Abstract
Background/Objectives:
Nutritional risk screening (NRS-2002) and routine clinical laboratory measurements (RCLMs) had been shown to have a predictive value in adverse outcomes in some studies, respectively. This study analyzed the association between NRS-2002 and RCLMs and estimated their prospective value in predicting adverse outcomes.
Subjects/Methods:
A total of 916 hospitalized patients were screened on admission with NRS-2002 and Subjective Global Assessment; RCLMs, which include blood test, kidney and liver function and electrolytes, were recorded. Diagnosis, nutritional support, surgery, radiotherapy, chemotherapy, complications, mortality and hospital stay during hospitalization were collected. The X2-test, odds ratios with 95% confidence intervals, kappa (k) statistic and regression analyses were conducted.
Results:
An overall 48.1% of the 916 patients were at nutritional risk on admission. Comparing ‘at risk' with ‘no risk', a significantly higher incidence of abnormality was found not only in nutritional markers but also in other parameters of RCLMs (OR ranged from 1.5 to 3.5). Regression analyses showed that ‘at risk' determined at admission was not a significant predictor of adverse outcomes after adjusting for other confounding factors, although it was a strong predictor in univariate analysis, whereas hypoalbuminemia, low total lymphocyte count, abnormality of hepatic and renal function were predictors after adjusting for confounders.
Conclusions:
The findings suggest that NRS-2002 might be a global index of ‘sickness' rather than be only a nutritional screening tool. It being rated once at admission is insufficient and should be repeated for using it as a predictor, whereas RCLMs routinely measured at admission may be able to be used to predict adverse outcomes.
Introduction
Although the prefix mal refers to both over and under, because of poor appetite and absorption caused by illness, malnutrition was often mentioned virtually synonymously with undernutrition in hospitals, and its prevalence has been reported between 10 and 80% depending on the population, pathology and test used.1 Malnutrition has been associated with higher rates of complications, increased length of hospital stay and ICU stay, increased morbidity and mortality and increased treatment costs.2, 3, 4, 5 Thus, special attention should be paid to patients' nutrition status, and proper nutritional support should be adopted timely to prevent the adverse outcomes.
Malnutrition should be identified before nutritional treatment. Out of more than 70 screening methods in existence, nutritional risk screening (NRS)-2002 is recommended by European Society of Parenteral and Enteral Nutrition for identifying patients at nutritional risk who may benefit from nutritional support in a hospital setting. NRS-2002 is straightforward, quick and easy to use, highly reliable and reproducible and has high practicability in different patient populations. NRS-2002 had been shown to have a high sensitivity (62%) and specificity (93%) in identifying malnutrition, and its score predicts clinical outcomes.3 However, nutritional screening is not part of the routine procedures in hospitals, and nutritional support is taken into consideration by some doctors only when a patient cannot eat, undergoes surgery, becomes skeletonized or has very low albumin and hemoglobin level. To improve further clinical nutrition supporting work, we conducted this study to explore the prevalence of malnutrition and nutritional support status in Wuhan Tongji Hospital.
Routine clinical laboratory measurements (RCLMs) had been demonstrated to have predictive value on mortality in older persons in the general population.6 The levels of serum albumin and cholesterol measured at hospital admission are predictors of in-hospital death, nosocomial infection and length of stay.7 Serum cholesterol, albumin, creatinine, hemoglobin and lymphocyte count that are nutritional makers are epidemiologically useful and correlate with morbidity and mortality.8 Given that NRS-2002 is a rapid and simple process conducted in busy clinics and RCLMs are routinely measured at hospital admission, we analyze the association between them and test their prospective value in predicting adverse outcomes.
Subjects and methods
Patients and data collection
This prospective observational study included consecutive patients admitted to medical and surgical wards in a tertiary teaching hospital with more than 4000 beds during a 6-month study period. Patients aged above 18 years, willing to give their informed consent and who had RCLMs measured at hospital admission were included in the study. We excluded patients who were clinically unstable, were pregnant or with a hospital stay <3 days. A total of 916 patients (551 men and 365 women), who had a mean age of 49±15.9 years (range: 18–88 years) were finally studied.
Patients were weighed and measured on admission. In the case of a patient unable to get actual weight, estimation was used instead. Sex, age, date of hospital admission, diagnosis, RCLMs, nutritional support, surgery, application of radiotherapy and chemotherapy, complications, mortality and date of discharge were collected.
Nutritional assessment
The nutritional state assessment was performed on admission, assessing on the following items: NRS-2002, Subjective Global Assessment (SGA), nutritional markers in RCLMs and a combined index.
NRS-2002
NRS-2002 was conducted according to the guideline provided by the European Society of Parenteral and Enteral Nutrition.9 As patients with the same diagnosis does not always mean the same severity, the severity of disease score is mainly based on the prototypes for severity of disease as described in the guideline. Patients are classified as no risk (NRS score <3) or as at risk (NRS score ⩾3).
Subjective Global Assessment
SGA was classified as outlined by Detsky.10 Weight loss pattern was considered, if an improvement in appetite and a recent weight gain that was neither caused by edema nor by tumor mass was observed, the patient was classified as being well nourished, even if the net loss was between 5 and 10% in 6 months. It was instructed to be less sensitive and more specific in assessment, therefore the patient was classified as well nourished if he had been well before but had been experiencing reduced intake recently because of acute disease. On the other hand, we classified the patient as malnourished if severe physical signs of undernutrition were exhibited even though the food intake and weight were stabilized.
Nutritional markers in RCLMs
Low level of serum total cholesterol, serum albumin, serum creatinine, hemoglobin or total lymphocyte count is a marker of malnutrition. These markers were obtained from RCLMs examined by the Department of Clinical Laboratory of Wuhan Tongji Hospital. Normal range for cholesterol, albumin, creatinine, hemoglobin and lymphocyte count was set at 2.9–5.2 mmol/l, 35–55 g/l, 44/53-97/106μmol/l, 110/120–160 g/l and 0.8–4 × 109/l, respectively.
A combined index
If the patient is indicated as malnutrition according to at least four out of the seven following indicators, low total cholesterol, hypoalbuminemia, anemia, low serum creatinine, low total lymphocyte count, the NRS-2002 score ⩾3 and malnutrition assessed by SGA, he/she was categorized as malnourished by the combined index.
Definition and classification of variables in multiple logistic regression analyses
Grading of surgery
Briefly, grade 0—no surgery; grade 1—surgery with small incision, such as laparoscopic cholecystectomy, thyroidectomy, inguinal hernia repair, etc; grade 2—surgery with large incision––for example, liver transplants, total gastrostomy, coronary bypass surgery, etc.
Grading of nutritional support during hospitalization
Grade 0—no support; grade 1—intravenously administered vitamins, amino acids or sugar (except 5% glucose used for medical preparation) alone or together for <5 days; grade 2—intravenously administered as in grade 1 but for >5 days or intravenously administered fat with vitamins, amino acid or sugar together ( ⩾500 kcal per day) for <5 days; grade 3—intravenously administered fat with vitamins, amino acids or sugar together for >5 days or enteral nutrition (⩾500 kcal per day) for >5 days(Enteral nutrition for <5 days was not taken into account, as too few patients received that in this study).
Grading of laboratory indexes in RCLMs
In multiple logistic regression analysis, the independent variables should not have a high degree of correlation between each other and should associate with the dependent variable (has statistics significance by the X2-test), hence the laboratory indexes were selected and graded in the model as shown in Table 1.
Table 1. Grading of laboratory indexes in RCLMs.
| Index |
Grading of abnormality |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| WBC (109/l) | 4–10 | 4–3 or 10–20 | 3–2 or 20–30 | 2–1 or 30–40 | <1 or>40 |
| Lym (109/l) | >0.8 | <0.8 | — | — | — |
| Hb (g/l) | 110/120–160 | 110/120–90 | 90–60 | 60–30 | <30 |
| Electrolytes (mmol/l) | 3.5–5.1 (K); | 3.0–3.5 or 5.1–6 (K); | 3.0–2.5 or 6–8 (K); | 2.5–2.0 or >8 (K); | <2.0 (K) |
| 135–145 (Na); | 130–135 or >145 (Na); | 120–130 (Na); | <120 (Na); | ||
| 98–106 (Cl); | <98 or >106 (Cl); | ||||
| 2.25–2.75 (Ca) | 2.25–2 or 2.75–3 (Ca) | 2–1.5 or 3–4 (Ca) | <1.5 or >4 (Ca) | ||
| Renal function Cr (μmol/l) | 53–106 for men 44–97 for women | 106–177 | 177–401 | 401–707 | >707 |
| Liver function | 4–41 μ/l (ALT, AST); | 1–3 ULN (ALT or AST); | 3–5 ULN (ALT or AST); | 5–20 ULN (ALT or AST); | > 20 ULN (ALT or AST); |
| 3.4–20.5 mmol/l (TBil);0–6.8 mmol/l (DBil); | 1–1.5 ULN (Bil); | 1.5–3 ULN (Bil); | 3–10 ULN (Bil); | > 10 ULN (Bil); | |
| 35–106 μ/l (ALP) | 1–2.5 ULN (ALP) | 2.5–5 ULN (ALP) | 5–20 ULN (ALP) | > 20 ULN (ALP) | |
| ALB (g/l) | >35 | 35–30 | 30–25 | <25–20 | <20 |
| TC (mmol/l) | >2.9 | <2.9 | — | — | — |
Abbreviations: ALB, albumin; Hb, hemoglobin level; Lym, lymphocyte counts; RCLM, routine clinical laboratory measurement; TC, total cholesterol; WBC, white blood cell counts. Renal function was reflected by creatinine (Cr). Liver function was reflected by alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) or bilirubin (bil). Electrolytes were potassium (K), chlorine (Cl), sodium (Na) and calcium (Ca). 1–3 ULN means 1–3 times the upper limit of normal (ULN). As for electrolyte abnormality grading, if more than one electrolyte is abnormal, only the highest-ranked abnormality is used in regression analyses.
Definition of adverse outcomes
A complication was defined as outlined by Naber.11 In this study, we used prolonged length of stay (greater than four times the mean length of stay for each departments12), complication and death as proxies of adverse outcomes. The incidence of adverse outcomes was the number of patients in a group divided by the number of patients who had adverse outcomes in the same group.
Statistical analysis
Statistical analysis was performed with SPSS 17.0 (SPSS Inc.,Chicago, IL, USA). Categorical variables were expressed as percentage and analyzed by the X2-test. Odds ratios (ORs) with 95% confidence intervals were calculated for the ratios of abnormalities of RCLMs and the incidence of adverse outcomes in ‘NRS ⩾3' compared with ‘NRS<3' patients. Sensitivity, specificity and predictive values were calculated to evaluate the nutritional indicators according to the combined index. The kappa (k) statistic was used to measure agreement between nutritional indicator and the combined index (0⩽k ⩽1, the larger k-value, the better agreement). To analyze the association between the independent variables (nutritional risk and RCLMs) and the dependent variable (incidence of adverse outcomes), multiple logistic regression analysis was performed. Results were considered statistically significant if P<0.05.
Results
The prevalence of nutritional risk and the nutritional support status
Of the total 916 patients, 503 patients were from surgical wards and 413 patients were from medical wards. Nearly 60% of the total patients were admitted for nontumor diseases, 32% for malignant tumor and 8% were for benign tumor. The patients' main admission diagnoses were classified into 12 categories: serious trauma (36 patients ), digestive disease (228 patients), hematologic disease (56 patients), neurological disease (92 patients), respiratory disease (133 patients), rheumatic disease (40 patients), cardiovascular disease (88 patients), nephropathy (73 patients), orthopedic disease (41 patients), urological disease (57 patients), endocrine system disease (23 patients) and thyroid breast surgical disease (49 patients).
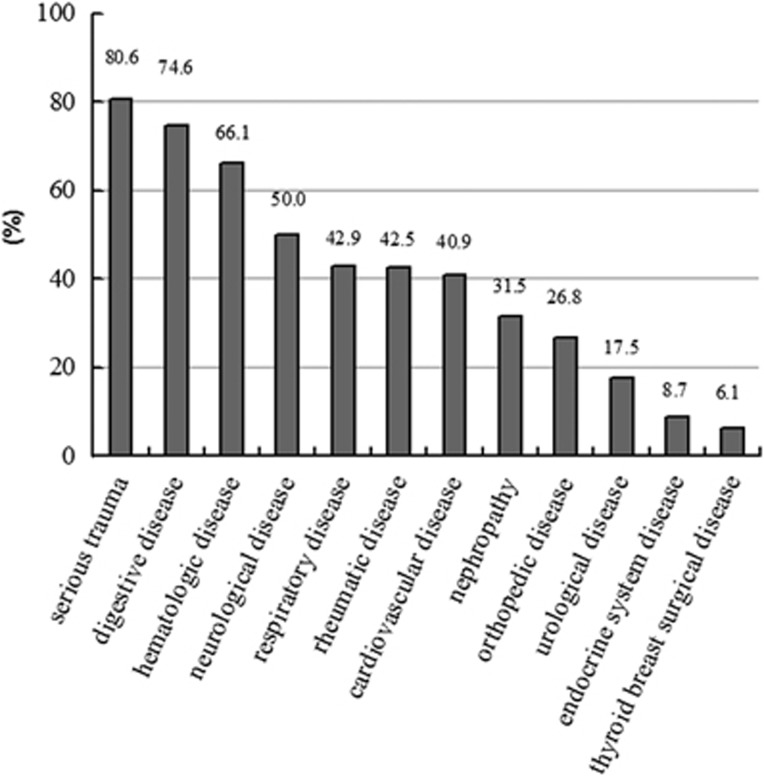
An overall 48.1% of the total patients were at nutritional risk at admission. No difference was found between the risk rate in surgical wards and that in medical wards (47.9% versus 48.4%). The risk rate was higher in malignant tumor patients than that with benign tumor and that of nontumor (54.9% versus 40% and 45.8%, P=0.014). With respect to diseases of different categories, nutritional risk varied greatly from 6.1% to 80.6% as presented in Figure 1.
Figure 1.
Prevalence of nutritional risk in diseases of different categories (%). It showed that nutritional risk rate diverged among diseases of different categories being highest in serious trauma and lowest in thyroid breast surgical disease (P=0.000).
In the time of admission, 13.5% (64 of 475) of the ‘no-risk' patients received nutritional support. Of the 64 patients, 42 patients received grade 1, 12 patients received grade 2 and 10 patients received grade 3 support. Of the ‘at-risk' patients, 43.5% (192 of 441) received nutritional support, including 34 cases of grade 1, 71 cases of grade 2 and 87 cases (19.7%) of grade 3. The proportion of subjects in each categorical variable levels of NRS score was listed in Table 2, and recent dietary intake was the most powerful trigger for nutritional support, followed by severity of disease and recent weight loss in descending order (standardized coefficient correlation was 0.355, 0.182 and 0.104 accordingly, all P <0.001), whereas BMI was not a trigger.
Table 2. Proportion of subjects in each categorical variable levels of the NRS score.
| Variables | Levels | Proportion (%) |
|---|---|---|
| Change in dietary intake in the preceding week | No reduction | 51.3 |
| Reduced 25–50% | 14.4 | |
| Reduced 50–75% | 14.1 | |
| Reduced 75–100% | 20.2 | |
| Weight loss in 3 months | No significant loss | 70.9 |
| loss >5% in 3 months | 6.3 | |
| loss >5% in 2 months | 6.9 | |
| loss >5% in 1 month | 15.9 | |
| Age | Age <70 years | 90.1 |
| Age ⩾70 years | 9.9 | |
| BMI | BMI ⩾18.5 | 84.7 |
| BMI <18.5 | 15.3 |
Abbreviations: BMI, body mass index; NRS, nutritional risk screening.
Comparison of nutritional markers with NRS-2002 in identifying malnutrition by the combined index
According to the combined index, 21.1% of the patients were malnourished. The frequency of malnutrition was highest as indicated by NRS-2002 (48.1%), followed by anemia (36.4%), SGA (35.8%), hypoalbuminemia (34.2%), low serum creatine (18.7%), low total lymphocyte count (11.3%) and low total cholesterol (10.1%) in the descending order. In comparison with nutritional markers in RCLMs, NRS-2002 has a higher sensitivity (94.5%) and less specificity (63.9%) in identifying malnutrition. Kappa (k) statistic revealed that SGA had the best agreement with the combined index (k=0.513; Table 3).
Table 3. Statistical evaluation of the efficacy of nutritional indicators, compared with the combined index.
| Nutritional indicators | Sensitivity (%) | Specificity (%) | k-value (P) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| NRS-2002 | 94.5 | 63.9 | 0.394 (0.000) | 40.7 | 97.8 |
| SGA | 87.5 | 77.6 | 0.513 (0.000) | 50.6 | 95.9 |
| Hypoalbuminemia | 81.5 | 78.3 | 0.486 (0.000) | 50.0 | 94.1 |
| Anemia | 80.2 | 75.1 | 0.433 (0.000) | 45.7 | 93.5 |
| Low serum creatinine | 40.1 | 87.0 | 0.284 (0.000) | 45.6 | 84.5 |
| Low total lymphocyte count | 33.3 | 94.5 | 0.333 (0.000) | 61.0 | 84.4 |
| Low total cholesterol | 30.9 | 95.3 | 0.325 (0.000) | 64.0 | 83.9 |
Abbreviations: NRS, nutritional risk screening; SGA, Subjective Global Assessment.
Association between nutritional risk and RCLMs and adverse outcomes
The X2-test and ORs with 95% confidence intervals showed that ‘NRS ⩾3' (versus NRS <3) was associated with a significantly higher incidence of abnormality of the laboratory indexes and the ORs for the incidence of abnormality increased from 1.5 times in uric acid to 3.5 times in serum albumin as shown in Table 4.
Table 4. The Chi-square test and odds ratios with 95% CIs calculated: incidence of abnormality of laboratory index in ‘NRS ⩾3' patients versus ‘NRS <3' patients.
| Laboratory index | Incidence in ‘NRS <3' (%) | Incidence in ‘NRS ⩾3' (%) | P-value | OR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 24.3 | 40.9 | 0.000 | 2.1 (1.6–2.8) |
| Neutrophils count | 23.4 | 38.6 | 0.000 | 2.0 (1.5–2.7) |
| Lymphocyte count | 9.0 | 17.1 | 0.000 | 2.0 (1.3–3.1) |
| Monocyte count | 11.2 | 19.4 | 0.000 | 1.9 (1.3–2.7) |
| Eosinophilia granulocyte count | 9.5 | 15.3 | 0.007 | 1.7 (1.1–2.6) |
| Basophil granulocyte count | 1.5 | 1.5 | 0.9 | — |
| Red blood cell count | 24.6 | 48.0 | 0.000 | 2.8 (2.1–3.7) |
| Hemoglobin level | 28.5 | 55.6 | 0.000 | 3.1 (2.3–4.1) |
| Platelet count | 19.7 | 36.7 | 0.000 | 2.3 (1.7–3.2) |
| Serum potassium | 6.5 | 13.8 | 0.000 | 2.3 (1.4–3.7) |
| Serum sodium | 5.1 | 13.8 | 0.000 | 2.9 (1.8–4.8) |
| Serum chlorine | 5.2 | 14.0 | 0.000 | 2.9 (1.8–4.8) |
| Serum calcium | 22.4 | 31.6 | 0.001 | 1.6 (1.1–2.1) |
| Serum creatinine | 24.1 | 34.2 | 0.001 | 1.6 (1.2–2.2) |
| Serum urea nitrogen | 12.7 | 15.8 | 0.223 | — |
| Serum uric acid | 20.1 | 28.9 | 0.002 | 1.5 (1.1–2.2) |
| Serum alanine aminotransferase | 11.8 | 19.5 | 0.001 | 1.8 (1.2–2.6) |
| Serum aspartate aminotransferase | 9.6 | 18.6 | 0.000 | 2.1 (1.4–3.2) |
| Serum total protein | 20.6 | 37.9 | 0.000 | 2.3 (1.7–3.2) |
| Serum albumen | 22.7 | 51.3 | 0.000 | 3.5 (2.6–4.8) |
| Serum total bilirubin | 7.2 | 10.9 | 0.048 | 1.6 (0.9–2.5) |
| Serum direct bilirubin | 5.8 | 13.8 | 0.000 | 2.6 (1.6–4.1) |
| Serum alkaline phosphatase | 9.8 | 19.7 | 0.000 | 2.3 (1.5–3.3) |
| Serum r-glutamyl trans peptidase | 17.4 | 27.6 | 0.000 | 1.8 (1.3–2.5) |
| Serum cholesterol | 22.1 | 25.0 | 0.251 | — |
Abbreviations: CI, confidence interval; NRS, nutritional risk screening; OR, odds ratio.
The ‘abnormality' of laboratory index meant beyond the normal range and dichotomous variables coded as 0 (normal) or 1 (abnormal) were used.
Although by the X2-test and ORs calculation, the OR for incidence of adverse outcomes in ‘NRS ⩾3' compared with ‘NRS <3' patients was 2.3 (95% confidence interval: 1.6–3.2; 28.6% in ‘NRS⩾3' versus 14.6% in ‘NRS <3', P=0.000), and a significantly higher incidence of adverse outcomes was also found in the ‘abnormal laboratory index' compared with the ‘normal laboratory index' (OR ranged from 1.1 to 3.4), several variables could confound this relation. The major potential confounders were nutritional support, surgery and application of radiotherapy or chemotherapy during hospitalization; hence, we entered those variables in the multiple logistic regression analysis. The analysis showed that nutritional support, radiotherapy or chemotherapy, serum albumin, total lymphocyte count, hepatic function and renal function were significantly related to the incidence of adverse outcomes (Table 5).
Table 5. Multiple logistic regression analysis: odds ratios for incidence of adverse outcomes.
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Nutritional risk on admission | 1.34 (0.89–1.99) | 0.155 |
| Nutritional support grading during hospitalization | 1.44 (1.21–1.71) | 0.000 |
| Surgery grading | 1.02 (0.87–1.19) | 0.860 |
| Application of radiotherapy or chemotherapy | 2.51 (1.41–4.46) | 0.002 |
| White blood cell count abnormality grading | 1.18 (0.93–1.50) | 0.189 |
| Low total lymphocyte count | 2.02 (1.20–3.35) | 0.007 |
| Low hemoglobin grading | 1.01 (0.88–1.16) | 0.911 |
| Electrolytes abnormality grading | 1.33 (0.91–1.98) | 0.148 |
| Hypoalbuminemia grading | 1.61 (1.30–1.97) | 0.000 |
| Low total cholesterol | 1.18 (0.78–1.78) | 0.443 |
| Liver function abnormality grading | 1.32 (1.01–1.73) | 0.035 |
| Renal function abnormality grading | 1.45 (1.10–1.92) | 0.009 |
Abbreviations: CI, confidence interval; OR, odds ratio.
The odds ratios for ‘nutritional risk', ‘application of radiotherapy or chemotherapy', ‘low total lymphocyte count' and ‘low total cholesterol' are categorical and dichotomous variables coded as 0 or 1 are used, whereas all the other variables are for each increase in the score unit.
Discussion
The present study might be the first investigation that reports objective correlations between nutritional risk and RCLMs and adverse outcomes. It further confirmed previous observations concerning the role of low lymphocyte count and hypoalbuminemia as predictors of complication, death and prolonged hospital stay7, 13 and demonstrated the predictive value of RCLMs in predicting adverse outcomes.
According to NRS-2002, 48.1% of patients were at nutritional risk on admission. The nutritional risk rate in this study is consistent with that reported in a recent review, which documented that 30–50% of patients in general hospitals were malnourished.14 No differences were found in the risk rate between medical and surgical departments. Our result was remarkably similar to that of Naber et al.11 and Vidal et al.15 However, in Velasco et al. study,16 a lower prevalence was found in surgical patients than in medical patients, which may be because most of these patients were admitted to the hospital for elective surgery in his study, as the risk rate was significantly lower in patients with grade 1 surgery than that with grade 2 surgery (39% versus 69.7%, P =0.000) in our study. Naber11 and Rocandio Pablo17 suggested that cancer weighed heavily in the diagnosis of malnutrition. Similarly, in this study, the risk rate was higher in cancer compared with other diseases, and, if patients with thyroid or breast cancer were excluded, the risk rate would be higher. As expected, nutritional risk is high in those disordered organ systems that may cause reduced oral intake and impaired gastrointestinal function. Significant differences in risk rate between diseases of different categories suggested that malnutrition was the result of the underlying disease. Estimated nutritional risk rate in this study would provide information for malnutrition prevalence studies. If malnutrition prevalence of each kind of disease, more specifically, of each severity degree, is established, it will be of great value. Such data would help clinicians distinguish malnutrition from underlying disease and allocate scarce resources where it is most needed.
About 30% patients had a weight loss >5% in 3 months, 48.7% had reduced food intake, with 20% having <1/4 and about 15% had a BMI <18.5 in this study. Our results are similar to that of Vidal et al.15 and Mercadal-Orfila et al.1 Among the obvious triggers for nutritional support (BMI, recent weight loss, recent dietary intake and severity of disease), reduced dietary intake was the most powerful trigger for nutritional support in this study. The patient was most likely to benefit from nutritional support when the NRS score depended mainly on the score of reduced oral intake.4 Actually, some patients were classified as at risk only because of BMI <18.5, without indication of malnutrition by any nutritional markers, and incidence of adverse outcomes was not increased in these patients in this study; moreover, in severe heart failure, a low BMI is adaptive as it reduces cardiac output and oxygen consumption. Some patients were classified as at risk only because of unintentional weight loss that was caused by disease–for example, serious diabetes mellitus. It is suggested that NRS-2002 classification was inferior to a single nutritional variable–namely, the recent dietary intake change for identifying patients at nutritional risk who may benefit from nutritional support just as Kuppinger claimed.18 The definition of nutritional support is inappropriate in this study as it is defined as nutrients administered that contain a combination of amino acids, carbohydrate and fat with nonprotein calories of at least 10 kcal/kg/day according to the guideline of the European Society of Parenteral and Enteral Nutrition; however, the duration is not defined, and Zhu-Ming Jiang defined it as at least 3 days in his study.4 In fact, it is impossible to define precisely the characteristics of an adequate regimen of nutritional support that must reach a compromise between a regimen of sufficient intensity and duration to normalize body composition and a regimen of such brevity that no detectable improvement in any malnutrition-related risk factors can be detected.19 It is concluded that the nutritional support was insufficient in the clinic, given that only 19.7% of at-risk patients and 60% of patients with reduced oral intake <1/4 recently were given grade 3 nutritional support.
This study first combined NRS-2002 and SGA with nutritional markers to produce a combined index used as a ‘gold standard'. Although it is somewhat arbitrary and open to criticism, it is not groundless as González Madroño et al.20 and Brugler et al.21 created nutritional screening tools that were based on nutritional markers in RCLMs, and they demonstrated substantial agreement between the created tools and SGA or the occurrence of malnutrition-related complications. Compared with other nutritional markers, NRS-2002 has better sensitivity and poorer specificity, which further confirmed that patients identified through NRS-2002 as ‘at risk' should subsequently be referred for further nutritional assessment to avoid being falsely classified as malnourished. Our results were similar to that of González Madroño et al.22 who found a sensitivity (27%) and specificity (91%) of low total lymphocyte count (<0.8 × 109/l) and a sensitivity (18%) and specificity (98%) of low total cholesterol (<2.5 mmol/l) according to SGA. One reason for the low sensitivity of nutritional markers was low cut points set for malnutrition, another reason was that these markers can be affected by many factors other than malnutrition such as inflammation, some drugs (corticosteroids, insulin, thyroid hormone, and so on), renal and liver disease. Further researches need to be conducted on validity of the marker in identifying malnutrition under specific condition to provide useful information regarding nutritional status.
In this study, it is demonstrated that ‘at risk' not only correlates with increased incidences of abnormalities of nutritional markers in agreement with prior studies but also closely relates to abnormalities of other parameters of RCLMs, which suggested that NRS-2002 provides a global index of ‘sickness' rather than be a nutritional screening tool only just as Jeejeebhoy23 said of SGA. After adjusting for confounders, not ‘at risk' determined at admission but nutritional support degree during hospitalization was significantly associated with adverse outcomes. It might be because patients' nutritional risk varies in the medical curriculum, it being rated once at admission is insufficient and should be repeated for using it as a predictor, whereas nutritional support grading during hospitalization is a better reflection of degree and duration of nutritional risk during hospitalization. It reminds us that it is important to select homogeneous patients in a nutrition-intervention outcome trial study, otherwise any potential benefit achieved by nutritional support will be obscured. Consistent with prior studies, our study further confirmed the value of lymphocyte count and albumin in predicting adverse outcomes and the rationality of some nutritional assessment tools derived from an equation, including serum albumin and lymphocyte count, as the purpose of nutritional screening tools is to identify those patients who are at nutritional risk and therefore at higher risk of complications. The predictive value of liver function and renal function highlights the importance of RCLMs.
This study has limitations that warrant consideration. First, lymphocyte count <1.5 × 109/l is defined as malnutrition in other literatures, as the lack of validation of such definition in chinese, <0.8 109/l is defined as malnutrition based on normal range used in clinic, this may influence the efficacy of the combined index. Second, the grading of some variables in multiple logistic regression analysis is too general. This would miss some valuable information. Third, patients being monitored intensively and rich clinical experience are required to make sure whether the complication is really a complication without being related specifically to the existing illness or a co-morbidity existing without discovered on admission. This is difficult to perform well in a study of a large sample of heterogeneous patients. Fourth, as logistic regressions are notoriously unstable, the associations demonstrated in this study need to be further confirmed in future study.
In conclusion, we showed that there are significant associations between nutritional risk and RCLMs and adverse outcomes; however, ‘at risk' determined at admission was insufficient for using it as a predictor and nutritional support degree during hospitalization was significantly associated with adverse outcomes in circumstances in which nutritional support was insufficient. After adjusting for confounders, hypoalbuminemia, low total lymphocyte count, abnormality of hepatic and renal function are predictors of adverse outcomes. In the absence of an ideal nutritional indicator to monitor the validity of nutritional treatment, it would be of value if these predictors can be improved with nutritional support.
The authors declare no conflict of interest.
References
- Mercadal-Orfila G, Lluch-Taltavull J, Campillo-Artero C, Torrent-Quetglas M. Association between nutritional risk based on the NRS-2002 test and hospital morbidity and mortality. Nutr Hosp. 2012;27:1248–1254. doi: 10.3305/nh.2012.27.4.5791. [DOI] [PubMed] [Google Scholar]
- Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krähenbühl L, Meier R, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27:340–349. doi: 10.1016/j.clnu.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Jie B, Jiang ZM, Nolan MT, Efron DT, Zhu SN, Yu K, et al. Impact of nutritional support on clinical outcome in patients at nutritional risk: a multicenter, prospective cohort study in Baltimore and Beijing teaching hospitals. Nutrition. 2010;26:1088–1093. doi: 10.1016/j.nut.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Smedley F, Bowling T, James M, Stokes E, Goodger C, O'Connor O, et al. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. Br J Surg. 2004;91:983–990. doi: 10.1002/bjs.4578. [DOI] [PubMed] [Google Scholar]
- Van Houwelingen AH, den Elzen Wendy PJ, Mooijaart SP, Heijmans M, Blom JW, de Craen Anton JM, et al. Predictive value of a profile of routine blood measurements on mortality in older persons in the general population: the Leiden 85-plus Study. PLoS One. 2013;8:e58050. doi: 10.1371/journal.pone.0058050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Rodríguez M, Medina-Cuadros M, Gómez-Ortega A, Martínez-Gallego G, Mariscal-Ortiz M, Martinez-Gonzalez MA, et al. Cholesterol and serum albumin levels as predictors of cross infection, death, and length of hospital stay. Arch Surg. 2002;137:805–812. doi: 10.1001/archsurg.137.7.805. [DOI] [PubMed] [Google Scholar]
- Kubota K, Kadomura T, Ohta K, Koyama K, Okuda H, Kobayashi M, et al. Analyses of laboratory data and establishment of reference values and intervals for healthy elderly people. J Nutr Health Aging. 2012;16:412–416. doi: 10.1007/s12603-011-0355-3. [DOI] [PubMed] [Google Scholar]
- Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN Guidelines for Nutrition Screening 2002. Clin Nutr. 2003;22:415–421. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status. JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- Naber TH, Schermer T, de Bree A, Nusteling K, Eggink L, Kruimel JW, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66:1232–1239. doi: 10.1093/ajcn/66.5.1232. [DOI] [PubMed] [Google Scholar]
- Shirodkar M, Mohandas KM. Subjective global assessment: a simple and reliable screening tool for malnutrition among Indians. Indian J Gastroenterol. 2005;24:246–250. [PubMed] [Google Scholar]
- Izaks Gerbrand J, Remarque Edmond J, Becker Sander V, Westendorp Rudi GJ. Lymphocyte count and mortality risk in older persons. The Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51:1461–1465. doi: 10.1046/j.1532-5415.2003.51467.x. [DOI] [PubMed] [Google Scholar]
- Barker Lisa A, Gout Belinda S, Crowe Timothy C. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8:514–527. doi: 10.3390/ijerph8020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal A, Iglesias MJ, Pertega S, Ayúcar A, Vidal O. Prevalence of malnutrition in medical and surgical wards of a university hospital. Nutr Hosp. 2008;23:263–267. [PubMed] [Google Scholar]
- Velasco C, García E, Rodríguez V, Frias L, Garriga R, Alvarez J, et al. Comparison of four nutritional screening tools to detect nutritional risk in hospitalized patients: a multicentre study. Eur J Clin Nutr. 2011;65:269–274. doi: 10.1038/ejcn.2010.243. [DOI] [PubMed] [Google Scholar]
- Rocandio Pablo AM, Arroyo Izaga M, Ansotegui Alday L. Assessment of nutritional status on hospital admission: nutritional scores. Eur J Clin Nutr. 2003;57:824–831. doi: 10.1038/sj.ejcn.1601616. [DOI] [PubMed] [Google Scholar]
- Kuppinger D, Hartl WH, Bertok M, Hoffmann JM, Cederbaum J, Bender A, et al. Nutritional screening for risk prediction in patients scheduled for extra-abdominal surgery. Nutrition. 2013;29:399–404. doi: 10.1016/j.nut.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Buzby GP, Williford WO, Peterson OL, Crosby LO, Page CP, Reinhardt GF, et al. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: the rationale and impact of previous clinical trials and pilot study on protocol design. Am J Clin Nutr. 1988;47:357–365. doi: 10.1093/ajcn/47.2.357. [DOI] [PubMed] [Google Scholar]
- González-Madroño A, Mancha A, Rodríguez FJ, Culebras J, de Ulibarri JI. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: comparison with two logistic regression models developed using SGA as the gold standard. Nutr Hosp. 2012;27:564–571. doi: 10.1590/S0212-16112012000200033. [DOI] [PubMed] [Google Scholar]
- Brugler L, Stankovic AK, Schlefer M, Bernstein L. A simplified nutrition screen for hospitalized patients using readily available laboratory and patient information. Nutrition. 2005;21:650–658. doi: 10.1016/j.nut.2004.10.012. [DOI] [PubMed] [Google Scholar]
- González Madroño A, Mancha A, Rodríguez FJ, de Ulibarri JI, Culebras J. The use of biochemical and immunological parameters in nutritional screening and assessment. Nutr Hosp. 2011;26:594–601. doi: 10.1590/S0212-16112011000300024. [DOI] [PubMed] [Google Scholar]
- Jeejeebhoy KN. Assessment of Nutritional Status. Saunders: Philadelphia, PA, USA; 1990. [Google Scholar]