Abstract
Currently, a comprehensive assessment between mitochondrial DNA (mtDNA) content and cancer risk is lacking. We designed this meta-analysis to test the hypothesis that altered mtDNA copy number might influence genetic susceptibility to some specific types of cancer. The processes of literature search, eligibility appraisal and data retrieval were independently completed in duplicate. The mtDNA copy number which was dichotomized or classified into tertiles was compared between cancer cases and controls. Twenty-six articles with 38 study groups were analyzed among 6682 cases and 9923 controls. When dichotomizing mtDNA copy number at the median value, there was an 11% increased cancer risk for carriers of high mtDNA content (P = 0.320). By cancer type, high mtDNA content was associated with an increased risk for lymphoma (OR = 1.76; P = 0.023) but a reduced risk for skeleton cancer (OR = 0.39; P = 0.001). Carriers of the 2nd and 3rd tertiles of mtDNA copy number had an 1.74-fold (P = 0.010) and 2.07-fold (P = 0.021) increased risk of lymphoma, respectively. By contrast, there was correspondingly a 56% (P < 0.001) and 80% (P < 0.001) reduced risk of skeleton cancer. Our findings suggested that elevated mtDNA content was associated with a higher risk for lymphoma, but a lower risk for skeleton cancer.
Mitochondrial DNA (mtDNA) is an extra-chromosomal circular, double-stranded, maternally-inherited DNA; it is 16.5 kb in length and encodes for 37 genes, including 2 rRNAs, 13 mRNAs and 22 tRNAs1. Lack of protective histones and deficiency in DNA repair capacity render the mtDNA more vulnerable to mutations, and as a feedback the cell will produce multiple copies of mtDNA molecule to antagonize this damage2. Somatic mtDNA mutations are frequently observed in many sites of human cancer3,4, and it gives a reason to expect that high mtDNA copy number might be a logical biomarker implicated in the onset and evolution of carcinogenesis. For example, elevated mtDNA copy number was pre-diagnostically identified in peripheral white blood cells of healthy subjects who developed B-cell non-Hodgkin lymphomas lately5. By contrast, subsequent observations argued against this observation by showing that low mtDNA copy number appeared to be associated with an increased risk of renal cell carcinoma6,7, leading to the existence of tumor site-specific heterogeneity. Even for the same cancer type, it was not without controversy. A recent study by Hofmann et al8 reported an opposite claim for renal cell carcinoma against two aforementioned previous studies9,10. However in medical literature a comprehensive assessment between mtDNA content and cancer risk thus far is lacking. To fill this gap in knowledge, we set up a systematic meta-analysis to test the hypothesis that altered mtDNA copy number might influence genetic susceptibility to some specific types of cancer. In addition, we tried to track potential sources of heterogeneity through subgroup and meta-regression analyses.
Methods & Materials
Literature search
Potentially eligible articles were obtained through searching the MEDLINE database (http://www.ncbi.nlm.nih.gov/pubmed) by two authors (Jia Mi and Geng Tian). The last update was on January 20, 2015. This meta-analysis collected articles that were exclusively published in English medical journals and involved human subjects only. The key subjects used in literature search were (‘mitochondrial DNA’ OR ‘mtDNA’) AND (‘copy number’ OR ‘content’) AND (‘cancer’ OR ‘carcinoma’ OR ‘neoplasia’ OR ‘adenoma’ OR ‘neoplasm’ OR ‘myeloma’ OR ‘melanoma’ OR ‘lymphoma’ OR ‘leukaemia’ OR ‘leiomyoma’). We also manually scanned the reference lists of major review articles and relevant original articles to find additional citations of interest. We implemented this meta-analysis of the summarized results of individual studies in accordance with the recommended guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement11.
Eligibility
The eligibility of all retrieved articles was appraised by the same two authors (Jia Mi and Geng Tian). If an article cannot be excluded with certainty from its title and/or abstract, full text and supplementary materials when necessary were read to fully interrogate its eligibility, and all uncertainties over eligibility were solved by a discussion in 100% agreement. In case of the same study group with the same clinical endpoint incorporated in more than one article, the article with the largest sample size took precedence.
Inclusion criteria
The included articles must meet the following necessary criteria concurrently, that is, all types of cancer except for skin cancer constituted the clinical end points (dependent variables); only retrospective or nested case-control studies were considered; distributions of mtDNA copy number should be dichotomized or categorized into tertiles, quartiles, quintiles or more quantiles in the controls, and provided in both cancer cases and controls.
Exclusion criteria
As most abstracts did not specifically address the topic of our analysis, they were excluded from our full-text review. Articles that provided only mean numbers of mtDNA copy or examined the association of mtDNA content with cancer severity or progression were excluded. In addition, case reports or series, editorial, reviews and non-English articles were also excluded from this meta-analysis.
Data retrieval
The relevant data from each qualified article were independently retrieved by two authors (Jia Mi and Geng Tian) according to a self-designed data collection form in Excel, including the first author, year of publication, ancestry of study subjects, cancer type, study design, source of controls, sample size of case and control groups, the distributions of mtDNA copy numbers between the two groups, the examined genes in both mtDNA and nuclear DNA and the assay method, and mean levels of age, male gender, body mass index, smoking and drinking in both groups. If an article provided mtDNA copy number between cancer cases and controls by gender, we retrieved them separately as independent study groups in the final analysis.
Statistics
The association between mtDNA content and cancer risk was investigated in a random-effects model using DerSimonian and Laird method12, and risk estimates were expressed as odds ratio (OR) and 95% confidence interval (95% CI). Heterogeneity arising from pooled individual studies was examined using the I2 statistic. This statistic is defined as the percentage of the observed between-study variability that is due to heterogeneity rather than chance, and it ranges from 0% to 100%. In this meta-analysis, we specified the I2 statistic of more than 50% as statistically significant, with higher values suggestive of the existence of heterogeneity.
We took two steps to explore the potential sources of heterogeneity. On one hand, we classified all qualified studies into two or more subgroups of homozygous host characteristics according to ancestry of study subjects (mainly Asian and White), gender (male and female), study design (prospective and retrospective case-control studies), source of controls (population-based controls and hospital-based controls) and cancer type, respectively. Only subgroups involving 2 or more groups were summarized. On the other hand, we constructed a meta-regression model by incorporating some continuous characteristics, such as age, gender (male in percentage), body mass index, smoking and drinking as independent variables.
We depicted the Begg’s funnel plot and computed the Egger regression asymmetry test to assess the probability of publication bias. The Egger’s test can identify the asymmetry of funnel plots by determining whether the intercept deviates significantly from zero in regressing the standardized effect estimates against their precision. In addition, the trim-and-fill method was also employed to estimate the number and outcomes of putatively missing studies stemming from publication bias. Significant publication bias was set at a 10% level of Egger test13. The above analyses were completed with the use of STATA software v12.0.
Results
Through layers of identification and assessment, a total of 26 articles were qualified that examined the association of altered mtDNA copy number with cancer risk5,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38. By gender, we classified these 26 articles into 38 independent study groups involving 6682 cancer cases and 9923 controls. Out of 38 study groups, 8 involved both genders, and 15 involved only males and females, respectively. By ancestry, 18 study groups were Asians, 8 whites, 1 African and 11 mixed populations. By cancer type, digestive cancer was examined in 11 study groups, respiratory cancer in 6 groups, urogenital cancer in 6 groups, head and neck cancer in 5 groups, lymphoma in 5 groups, breast cancer in 3 groups and skeleton cancer in 2 groups. By study design, 21 study groups designed prospectively, and 17 study groups retrospectively. By source of controls, 35 study groups involved controls from populations and 3 from hospitals. Baseline characteristics of all qualified studies are shown in Table 1.
Table 1. Baseline characteristics of all qualified studies in this meta-analysis.
| Author, year | Ancestry | Control source | Match |
Cancer |
Gender | Study design | Sample | mtDNA gene | Nuclear gene | |
|---|---|---|---|---|---|---|---|---|---|---|
| Detailed type | Classification | |||||||||
| Lan, 2008 | White | Population | Yes | Non-Hodgkin lymphoma | Lymphoma | Male | Prospective | PBL | ND1 | HGB |
| Xing, 2008 | White | Population | Yes | Renal cell carcinoma | Urogenital | Both | Retrospective | PBL | ND1 | HGB |
| Bonner, 2009 M | Asian | Population | Yes | Lung cancer | Respiratory | Male | Prospective | Sputum | ND1 | HGB |
| Bonner, 2009 F | Asian | Population | Yes | Lung cancer | Respiratory | Female | Prospective | Sputum | ND1 | HGB |
| Hosgood, 2010 | White | Population | Yes | Lung cancer | Respiratory | Male | Prospective | PBL | ND1 | HGB |
| Shen, 2010 | Mixed | Population | Yes | Breast cancer | Breast | Female | Retrospective | PBL | ND1 | HGB |
| Liao, 2011 | Asian | Population | Yes | Gastric cancer | Digestive | Female | Prospective | PBL | ND1 | HGB |
| Lynch, 2011 | White | Population | Yes | Pancreatic cancer | Digestive | Male | Prospective | PBL | ND1 | HGB |
| Qu, 2011 Overall | Asian | Population | Yes | Colorectal cancer | Digestive | Both | Retrospective | PBL | ND1 | HGB |
| Qu, 2011 M | Asian | Population | Yes | Colorectal cancer | Digestive | Male | Retrospective | PBL | ND1 | HGB |
| Qu, 2011 F | Asian | Population | Yes | Colorectal cancer | Digestive | Female | Retrospective | PBL | ND1 | HGB |
| Zhao, 2011 | Asian | Population | Yes | Hepatocellular carcinoma | Digestive | Both | Retrospective | PBL | ND1 | HGB |
| Purdue, 2012 | White | Population | Yes | Renal cell carcinoma | Urogenital | Both | Prospective | PBL | ND1 | HGB |
| Purdue, 2012 | African | Population | Yes | Renal cell carcinoma | Urogenital | Both | Prospective | PBL | ND1 | HGB |
| Thyagarajan, 2012 M | Asian | Population | NA | Colorectal cancer | Digestive | Male | Prospective | PBL | ND1 | 18s |
| Thyagarajan, 2012 F | Asian | Population | NA | Colorectal cancer | Digestive | Female | Prospective | PBL | ND1 | 18s |
| Mondal, 2013 | Asian | Hospital | Yes | Oral cancer | Head/neck cancer | Both | Retrospective | PBL | D-loop | GAPDH |
| Thyagarajan, 2013 | Asian | Population | Yes | Breast cancer | Breast | Female | Prospective | PBL | ND1 | 18s |
| Xie, 2013 M | Mixed | Population | Yes | Soft tissue sarcoma | Skeleton | Male | Retrospective | PBL | ND1 | HGB |
| Xie, 2013 F | Mixed | Population | Yes | Soft tissue sarcoma | Skeleton | Female | Retrospective | PBL | ND1 | HGB |
| Xu, 2013 M | Mixed | Population | Yes | Esophageal cancer | Digestive | Male | Retrospective | PBL | ND1 | HGB |
| Xu, 2013 F | Mixed | Population | Yes | Esophageal cancer | Digestive | Female | Retrospective | PBL | ND1 | HGB |
| Cheau-Feng, 2014 | Asian | Hospital | NA | Head/neck cancer | Head/neck cancer | Male | Retrospective | PBL | tRNAleu | 18s |
| Ghosh, 2014 | Asian | Hospital | Yes | Nasopharyngeal carcinoma | Head/neck cancer | Both | Retrospective | PBL | D-loop | GAPDH |
| Hofmann, 2014 M | Mixed | Population | Yes | Renal cell carcinoma | Urogenital | Male | Prospective | PBL | ND1 | HGB |
| Hofmann, 2014 F | Mixed | Population | Yes | Renal cell carcinoma | Urogenital | Female | Prospective | PBL | ND1 | HGB |
| Hosnijeh, 2014 | White | Population | Yes | Non-Hodgkin lymphoma | Lymphoma | Both | Prospective | PBL | ND1 | HGB |
| Huang, 2014 | Asian | Population | Yes | Colorectal cancer | Digestive | Female | Prospective | PBL | ND1 | BRCA1 |
| Jiang, 2014 | Asian | Population | Yes | Breast cancer | Breast | Female | Retrospective | PBL | ND1 | HGB |
| Kim, 2014 PLCO M | Mixed | Population | Yes | Non-Hodgkin lymphoma | Lymphoma | Male | Prospective | PBL | ND1 | HGB |
| Kim, 2014 PLCO F | Mixed | Population | Yes | Non-Hodgkin lymphoma | Lymphoma | Female | Prospective | PBL | ND1 | HGB |
| Kim, 2014 ATBC | White | Population | Yes | Non-Hodgkin lymphoma | Lymphoma | Male | Prospective | PBL | ND1 | HGB |
| Kim, 2014 PLCO-M | Mixed | Population | Yes | Lung cancer | Respiratory | Male | Prospective | PBL | ND1 | HGB |
| Kim, 2014 PLCO-F | Mixed | Population | Yes | Lung cancer | Respiratory | Female | Prospective | PBL | ND1 | HGB |
| Kim, 2014 SWHS | Asian | Population | Yes | Lung cancer | Respiratory | Female | Prospective | PBL | ND1 | HGB |
| Sun, 2014 | White | Population | Yes | Gastric cancer | Digestive | Both | Retrospective | PBL | ND1 | HGB |
| Zhang, 2014 Overall | Asian | Population | Yes | Glioma | Head/neck cancer | Both | Retrospective | PBL | ND1 | HGB |
| Zhang, 2014 M | Asian | Population | Yes | Glioma | Head/neck cancer | Male | Retrospective | PBL | ND1 | HGB |
| Zhang, 2014 F | Asian | Population | Yes | Glioma | Head/neck cancer | Female | Retrospective | PBL | ND1 | HGB |
| Zhou, 2014 | Asian | Population | Yes | Prostate cancer | Urogenital | Male | Retrospective | PBL | ND1 | HGB |
Abbreviations: M, male; F, female; PBL, peripheral blood lymphocytes.
Overall, cancer cases were slightly older than controls (age: 59.50 versus 58.53 years, P = 0.024), and no significant differences were observed in gender and body mass index. Cancer cases were more likely to be smokers (53.61% versus 47.48%, P = 0.005) and drinkers (26.92% versus 21.93%, P = 0.090) than controls. Host characteristics of all study populations are shown in Table 2.
Table 2. Host characteristics of study groups in this meta-analysis.
| Author, year |
Sample size |
Age, yrs |
Male, % |
BMI, kg/m2 |
Smoking, % |
Drinking, % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| Lan, 2008 | 104 | 104 | 58.0 | 57.0 | 100.0 | 100.0 | 25.5 | 25.4 | 100.0 | 100.0 | NR | NR |
| Xing, 2008 | 260 | 281 | 59.2 | 59.5 | 66.0 | 62.0 | NR | NR | 48.0 | 55.0 | NR | NR |
| Bonner, 2009 M | 73 | 68 | 54.9 | 54.5 | 100.0 | 100.0 | NR | NR | NR | NR | NR | NR |
| Bonner, 2009 F | 40 | 39 | 54.9 | 54.5 | 0.0 | 0.0 | NR | NR | NR | NR | NR | NR |
| Hosgood, 2010 | 227 | 227 | 58.7 | 58.4 | 100.0 | 100.0 | 25.6 | 26.3 | 100.0 | 100.0 | NR | NR |
| Shen, 2010 | 103 | 103 | 58.0 | 56.0 | 0.0 | 0.0 | 27.8 | 26.4 | 48.5 | 47.6 | 72.8 | 75.8 |
| Liao, 2011 | 162 | 299 | 61.0 | 61.0 | 0.0 | 0.0 | NR | NR | 5.6 | 4.4 | 1.9 | 2.0 |
| Lynch, 2011 | 203 | 656 | 58.0 | 58.0 | 100.0 | 100.0 | 26.2 | 25.7 | 100.0 | 100.0 | NR | NR |
| Qu, 2011 Overall | 58.4 | 58.2 | 52.8 | 52.8 | 23.8 | 23.6 | 39.4 | 32.8 | 13.1 | 11.9 | ||
| Qu, 2011 M | 169 | 169 | 58.4 | 58.2 | 100.0 | 100.0 | 23.8 | 23.6 | 39.4 | 32.8 | 13.1 | 11.9 |
| Qu, 2011 F | 151 | 151 | 58.4 | 58.2 | 0.0 | 0.0 | 23.8 | 23.6 | 39.4 | 32.8 | 13.1 | 11.9 |
| Zhao, 2011 | 274 | 384 | 50.1 | 48.7 | 86.1 | 84.4 | NR | NR | 56.2 | 49.7 | 35.8 | 15.1 |
| Purdue, 2012 | 445 | 379 | NR | NR | 58.0 | 65.0 | NR | NR | 66.0 | 61.0 | NR | NR |
| Purdue, 2012 | 158 | 224 | NR | NR | 69.0 | 49.0 | NR | NR | 71.0 | 69.0 | NR | NR |
| Thyagarajan, 2012 M | 92 | 379 | 66.1 | 57.6 | 100.0 | 100.0 | 23.0 | 22.8 | 42.9 | 26.1 | 23.7 | 19.5 |
| Thyagarajan, 2012 F | 76 | 495 | 66.1 | 57.6 | 0.0 | 0.0 | 23.0 | 22.8 | 42.9 | 26.1 | 23.7 | 19.5 |
| Mondal, 2013 | 124 | 140 | 58.0 | 56.0 | 79.0 | 72.1 | NR | NR | 71.7 | 53.4 | NR | NR |
| Thyagarajan, 2013 | 183 | 529 | 61.1 | 61.1 | 0.0 | 0.0 | NR | NR | NR | NR | NR | NR |
| Xie, 2013 M | 174 | 180 | 58.2 | 58.5 | 100.0 | 100.0 | NR | NR | 37.5 | 35.5 | NR | NR |
| Xie, 2013 F | 151 | 150 | 58.2 | 58.5 | 0.0 | 0.0 | NR | NR | 37.5 | 35.5 | NR | NR |
| Xu, 2013 M | 173 | 173 | 62.1 | 60.9 | 100.0 | 100.0 | NR | NR | 68.8 | 56.0 | NR | NR |
| Xu, 2013 F | 45 | 45 | 62.1 | 60.9 | 0.0 | 0.0 | NR | NR | 68.8 | 56.0 | NR | NR |
| Cheau-Feng, 2014 | 67 | 79 | 56.0 | 59.6 | 100.0 | 100.0 | NR | NR | NR | NR | NR | NR |
| Ghosh, 2014 | 64 | 100 | NR | NR | 76.6 | 79.0 | NR | NR | 67.2 | 26.0 | 68.8 | 49.0 |
| Hofmann, 2014 M | 164 | 231 | NR | NR | 100.0 | 100.0 | NR | NR | 60.0 | 54.7 | NR | NR |
| Hofmann, 2014 F | 67 | 137 | NR | NR | 0.0 | 0.0 | NR | NR | 60.0 | 54.7 | NR | NR |
| Hosnijeh, 2014 | 469 | 469 | 56.6 | 56.6 | 49.3 | 49.3 | 26.9 | 26.6 | 57.9 | 56.3 | NR | NR |
| Huang, 2014 | 444 | 1423 | 58.6 | 55.2 | 0.0 | 0.0 | 24.6 | 24.4 | 2.5 | 3.2 | 3.2 | 2.7 |
| Jiang, 2014 | 506 | 520 | 50.0 | 51.0 | 0.0 | 0.0 | NR | NR | NR | NR | NR | NR |
| Kim, 2014 PLCO M | 57 | 185 | 63.9 | 63.8 | 100.0 | 100.0 | 27.0 | 27.3 | 48.0 | 51.0 | NR | NR |
| Kim, 2014 PLCO F | 38 | 116 | 63.9 | 63.8 | 0.0 | 0.0 | 27.0 | 27.3 | 48.0 | 51.0 | NR | NR |
| Kim, 2014 ATBC | 33 | 97 | 59.3 | 57.6 | 100.0 | 100.0 | 26.1 | 26.4 | 100.0 | 100.0 | NR | NR |
| Kim, 2014 PLCO-M | 259 | 267 | 64.1 | 63.7 | 100.0 | 100.0 | 26.8 | 27.4 | 89.2 | 54.6 | NR | NR |
| Kim, 2014 PLCO-F | 167 | 169 | 64.1 | 63.7 | 0.0 | 0.0 | 26.8 | 27.4 | 89.2 | 54.6 | NR | NR |
| Kim, 2014 SWHS | 221 | 222 | 59.2 | 59.2 | 0.0 | 0.0 | 24.6 | 25.0 | 7.7 | 5.0 | NR | NR |
| Sun, 2014 | 132 | 125 | 58.2 | 55.5 | 56.1 | 52.0 | NR | NR | 41.7 | 65.1 | NR | NR |
| Zhang, 2014 Overall | NR | NR | 58.2 | 58.2 | NR | NR | 23.2 | 20.5 | NR | NR | ||
| Zhang, 2014 M | 241 | 241 | NR | NR | 100.0 | 100.0 | NR | NR | 23.2 | 20.5 | NR | NR |
| Zhang, 2014 F | 173 | 173 | NR | NR | 0.0 | 0.0 | NR | NR | 23.2 | 20.5 | NR | NR |
| Zhou, 2014 | 193 | 194 | 70.3 | 70.1 | 100.0 | 100.0 | 24.1 | 23.2 | 51.8 | 50.5 | NR | NR |
Abbreviations: M, male; F, female; BMI, body mass index; NR, not reported.
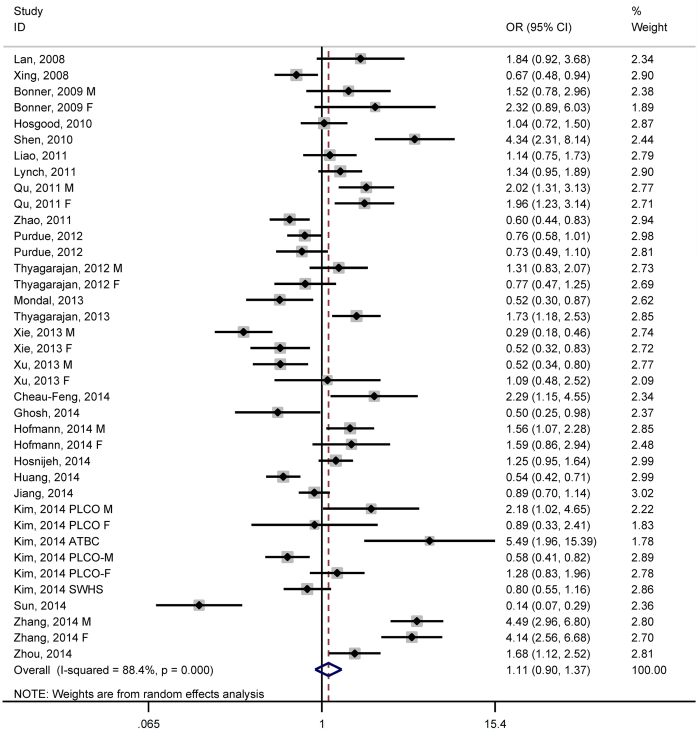
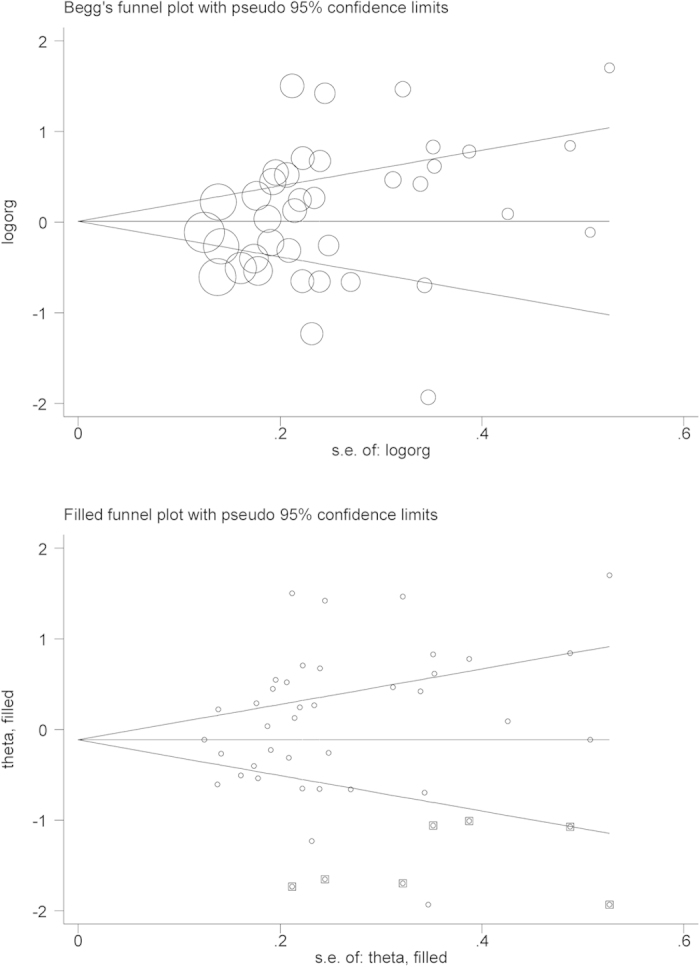
When all study groups were pooled together, dichotomizing mtDNA copy number at the median value in the controls identified an 11% increase in overall cancer risk for carriers of high mtDNA content (95% CI: 0.92 to 1.37; P = 0.320) (Fig. 1), with strong evidence of heterogeneity (I2 = 88.4%) but a low probability of publication bias (P for Egger test = 0.119), although it was estimated to have potentially 7 missing studies stemming from publication bias by the trim-and-fill method (Fig. 2).
Figure 1. Forest plot of mtDNA and overall cancer risk in median comparison.
Figure 2. The Begg’s and the Filled funnel plot in median comparison.
A set of subgroup analyses were conducted to account for this evident heterogeneity (Table 3). By ancestry, high mtDNA content appeared to be neutral in populations of White ancestry (OR = 0.97; 95% CI: 0.64 to 1.47; P = 0.894), yet a marginally increased risk was observed in populations of Asian ancestry (OR = 1.29; 95% CI: 0.94 to 1.76; P = 0.116), and no improvement was observed in heterogeneity. By gender, high mtDNA content was consistently overrepresented in cancer cases relative to controls in both genders, especially in males (OR = 1.40; 95% CI: 0.97 to 2.04; P = 0.076), with evident heterogeneity. Grouping studies by study design and source of controls failed to identify any significance. By cancer type, high mtDNA content was associated with an increased risk for lymphoma (OR = 1.76; 95% CI: 1.08 to 2.85; P = 0.023) but a reduced risk for skeleton cancer (OR = 0.39; 95% CI: 0.22 to 0.68; P = 0.001), accompanying moderate heterogeneity (I2 = 60.3% and 66.7%).
Table 3. Subgroup analyses of mtDNA copy number with cancer risk in median.
| Study groups | Studies (n) | OR | 95% CI | P | I2 |
|---|---|---|---|---|---|
| Ancestry | |||||
| Asian | 18 | 1.29 | 0.94 to 1.76 | 0.116 | 89.7% |
| White | 8 | 0.97 | 0.64 to 1.47 | 0.894 | 88.0% |
| Gender | |||||
| Male | 15 | 1.40 | 0.97 to 2.04 | 0.076 | 89.5% |
| Female | 15 | 1.27 | 0.92 to 1.75 | 0.151 | 86.8% |
| Study design | |||||
| Prospective | 21 | 1.15 | 0.94 to 1.39 | 0.169 | 75.8% |
| Retrospective | 17 | 1.02 | 0.67 to 1.55 | 0.917 | 93.2% |
| Control source | |||||
| Population | 35 | 1.14 | 0.92 to 1.41 | 0.240 | 88.8% |
| Hospital | 3 | 0.83 | 0.32 to 2.11 | 0.689 | 85.1% |
| Cancer type | |||||
| Digestive | 11 | 0.86 | 0.59 to 1.25 | 0.432 | 88.3% |
| Urogenital | 6 | 1.05 | 0.74 to 1.49 | 0.782 | 79.9% |
| Lymphoma | 5 | 1.76 | 1.08 to 2.85 | 0.023 | 60.3% |
| Respiratory | 5 | 1.02 | 0.73 to 1.43 | 0.904 | 68.0% |
| Head/neck | 5 | 1.63 | 0.62 to 4.31 | 0.323 | 93.9% |
| Breast | 3 | 1.80 | 0.81 to 4.01 | 0.152 | 92.0% |
| Skeleton | 2 | 0.39 | 0.22 to 0.68 | 0.001 | 66.7% |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
To investigate the possible nonlinear relationship between mtDNA content and cancer risk, we categorized mtDNA copy number into tertiles in the controls and assigned the 1st tertile as the reference (Table 4). Carriers of the 2nd and 3rd tertiles had a linear increase in overall cancer risk, with odds of being 1.31 (95% CI: 0.87 to 1.95; P = 0.192) and 1.45 (95% CI: 0.70 to 2.99; P = 0.313), respectively, without publication bias (P for Egger test = 0.271 and 0.651, respectively). Accordingly as reflected by the trim-and-fill method, there were 2 and 3 missing studies required to achieve symmetry of the Filled funnel plot, respectively (Figures not shown). Still heterogeneity was a disturbing issue for both comparisons (I2 > 80%).
Table 4. Overall and subgroup analyses of mtDNA copy number with cancer risk in tertiles.
| Study groupsStudies (n) |
High-tertile versus low-tertile |
Middle-tertile versus low-tertile |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | I2 | OR | 95% CI | P | I2 | ||
| Overall | 11 | 1.45 | 0.70 to 2.99 | 0.313 | 95.3% | 1.31 | 0.87 to 1.95 | 0.192 | 84.5% |
| Ancestry | |||||||||
| Asian | 4 | 1.97 | 0.66 to 5.92 | 0.227 | 97.3% | 1.46 | 0.85 to 2.51 | 0.171 | 88.7% |
| White | 2 | 2.67 | 1.02 to 8.61 | 0.045 | 66.5% | 1.83 | 0.93 to 3.59 | 0.081 | 17.6% |
| Gender | |||||||||
| Male | 4 | 1.34 | 0.28 to 6.41 | 0.711 | 94.4% | 1.22 | 0.46 to 3.20 | 0.689 | 85.6% |
| Female | 3 | 0.88 | 0.29 to 2.64 | 0.815 | 90.4% | 1.07 | 0.57 to 2.01 | 0.830 | 36.5% |
| Study design | |||||||||
| Prospective | 5 | 1.53 | 0.64 to 3.64 | 0.338 | 88.1% | 1.39 | 0.83 to 2.33 | 0.213 | 65.2% |
| Retrospective | 6 | 1.37 | 0.45 to 4.17 | 0.578 | 96.7% | 1.22 | 0.65 to 2.30 | 0.533 | 90.0% |
| Control source | |||||||||
| Population | 11 | 1.45 | 0.70 to 2.99 | 0.313 | 95.3% | 1.31 | 0.87 to 1.95 | 0.192 | 84.5% |
| Cancer type | |||||||||
| Lymphoma | 4 | 2.07 | 1.11 to 3.84 | 0.021 | 52.6% | 1.74 | 1.14 to 2.65 | 0.010 | 0.0% |
| Digestive | 3 | 1.34 | 0.49 to 3.67 | 0.571 | 95.9% | 1.26 | 0.71 to 2.26 | 0.434 | 87.8% |
| Skeleton | 2 | 0.20 | 0.13 to 0.30 | <0.001 | 0.0% | 0.44 | 0.30 to 0.63 | <0.001 | 0.0% |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
After grouping studies by ancestry, risk estimates were potentiated for the comparisons of the 2nd and 3rd tertiles with the 1st tertile of mtDNA copy number in populations of both White and Asian ancestries, especially in the former (OR = 1.83 and 2.97; P = 0.081 and 0.045, respectively), with improved heterogeneity. All qualified studies with tertile comparisons enrolled population-based controls. By gender and study design, the association of mtDNA content in tertiles with overall cancer risk was more obvious in males than in females, and in prospective studies than in retrospective studies, while there was no observable significance. By cancer type, carriers of the 2nd and 3rd tertiles of mtDNA copy number had a 1.74-fold (95% CI: 1.14 to 2.65; P = 0.010) and 2.07-fold (95% CI: 1.11 to 3.84; P = 0.021) increased risk of lymphoma, respectively, and heterogeneity was greatly improved (I2 = 0.0% and 52.6%, respectively). In contrast, there was a 56% (95% CI: 0.30 to 0.63; P < 0.001) and 80% (95% CI: 0.13 to 0.30; P < 0.001) reduced risk of skeleton cancer for the comparisons of the 2nd and 3rd tertiles with the 1st tertile of mtDNA copy number, respectively, and there was no indicative of heterogeneity (both I2 = 0.0%).
To further study heterogeneity, regression of various study-level covariates was conducted in a multivariable meta-regression model. For both comparisons by median and in tertiles, differences in body mass index explained a marginally significant part of heterogeneity for the association of mtDNA copy number with overall cancer risk (P = 0.065 and 0.081, respectively). In addition, for the comparisons in tertiles, drinking was identified as a potential source of heterogeneity (P = 0.043).
Discussion
To the best of our knowledge, this is the first systematic meta-analysis covering available English literature to date demonstrating that carriers of high mtDNA content had a higher risk for lymphoma, but a lower risk for skeleton cancer, and this risk prediction might follow a dose-dependent pattern. In spite of a large panel of subgroup and meta-regression analyses, there was no substantial improvement in overall evident heterogeneity for a majority of comparisons in this meta-analysis.
Generally, mitochondria are likened to the energy factories of the cells, and they produce a usable form of energy, adenosinetriphosphate (ATP) through oxidative phosphorylation. Lowered mtDNA copy number can cause a deficiency in oxidative phosphorylation and a resultant enhanced generation of ATP by glycolysis, these changes often implicating cancer development39. There is also evidence that mitochondria play a key role in activating apoptosis in mammalian cells40 and are the primary target of reactive oxygen species (ROS)41. High mtDNA content can be indicative of ROS-mediated oxidative stress, which is thought to be involved in the molecular mechanisms of carcinogenesis42,43. These observations altogether suggest a biologically plausible role for the changes of mtDNA copy number in the modulation of cancer risk. With the above information in mind, we in the present meta-analysis provided a comprehensive assessment to enrich our understandings of altered mtDNA content in predisposition to overall and specific cancer types.
Of note, our findings revealed a cancer site-specific effect of high mtDNA copy number on the risk of different types of cancer. Our subgroup analysis by cancer type detected a totally opposite outcome between lymphoma and skeleton cancer, with higher mtDNA copy number gradually associating with an increased risk for lymphoma but a reduced risk for skeleton cancer. Understanding tumor heterogeneity may be the next big quest in cancer sciences, which is beyond the capability of the present meta-analysis. However, a phenomenon that cannot be overlooked for lymphoma is that lymphoma is a kind of blood cancer that affects the lymphatic system, and a majority of involved studies quantified mtDNA copy number in peripheral blood lymphocytes, which may better reflect the underlying association between mitochondrial dysfunction and the initiation and progression of lymphoma. This significant association is not surprising, but emphasizes the importance of quantifying mtDNA content in the targeted tissue of each specific cancer, which might be a putative explanation for the neutral associations with the other types of cancer in this meta-analysis. On the other hand, we must have reservations with regard to the association between mtDNA content and skeleton cancer, because this conclusion was only based on two underpowered groups stratified by gender in the study by Xie et al44. Therefore, the jury must refrain from drawing a firm conclusion until the confirmation from large, well-performed prospective studies with tissue mtDNA content.
Potential biases and limitations
This meta-analysis is only based on the summary results of published articles, and it is possible some small studies with negative findings are missing. So we cannot exclude the existence of selection bias. Although there was no indication of publication bias according to Egger’s test, the power of identifying significance might be low especially if the total number of studies involved in a meta-analysis is 10 or fewer45. The interpretation of our findings might be limited by the strong or moderate evidence of heterogeneity, and this is a common issue with most available meta-analyses in medical literature, leaving further explorations of disturbing heterogeneity open. In addition, the moderate sample size of the current meta-analysis, especially in some subgroups made our findings preliminary and required future confirmation. Furthermore, assay of mtDNA content in peripheral blood lymphocytes may have clouded the true effect of mtDNA copy changes.
Clinical importance
The utility of mtDNA copy number to indicate the potential for cancer incidence in future screening programs may enable clinical practitioners to better refine individuals at risk for cancer and develop approaches for tailoring antitumor therapy.
In summary, we through a comprehensive meta-analysis demonstrated that elevated mtDNA content was associated with a higher risk for lymphoma, yet a lower risk for skeleton cancer, and the risk prediction followed a dose-dependent pattern. For practical reasons, we hope this study will enrich our understandings of mtDNA content alterations in molecular carcinogenesis. Future investigations to elucidate the specific role of mtDNA in specific cancer are warranted.
Author Contributions
G.T. and B.W. conceived and designed the experiments; J.M., G.T. and S.L. performed the experiments; G.T. and S.L. analyzed the data; S.L., X.L., T.N. and L.Z. contributed materials/analysis tools; J.M. and B.W. wrote and revised the manuscript. All authors reviewed and approved the manuscript prior to submission.
Additional Information
How to cite this article: Mi, J. et al. The Relationship Between Altered Mitochondrial DNA Copy Number And Cancer Risk: A Meta-Analysis. Sci. Rep. 5, 10039; doi: 10.1038/srep10039 (2015).
Acknowledgments
Financial Support: This work was supported by the National Natural Science Foundation of China (81400771 and 81171303), Taishan Scholars Construction Engineering and the Science, Natural Science Foundation of Shandong Province (ZR2014HL028), Technology Project for the Universities of Shandong Province (J14LE01) and Binzhou Medical University Scientific Research Funds (BY2013KYQD18 and BY2013KYQD17).
References
- Raffoul J. J., Heydari A. R. & Hillman G. G. DNA Repair and Cancer Therapy: Targeting APE1/Ref-1 Using Dietary Agents. J. Oncol. 2012, 370481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H. et al. The APE1 Asp/Asp genotype and the combination of APE1 Asp/Asp and hOGG1-Cys variants are associated with increased p53 mutation in non-small cell lung cancer. J. Epidemiol. 22, 537–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbay E. et al. Association of APE1 and hOGG1 polymorphisms with colorectal cancer risk in a Turkish population. Curr. Med. Res. Opin. 27, 1295–1302 (2011). [DOI] [PubMed] [Google Scholar]
- Wang M. et al. Genetic variants of XRCC1, APE1, and ADPRT genes and risk of bladder cancer. DNA Cell. Biol. 29, 303–311 (2010). [DOI] [PubMed] [Google Scholar]
- De Ruyck K. et al. Polymorphisms in base-excision repair and nucleotide-excision repair genes in relation to lung cancer risk. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 631, 101–110 (2007). [DOI] [PubMed] [Google Scholar]
- Deng Q. et al. Genetic polymorphisms in ATM, ERCC1, APE1 and iASPP genes and lung cancer risk in a population of southeast China. Medical Oncology 28, 667–672 (2010). [DOI] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Thakkinstian A. et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am. J. Epidemiol. 162, 201–211 (2005). [DOI] [PubMed] [Google Scholar]
- Bowden J., Tierney J. F., Copas A. J. & Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 11, 41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R. R. et al. Polymorphisms in the DNA repair genes XPD, XRCC1, XRCC3, and APE/ref-1, and the risk of lung cancer amongmale smokers in Finland. Cancer Letters 191, 171–178 (2003). [DOI] [PubMed] [Google Scholar]
- Ito H. et al. Gene-environment interactions between the smoking habit and polymorphisms in the DNA repair genes, APE1 Asp148Glu and XRCC1 Arg399Gln, in Japanese lung cancer risk. Carcinogenesis 25, 1395–1401 (2004). [DOI] [PubMed] [Google Scholar]
- Popanda O. et al. Specific combinations of DNA repair gene variants and increased risk for non-small cell lung cancer. Carcinogenesis 25, 2433–2441 (2004). [DOI] [PubMed] [Google Scholar]
- Broberg K., Bjork J., Paulsson K., Hoglund M. & Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis 26, 1263–1271 (2005). [DOI] [PubMed] [Google Scholar]
- Shen M. et al. Polymorphisms in the DNA base excision repair genes APEX1 and XRCC1 and lung cancer risk in Xuan Wei, China. Anticancer Res. 25, 537–542 (2005). [PubMed] [Google Scholar]
- Chen L. et al. Association Between Polymorphisms in the DNA Repair Genes XRCC1 and APE1, and the Risk of Prostate Cancer in White and Black Americans. The Journal of Urology 175, 108–112 (2006). [DOI] [PubMed] [Google Scholar]
- Li C. et al. Genetic variants of the ADPRT, XRCC1 and APE1 genes and risk of cutaneous melanoma. Carcinogenesis 27, 1894–1901 (2006). [DOI] [PubMed] [Google Scholar]
- Moreno V. et al. Polymorphisms in genes of nucleotide and base excision repair: risk and prognosis of colorectal cancer. Clin Cancer Res. 12, 2101–2108 (2006). [DOI] [PubMed] [Google Scholar]
- Terry P. D., Umbach D. M. & Taylor J. A. APE1 genotype and risk of bladder cancer: Evidence for effect modification by smoking. International Journal of Cancer 118, 3170–3173 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang Y. Genetic Polymorphisms in Base-Excision Repair Pathway Genes and Risk of Breast Cancer. Cancer Epidemiology Biomarkers & Prevention 15, 353–358 (2006). [DOI] [PubMed] [Google Scholar]
- Zienolddiny S. et al. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis 27, 560–567 (2006). [DOI] [PubMed] [Google Scholar]
- Berndt S. I. et al. Genetic Variation in Base Excision Repair Genes and the Prevalence of Advanced Colorectal Adenoma. Cancer Research 67, 1395–1404 (2007). [DOI] [PubMed] [Google Scholar]
- Figueroa J. D. et al. Genetic variation in the base excision repair pathway and bladder cancer risk. Human Genetics 121, 233–242 (2007). [DOI] [PubMed] [Google Scholar]
- Huang M. et al. High-Order Interactions among Genetic Variants in DNA Base Excision Repair Pathway Genes and Smoking in Bladder Cancer Susceptibility. Cancer Epidemiology Biomarkers & Prevention 16, 84–91 (2007). [DOI] [PubMed] [Google Scholar]
- Li C. et al. Genetic polymorphisms in DNA base-excision repair genes ADPRT, XRCC1, and APE1 and the risk of squamous cell carcinoma of the head and neck. Cancer 110, 867–875 (2007). [DOI] [PubMed] [Google Scholar]
- Sangrajrang S. et al. Polymorphisms in three base excision repair genes and breast cancer risk in Thai women. Breast Cancer Research and Treatment 111, 279–288 (2007). [DOI] [PubMed] [Google Scholar]
- Andrew A. S. et al. DNA Repair Polymorphisms Modify Bladder Cancer Risk: A Multi-factor Analytic Strategy. Human Heredity 65, 105–118 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. S. et al. Base excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African-Americans. Carcinogenesis 30, 78–87,(2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang F. Y. et al. Association between Polymorphisms in DNA Base Excision Repair Genes XRCC1, APE1, and ADPRT and Differentiated Thyroid Carcinoma. Clinical Cancer Research 14, 5919–5924 (2008). [DOI] [PubMed] [Google Scholar]
- Huang W. Y. et al. Selected base excision repair gene polymorphisms and susceptibility to biliary tract cancer and biliary stones: a population-based case-control study in China. Carcinogenesis 29, 100–105 (2008). [DOI] [PubMed] [Google Scholar]
- Kasahara M. et al. Association of MUTYH Gln324His and APEX1 Asp148Glu with colorectal cancer and smoking in a Japanese population. Journal of Experimental &Clinical Cancer Research 27, 49 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A. K. et al. Association of polymorphisms in base excision repair genes with the risk of breast cancer: a case-control study in North Indian women. Oncol Res. 17, 127–135 (2008). [DOI] [PubMed] [Google Scholar]
- Pardini B. et al. DNA repair genetic polymorphisms and risk of colorectal cancer in the Czech Republic. Mutat Res. 638, 146–153 (2008). [DOI] [PubMed] [Google Scholar]
- Shekari M. et al. Association of genetic polymorphism of the DNA base excision repair gene (APE-1 Asp/148 Glu) and HPV type (16/18) with the risk of cervix cancer in north Indian population. Cancer Biomark 4, 63–71 (2008). [DOI] [PubMed] [Google Scholar]
- Smith T. R. et al. Polygenic model of DNA repair genetic polymorphisms in human breast cancer risk. Carcinogenesis 29, 2132–2138 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D. et al. Polymorphisms of the NER pathway genes, ERCC1 and XPD are associated with esophageal adenocarcinoma risk. Cancer Causes & Control 19, 1077–1083 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R. et al. Polymorphisms and haplotypes of XRCC1 and APE1 and risk of childhood leukaemia in China: A case-control analysis. European Journal of Oncology 13, 187–192 (2008). [Google Scholar]
- Agachan B. et al. Apurinic/apyrimidinic endonuclease (APE1) gene polymorphisms and lung cancer risk in relation to tobacco smoking. Anticancer Res. 29, 2417–2420 (2009). [PubMed] [Google Scholar]
- Gangwar R., Ahirwar D., Mandhani A. & Mittal R. D. Influence of XPD and APE1 DNA Repair Gene Polymorphism on Bladder Cancer Susceptibility in North India. Urology 73, 675–680 (2009). [DOI] [PubMed] [Google Scholar]
- Ji L. Single-nucleotide polymorphisms in DNA repair gene hOGG1 and APE1 and susceptibility to hepatocellular carcinoma , Guangxi Medical University (2009). [Google Scholar]
- Liu Y. et al. Association and Interactions between DNA Repair Gene Polymorphisms and Adult Glioma. Cancer Epidemiology Biomarkers & Prevention 18, 204–214 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y. L. et al. A Polymorphism in the APE1 Gene Promoter is Associated with Lung Cancer Risk. Cancer Epidemiology Biomarkers & Prevention 18, 223–229 (2009). [DOI] [PubMed] [Google Scholar]
- Lu J. et al. Functional characterization of a promoter polymorphism in APE1/Ref-1 that contributes to reduced lung cancer susceptibility. The FASEB Journal 23, 3459–3469 (2009). [DOI] [PubMed] [Google Scholar]
- Narter K. F. et al. Bladder cancer and polymorphisms of DNA repair genes (XRCC1, XRCC3, XPD, XPG, APE1, hOGG1). Anticancer Res. 29, 1389–1393 (2009). [PubMed] [Google Scholar]
- Agalliu I. et al. Genetic variation in DNA repair genes and prostate cancer risk: results from a population-based study. Cancer Causes Control 21, 289–300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah R. R., Alexander J. S. & Michael B. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Publisher: WileyP105, (2005).