] [Google Scholar]
- 35.Acosta A, Kolar G, Iturrino J, Szarka L, Boldingh A, Burton D, Ryks M, Rhoten D, Zinsmeister AR, Camilleri M. A phase II, single-center, randomized, double-blind, placebo-controlled, multiple-dose, 2-period, parallel-group study to evaluate the efficacy, safety, and pharmacodynamics of RM 131 administered to patients with chronic constipation. Gastroenterology. 2014;146:S364. [Google Scholar]
- 36.Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57:3205–3210. doi: 10.2337/db08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garin MC, Burns CM, Kaul S, Cappola AR. Clinical review: The human experience with ghrelin administration. The Journal of clinical endocrinology and metabolism. 2013;98:1826–1837. doi: 10.1210/jc.2012-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014;63:687–695. doi: 10.1136/gutjnl-2013-306235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molfino A, Formiconi A, Fanelli FR, Muscaritoli M. Ghrelin: from discovery to cancer cachexia therapy. Current opinion in clinical nutrition and metabolic care. 2014 doi: 10.1097/MCO.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 40.Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, Kangawa K. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- 41.Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 42.Chopin L, Walpole C, Seim I, Cunningham P, Murray R, Whiteside E, Josh P, Herington A. Ghrelin and cancer. Mol Cell Endocrinol. 2011;340:65–69. doi: 10.1016/j.mce.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 43.Chopin LK, Seim I, Walpole CM, Herington AC. The ghrelin axis--does it have an appetite for cancer progression? Endocr Rev. 2012;33:849–891. doi: 10.1210/er.2011-1007. [DOI] [PubMed] [Google Scholar]
- 44.Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011;340:44–58. doi: 10.1016/j.mce.2011.04.019. [DOI] [PubMed] [Google Scholar]