Abstract
Atypical hemolytic uremic syndrome (aHUS) is a rare, possibly life-threatening disease characterized by platelet activation, hemolysis and thrombotic microangiopathy (TMA) leading to renal and other end-organ damage. We originally conducted two phase 2 studies (26 weeks and 1 year) evaluating eculizumab, a terminal complement inhibitor, in patients with progressing TMA (trial 1) and those with long duration of aHUS and chronic kidney disease (trial 2). The current analysis assessed outcomes after 2 years (median eculizumab exposure 100 and 114 weeks, respectively). At all scheduled time points, eculizumab inhibited terminal complement activity. In trial 1 with 17 patients, the platelet count was significantly improved from baseline, and hematologic normalization was achieved in 13 patients at week 26, and in 15 patients at both 1 and 2 years. The estimated glomerular filtration rate (eGFR) was significantly improved compared with baseline and year 1. In trial 2 with 20 patients, TMA event-free status was achieved by 16 patients at week 26, 17 patients at year 1, and 19 patients at year 2. Criteria for hematologic normalization were met by 18 patients at each time point. Improvement of 15 ml/min per 1.73 m2 or more in eGFR was achieved by 1 patient at week 26, 3 patients at 1 year, and 8 patients at 2 years. The mean change in eGFR was not significant compared with baseline, week 26, or year 1. Eculizumab was well tolerated, with no new safety concerns or meningococcal infections. Thus, a 2-year analysis found that the earlier clinical benefits achieved by eculizumab treatment of aHUS were maintained at 2 years of follow-up.
Keywords: aHUS, chronic kidney disease, complement, eculizumab
Atypical hemolytic uremic syndrome (aHUS) is a rare, progressive, and possibly life-threatening disease of chronic, uncontrolled complement activation, which affects patients of all ages.1 aHUS is caused by dysregulation of the complement alternative pathway, leading to persistent cleavage of complement protein C5, generation of proinflammatory C5a and lytic C5b proteins, and the formation of the membrane attack complex (C5b–9), which in turn leads to endothelial cell activation, injury, and death.1, 2, 3 Patients with complement alternative pathway dysregulation are at lifelong risk of thrombocytopenia, hemolysis, and renal impairment that may also occur with extrarenal (i.e., neurological, cardiovascular, pulmonary, and gastrointestinal) organ damage.1, 4, 5 Mutations in complement genes (e.g., CFH, MCP, CFI, CFB, and C3) or complement factor H (CFH) autoantibodies are identified in ∼50–70% of patients with aHUS.4, 6, 7 Components of the coagulation pathway can also modulate complement activation, and abnormalities in genes encoding thrombomodulin (THBD/CD146) and plasminogen (PLG)8, 9 have been identified in small numbers of patients. Rare cases of thrombotic microangiopathy (TMA) are caused by recessive diacylglycerol kinase ɛ (DGKE) mutations;10 thus far, instances have been limited to infants <1 year of age, and have not been reported in adolescent- and adult-onset aHUS. Although TMA complications observed in DGKE mutation carriers indicate complement-independent pathogenesis,10 a recent report identified a consanguineous family with DGKE mutations and evidence of significantly decreased C3 levels.11
Plasma exchange/plasma infusion (PE/PI) has historically been used to manage aHUS, yet 67% of adult patients required dialysis or died within 3 years, with variations in outcome by genotype.6 Registry and observational studies have demonstrated mortality rates of 8% at the first manifestation and 11% at 3 years of follow-up in one series,6 and 2% in adults and 8% in children at ⩾45 months of follow-up in another series.4 In addition, many patients with aHUS require kidney transplantation6 but experience high rates (68%) of post-transplant recurrence of TMA leading to graft failure; rates vary by genotype but graft failure occurs in ∼70% within 5 years.12 Thus, although PE/PI may temporarily maintain hematologic parameters, it does not inhibit the underlying complement-mediated pathogenic mechanism of TMA,4, 6, 13, 14 it does not block the terminal complement pathway, or it does not efficiently prevent progression of tissue damage and substantial morbidity and mortality.
Eculizumab (Soliris), the only approved treatment for aHUS,15, 16 is a humanized monoclonal antibody that binds with high affinity to the human C5 complement protein and blocks the formation of proinflammatory C5a and lytic C5b.7, 17, 18, 19, 20 The efficacy and safety of eculizumab were demonstrated in two prospective 26-week phase 2 studies with 1-year extension phases: one in patients with aHUS with clinical evidence of progressing TMA (trial 1; N=17; ClinicalTrials.gov numbers NCT00844545 (adults) and NCT00844844 (adolescents)), and one in patients with aHUS with long duration of disease and chronic kidney damage receiving prolonged PE/PI (trial 2; N=20; ClinicalTrials.gov numbers NCT00838513 (adults) and NCT00844428 (adolescents)).21 In both studies, eculizumab inhibited complement activation within 1 h of the first dose. Treatment resulted in significant improvements in platelet count and renal function by 26 weeks (primary analysis) and at the 1-year data cutoff. In addition, patients treated with eculizumab did not have additional TMA events, and PE/PI and dialysis were decreased or eliminated.
Longer-term results are needed to establish the ongoing efficacy and safety of eculizumab. Here we present outcomes after 2 years of eculizumab therapy in patients with aHUS in trial 1 and trial 2.
RESULTS
Patients
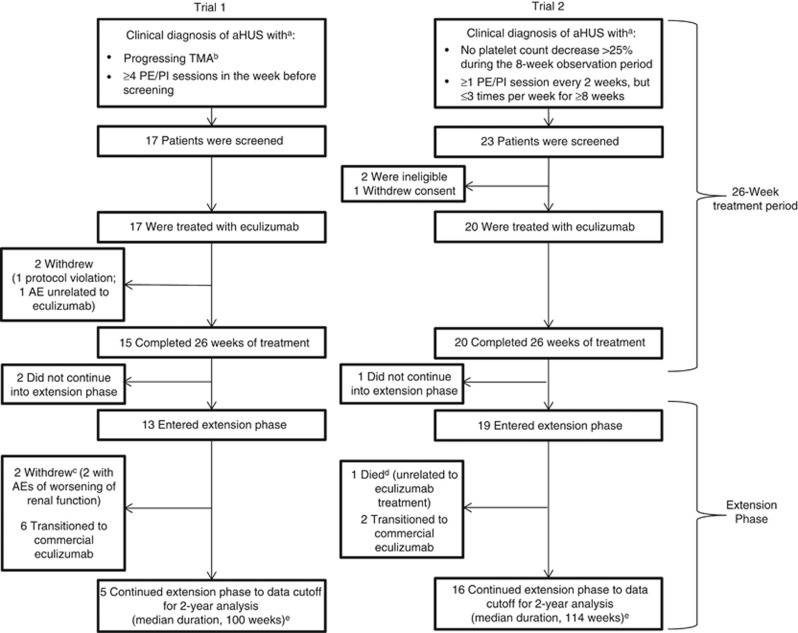
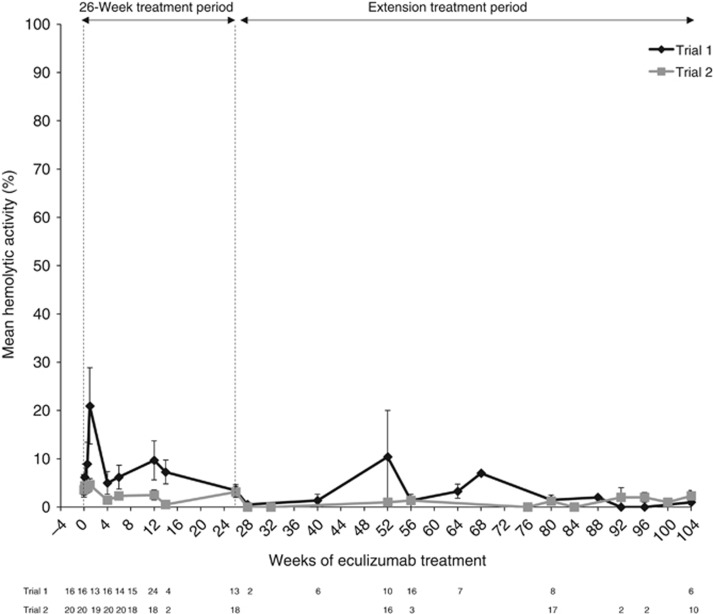
In trial 1, 17 patients (16 adults and one adolescent) with aHUS and progressing TMA were enrolled and 13 entered the extension phase (Figure 1). At the 2-year data cutoff, 11 patients remained on eculizumab (five on study drug in the trial; six transitioned to commercial eculizumab) and two had withdrawn due to worsening renal function. The median (range) duration of eculizumab exposure in trial 1 was 100 (2–145) weeks. In trial 2, 20 patients (15 adults and five adolescents) were enrolled in and completed the initial 26-week study. Nineteen patients entered the extension period and continued eculizumab treatment for ⩾78 weeks (Figure 1). At the 2-year data cutoff, 18 patients remained on eculizumab (16 on study drug in the trial, and two transitioned to commercial eculizumab). The median (range) duration of eculizumab exposure in trial 2 was 114 (26–129) weeks. In both trials, data were not available after patients transferred to commercial eculizumab. Terminal complement activity was sufficiently inhibited at all scheduled time points over 2 years in both studies (Figure 2).
Figure 1.
Patient disposition in trial 1 and trial 2. aAdditional inclusion and exclusion criteria were reported previously.21 bMeasured by low platelet count (<150 × 109/l) and a decrease of at least 25% lower than the average of three measures before the most recent TMA complication. cBoth patients who withdrew during the extension study had a history of kidney transplant, poor renal function, and CKD at the start of eculizumab treatment. These patients discontinued from the study after 67 and 82 weeks of treatment because of worsening of severe CKD due to aHUS before eculizumab initiation. dThe patient was hospitalized for pyrexia; while hospitalized, the patient experienced intestinal hemorrhage (distal ileum), which was considered a severe adverse event unrelated to eculizumab. The exact cause of the intestinal hemorrhage remains unknown. The patient died 37 days after admission. ePatients may have had greater than 104 weeks of data at the time of the cutoff. AE, adverse event; aHUS, atypical hemolytic uremic syndrome; CKD, chronic kidney disease; PE/PI, plasma exchange/plasma infusion; TMA, thrombotic microangiopathy.
Figure 2.
Effect of eculizumab on serum complement inhibition over 2 years of treatment in trial 1 and trial 2. Results from a pharmacodynamic assay that quantified serum complement activity by measuring the degree of hemolysis, as determined by means of a spectrophotometer. Inhibition of complement activity is indicated by 20% or lower hemolysis. Bars represent s.e.
Baseline demographics and disease characteristics were previously reported and are summarized in Table 1. The median time from onset of the current clinical manifestation of aHUS to screening for the trial was 0.8 months in trial 1 and 8.6 months in trial 2. PE/PI was received by 17 patients (100%) in trial 1 and by 20 patients (100%) in trial 2 before the initiation of eculizumab, and estimated glomerular filtration rate (eGFR) was <60 ml/min per 1.73 m2 in 17 (100%) and 18 patients (90%), respectively. Six patients (35%) in trial 1 and two patients (10%) in trial 2 were on dialysis before the first dose of eculizumab; in trial 1, 5 patients (29%) were on dialysis at the initiation of therapy. The median (range) duration of dialysis during the current manifestation was 22 (1−27) days in trial 1 and 625 (120−1129) days in trial 2. In trial 1, six patients (35%) had one current kidney transplant and one patient (6%) had two transplants. In trial 2, three patients (15%) had one current transplant, three patients (15%) had two transplants, and two patients (10%) had four transplants each.
Table 1. Baseline demographics and disease characteristics.
| Parameter | Trial 1 (N=17) | Trial 2 (N=20) |
|---|---|---|
| Age, years, median (range) | 28 (17–68) | 28 (13–63) |
| Female sex, n (%) | 12 (71) | 12 (60) |
| Presence of ⩾1 complement gene mutation and/or factor H autoantibody, n/N (%) | 13/17 (76)a | 14/20 (70)b |
| Time from diagnosis of aHUS to screening, months, median (range) | 9.7 (0.3–235.9) | 48.3 (0.7–285.8) |
| Time from current clinical presentation of aHUS to screening, months, median (range) | 0.8 (0.2–3.7) | 8.6 (1.2–45.0) |
| Received PE/PI within 1 week before eculizumab initiation, n (%) | 17 (100) | 20 (100) |
| Duration of PE/PI, months, median (range) | 0.7 (0.1–3.2) | 10.1 (2.4–47.0) |
| On dialysis before the first dose of eculizumab, n/N (%) | 6/17 (35)c | 2/20 (10) |
| ⩾1 Prior kidney transplant, n/N (%) | 7/17 (41) | 8/20 (40) |
| Platelet count, × 109/l, median (range) | 118 (62–161) | 218 (105–421) |
| Patients with platelet count <150 × 109/l, n (%) | 15 (88) | 3 (15) |
| LDH level, U/l, median (range) | 269 (134–634) | 200 (151–391) |
| LDH>ULNd, n (%) | 10 (59) | 4 (20) |
| Hemoglobin level, g/l, median (range) | 87 (67–126) | 108 (79–131) |
| Serum creatinine, μmol/l, median (range) | 256 (124–787) | 234 (106–893) |
| eGFR, ml/min per 1.73 m2 | ||
| Mean (s.d.) | 23 (15) | 31 (19) |
| Median | 19 | 28 |
| Range | 5–59 | 6–72 |
| CKD stage, n (%) | ||
| 1–2 | 0 | 2 (10) |
| 3a/b | 5 (29) | 8 (40) |
| 4 | 5 (29) | 6 (30) |
| 5 | 7 (41) | 4 (20) |
| EQ-5D scoree, median (range) | 0.8f (0.3−1.0) | 0.9 (0.2−1.0) |
Abbreviations: aHUS, atypical hemolytic uremic syndrome; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; EQ-5D, EuroQol 5-Dimension Questionnaire; LDH, lactate dehydrogenase; PE/PI, plasma exchange/plasma infusion; ULN, upper limit of normal.
CFH (n=4); CFI (n=3); anti-factor H autoantibodies and homozygous deletion of CFHR1 and CFHR3 (n=2); C3 (n=1); CD46 (n=1); CFH and homozygous deletion of CFHR1 and CFHR3 (n=1); and CFH, CFI, and homozygous deletion of CFHR1 and CFHR3 (n=1).
CFH (n=3); CFI (n=2); CD46 (n=1); CD46 and CFI (n=1); CFB (n=1); CFH and C3 (n=1); CFI and C3 (n=1); anti-factor H autoantibodies (n=1); homozygous deletion of CFHR1 and CFHR3 (n=1); CFH and homozygous deletion of CFHR1 and CFHR3 (n=1); and anti-factor H autoantibodies, heterozygous deletion of CFHR3, and homozygous deletion of CFHR1 (n=1).
One of the six patients who had been receiving dialysis within 8 weeks before eculizumab treatment discontinued dialysis 5 weeks before the first dose of eculizumab.
Definitions of the ULN for LDH levels varied by trial, gender, and age. For adult patients, ULN was defined as 261 U/l in both trials. For adolescent patients, ULN was defined as 230−261 U/l in trial 1 and 230−290 U/l in trial 2.
The EQ-5D scores range from 0 to 1, with higher scores indicating a better quality of life.
n=16.
Primary end points
All efficacy outcomes, calculated based on the intent-to-treat populations and defined in Table 2,22, 23, 24, 25 are reported in Table 3.
Table 2. Definitions of primary and secondary efficacy end points.
| End points | Definition |
|---|---|
| Primary | |
| Trial 1 | Platelet count increase: change in platelet count from baseline to week 26 and the proportion of patients with platelet count normalization (⩾150 × 109/l; proportion of patients with platelet count normalization sustained for at least two consecutive measurements for ⩾4 weeks was an additional analysis) |
| Trial 2 | TMA event-free status: absence of all the following for ⩾12 consecutive weeks: (1) a decrease in platelet count of >25%, (2) plasma exchange/infusion, and (3) new dialysis |
| Trials 1 and 2 | Hematologic normalization: platelet count normalization (⩾150 × 109/l) and LDH⩽ULN sustained for at least two consecutive measurements, which span a period of ⩾4 weeks |
| Secondary | |
| TMA outcomes | TMA event-free status (secondary in trial 1 only) |
| TMA intervention rate: the number of PE/PIs and new dialysis (interventions/patient per day); rate during the pre-eculizumab period compared with the rate during eculizumab treatment period | |
| Complete TMA response: hematologic normalization plus improvement in renal function (25% reduction from baseline in serum creatinine in two consecutive measurements for ⩾4 weeks) | |
| Hematologic outcomes | Change in hemoglobin ⩾20 g/l from baseline |
| LDH⩽ULN | |
| Renal function parametersa | eGFR increase ⩾15 ml/min per 1.73 m2b. Serum creatinine decrease ⩾25%. Improvement in proteinuria by ⩾1 grade. Chronic kidney disease improvement of ⩾1 stage23 |
| Change in HRQoL | Change in EQ-5D22 using TTO value set for US population. Attainment of MID in US TTO value (0.06)24 |
| Pharmacokinetics/pharmacodynamics | Peak and minimum serum eculizumab concentrations. Hemolysis assay25 |
| Additional | Proteinuria: change in grade according to dipstick measurement (negative, trace 1+, 2+, 3+, and 4+) |
| Urine protein-to-creatinine ratio | |
| Change in urine protein-to-creatinine ratio from baseline | |
Abbreviations: eGFR, estimated glomerular filtration rate; EQ-5D, 5-dimension EuroQoL questionnaire; HRQoL, health-related quality of life; LDH, lactate dehydrogenase; MID, minimally important difference; PE/PI, plasma exchange/plasma infusion; TMA, thrombotic microangiopathy; TTO, time trade-off; ULN, upper limit of normal.
Criteria were required to be sustained for ⩾2 consecutive measurements, which span a period of ⩾4 weeks.
For patients on dialysis, eGFR was calculated with the creatinine value immediately before dialysis or a fixed value of 10 ml/min per 1.73 m2.
Table 3. Efficacy outcomes.
|
Trial 1 |
Trial 2 |
|||||
|---|---|---|---|---|---|---|
| Parameter | 26-Week analysis (N=17) | 1-Year analysisa (N=17) | 2-Year analysisb (N=17) | 26-Week analysis (N=20) | 1-Year analysisa (N=20) | 2-Year analysisb (N=20) |
| Primary end points | ||||||
| Mean change from baseline in platelet count, × 109/l (95% CI) | 73c (40–105) | 91c (67–116)d | 75c (54–96) | NA | NA | NA |
| Normalization of platelet count, n/N (%) | 14/17 (82) | 15/17 (88) | 15/17 (88) | 18/20 (90) | 18/20 (90) | 18/20 (90) |
| TMA event-free status, n/N (%) | 15/17 (88) | 15/17 (88) | 15/17 (88) | 16/20 (80) | 17/20 (85) | 19/20 (95) |
| Hematologic normalization, n/N (%) | 13/17 (76) | 15/17 (88) | 15/17 (88) | 18/20 (90) | 18/20 (90) | 18/20 (90) |
| Secondary end points | ||||||
| TMA and hematologic outcomes | ||||||
| Complete TMA response, n/N (%) | 11/17 (65) | 13/17 (76) | 13/17 (76) | 5/20 (25) | 7/20 (35) | 11/20 (55) |
| LDH⩽ULN, n/N (%) | 14/17 (82) | 15/17 (88) | 15/17 (88) | 19/20 (95) | 19/20 (95) | 19/20 (95) |
| Increase in hemoglobin concentration of ⩾20 g/l from baseline, n/N (%) | 11/17 (65) | 13/17 (76) | 13/17 (76) | 9/20 (45) | 10/20 (50) | 13/20 (65) |
| Mean change in haptoglobin level from baseline, g/l (s.d.) | 0.5 (0.44) | 0.6 (0.41) | 0.9 (0.38) | –0.1 (0.52) | 0.3 (0.61) | 0.5 (0.64) |
| Renal outcomes | ||||||
| Increase in eGFR of ⩾15 ml/min per 1.73 m2, n/N (%)e | 8/17 (47) | 9/17 (53) | 10/17 (59) | 1/20 (5) | 3/20 (15) | 8/20 (40) |
| Decrease in serum creatinine level of ⩾25%, n/N (%)e | 11/17 (65) | 13/17 (76) | 13/17 (76) | 3/20 (15) | 7/20 (35) | 11/20 (55) |
| Improvement in proteinuria by ⩾1 grade, n/N (%)e,f | 12/16 (75) | 13/16 (81) | 14/16 (88) | 6/11 (55) | 7/11 (64) | 9/11 (82) |
| Improvement in CKD by ⩾1 stage, n/N (%)e | 10/17 (59) | 11/17 (65) | 12/17 (71) | 7/20 (35) | 9/20 (45) | 12/20 (60) |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; NA, not applicable; TMA, thrombotic microangiopathy; ULN, upper limit of normal range.
Median (range) treatment duration of 64 (2–90) weeks in trial 1 and 62 (26–74) weeks in trial 2.
Median (range) treatment duration of 100 (2–145) weeks in trial 1 and 114 (26–129) weeks in trial 2.
P<0.001 vs baseline.
Data were from week 60.
Criteria were required to be sustained for ⩾2 consecutive measurements, which span a period of ⩾4 weeks.
Proteinuria data were not available for all patients.
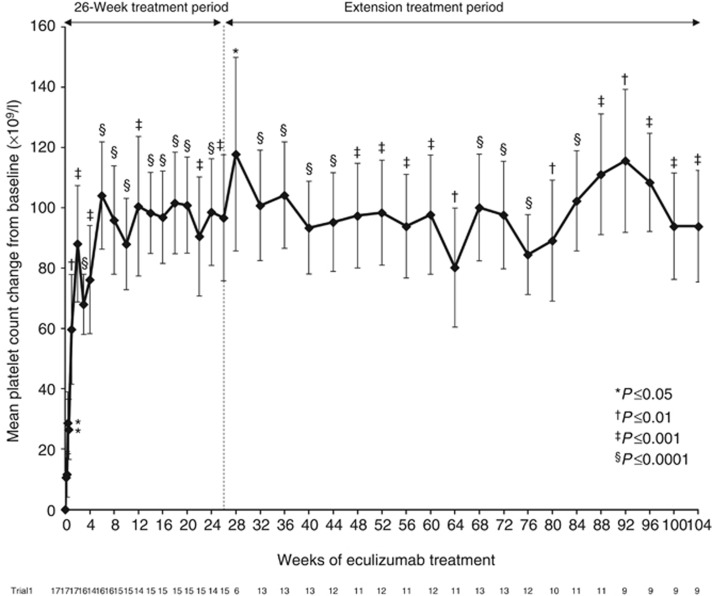
In trial 1, eculizumab treatment was associated with a significant increase in platelet count from baseline at week 26 (P<0.001), 1 year (P<0.001), and at the 2-year cutoff (P⩽0.001; Figure 3), indicating the inhibition of complement-mediated TMA throughout treatment. Platelet count was normalized in 14 patients (82%) at 26 weeks and in 15 patients (88%) at the 1- and 2-year cutoffs. Criteria for hematologic normalization were met by 13 patients (76%) at week 26 and by 15 patients (88%) at the 1- and 2-year cutoffs. The two patients who did not achieve hematologic normalization withdrew from the study within the initial 26-week treatment period.
Figure 3.
Mean change from baseline in platelet counts over 2 years of eculizumab treatment in trial 1 (P=0.001 at the 2-year cutoff). Bars represent s.e.
In trial 2, criteria for TMA event-free status were met by 16 patients (80%) at week 26, 17 patients (85%) at year 1, and 19 patients (95%) by the 2-year cutoff. Hematologic normalization was achieved by 18 patients (90%) at all three time points.
Secondary end points
TMA outcomes
In trial 1, 15 patients (88%) achieved TMA event-free status by 26 weeks, 1 year, and the 2-year cutoff. In comparison with a median (range) pretreatment rate of 0.88 (0.04−1.59), the median (range) TMA intervention rate decreased to 0 (0−0.31) events/patient per day at week 26, 1 year, and at the 2-year cutoff (P<0.001 for all time points). Complete TMA response was achieved by 11 patients (65%) at week 26 and by 13 patients (76%) at the 1- and 2-year cutoffs. In trial 2, the median (range) TMA intervention pretreatment rate (0.23 (0.05−1.09)) decreased to 0 (0−0) events/patient per day at 26 weeks, 1 year, and at the 2-year cutoff (P<0.001 for all time points). Complete TMA response was achieved by five patients (25%) at 26 weeks, by seven patients (35%) at 1 year, and by 11 patients (55%) at the 2-year cutoff. In both trials, patients with and without identified complement abnormalities met criteria for TMA event-free status and complete TMA response at the 2-year cutoff (Table 4).
Table 4. Efficacy outcomes at the 2-year cutoff in patients with and without identified complement abnormalitiesa.
|
Trial 1 (N=17) |
Trial 2 (N=20) |
|||
|---|---|---|---|---|
| Outcome, n (%) | Patients with identified abnormalities (n=13) | Patients with no identified abnormalities (n=4) | Patients with identified abnormalities (n=14) | Patients with no identified abnormalities (n=6) |
| TMA event-free status | 12 (92) | 3 (75) | 14 (100) | 5 (83) |
| Complete TMA response | 11 (85) | 2 (50) | 7 (50) | 4 (67) |
Abbreviation: TMA, thrombotic microangiopathy.
Includes complement gene mutations and polymorphisms and complement factor H autoantibodies.
Hematologic outcomes
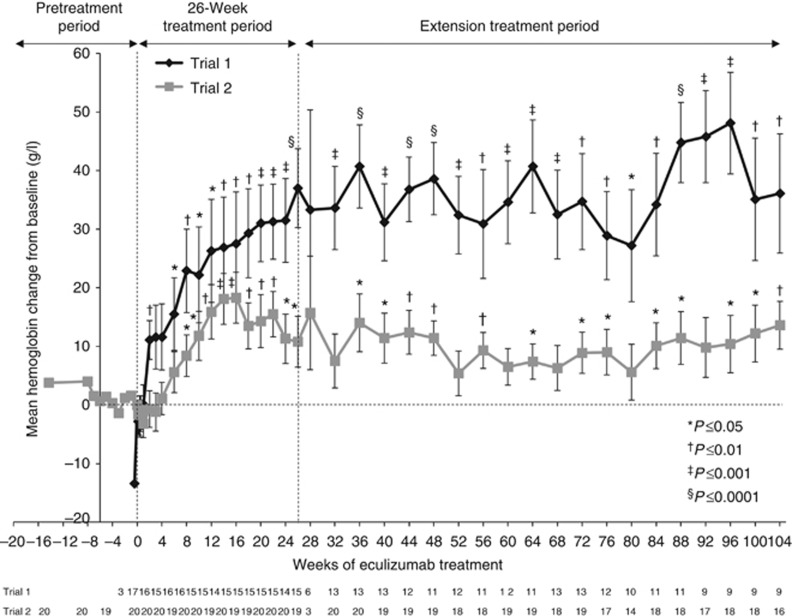
In trial 1, lactate dehydrogenase (LDH) levels less than or equal to the upper limit of normal were achieved by 14 patients (82%) at 26 weeks and by 15 patients (88%) at 1 and 2 years. The mean (s.d.) change from baseline in LDH level was –129 (161) U/l (P=0.0077) at 26 weeks, −27 (42) U/l (P=0.0860) at 1 year, and –205 (107) U/l (P=0.013) at the 2-year cutoff. The mean (s.d.) change from baseline in hemoglobin levels was 37 (26) g/l (P<0.0001) at 26 weeks, 32 (23) g/l (P=0.0005) at 1 year, and 36 (31) g/l (P=0.0075) at the 2-year cutoff (Figure 4).
Figure 4.
Mean change from baseline in hemoglobin concentration over 2 years of eculizumab treatment in trial 1 and trial 2 (P=0.0075 and P=0.0044, respectively, at the 2-year cutoff). Bars represent s.e.
In trial 2, 17 patients retained normal platelet counts and 14 maintained normal LDH levels throughout the study. At the 2-year data cutoff, 18 patients (90%) met the criteria for normalization of platelet count. Nineteen patients (95%), including all four with abnormal baseline values, achieved normal LDH levels at week 26 and at the 1- and 2-year cutoffs. The mean (s.d.) change from baseline in hemoglobin levels was 11 (19) g/l (P=0.0231) at 26 weeks, 5 (16) g/l (P=0.1751) at 1 year, and 14 (16) g/l (P=0.0044) at the 2-year cutoff (Figure 4).
Renal outcomes
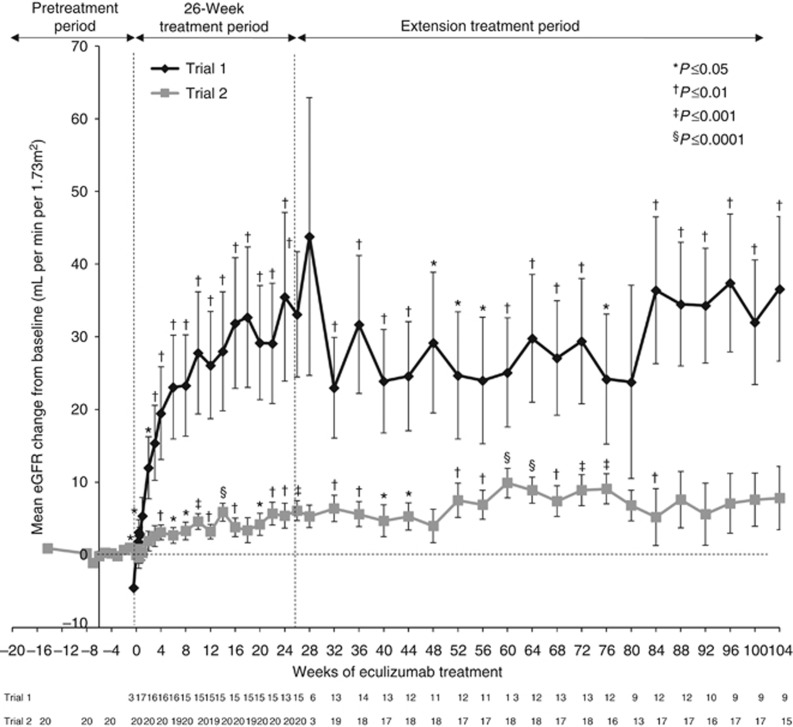
Improvements in eGFR from baseline levels that were observed at week 26 and at 1 year were maintained beyond 1 year of eculizumab treatment in trial 1 (Figure 5). Mean changes from baseline in eGFR at week 26, year 1, and the 2-year cutoff are shown in Table 5. An ad hoc pairwise comparison showed further improvement in eGFR between years 1 and 2 in trial 1 (P=0.0285). In trial 2, there were no significant differences between changes from baseline in eGFR at year 2 compared with week 26 or year 1 (P=NS). In a subgroup analysis (not shown), changes from baseline in eGFR were significant (P<0.05) in patients with shorter disease duration at 26 weeks, 1 year, and 2 years in Trial 1, but only at 26 weeks in patients with longer disease duration in trial 2. The absolute mean (s.d.) eGFR was 56 (40) ml/min per 1.73 m2 at 26 weeks, 42 (32) ml/min per 1.73 m2 at 1 year, and 56 (30) ml/min per 1.73 m2 at the 2-year cutoff in trial 1, and 37 (21) ml/min per 1.73 m2 at 26 weeks, 39 (20) ml/min per 1.73 m2 at 1 year, and 40 (18) ml/min per 1.73 m2 at the 2-year cutoff in trial 2.
Figure 5.
Mean change from baseline in eGFR over 2 years of eculizumab treatment in trial 1 (P=0.0062) and trial 2 (P=0.0959 at the 2-year cutoff). Bars represent s.e. eGFR, estimated glomerular filtration rate.
Table 5. Mean change from baseline in eGFR in trial 1 and trial 2.
|
P-value |
||||
|---|---|---|---|---|
| Time point | Change from baseline in eGFR (ml/min per 1.73 m2) | Compared with baseline eGFR | Compared with change from baseline at week 26 | Compared with change from baseline at the 1-year cutoff |
| Trial 1 | ||||
| Week 26 | ||||
| n | 15 | — | — | |
| Mean (s.d.) | 33 (33) | 0.0018 | ||
| Median (range) | 20 (−1 to 98) | |||
| Year 1 | ||||
| n | 12 | — | ||
| Mean (s.d.) | 25 (30) | 0.0164 | 0.6299 | |
| Median (range) | 15 (−8 to 82) | |||
| Year 2 | ||||
| n | 9 | |||
| Mean (s.d.) | 37 (30) | 0.0062 | 0.2099 | 0.0285 |
| Median (range) | 29 (3 to 82) | |||
| Trial 2 | ||||
| Week 26 | ||||
| n | 20 | — | — | |
| Mean (s.d.) | 6 (6) | 0.0003 | ||
| Median (range) | 5 (−1 to 20) | |||
| Year 1 | ||||
| n | 17 | — | ||
| Mean (s.d.) | 7 (10) | 0.0057 | 0.5264 | |
| Median (range) | 5 (−14 to 23) | |||
| Year 2 | ||||
| n | 15 | |||
| Mean (s.d.) | 8 (17) | 0.0959 | 0.8689 | 0.7700 |
| Median (range) | 11 (−42 to 30) | |||
Abbreviations: eGFR, estimated glomerular filtration rate.
Over 2 years of study, discontinuation of dialysis was observed in both trials. In trial 1, four out of the five patients (80%) on dialysis at baseline discontinued use, including one who discontinued before the first dose of eculizumab, and three who discontinued at a mean of 7.7 days after the treatment was initiated. New dialysis occurred in one patient during the 26-week study period, but the patient discontinued the study before the extension phase and was not included in this analysis. New dialysis also was initiated on study day 444 in one patient, who subsequently discontinued the study. Thus, two patients (11.8%) were on chronic dialysis at the 2-year cutoff. In trial 2, of the two patients who required dialysis at baseline, one continued dialysis at the 2-year cutoff and the other received dialysis until renal transplantation occurred on day 217. During the 2-year study period, one patient required new dialysis (days 695–696) during hospitalization for an intestinal hemorrhage and subsequently died as a result of this complication.
In trial 1, the number of patients who had transplants did not change between baseline and the 2-year cutoff, and no patient lost an existing renal graft. In trial 2, no patient underwent renal transplantation or lost an existing renal graft between years 1 and 2 of the study (as reported above, one patient received a renal transplant on study day 217).
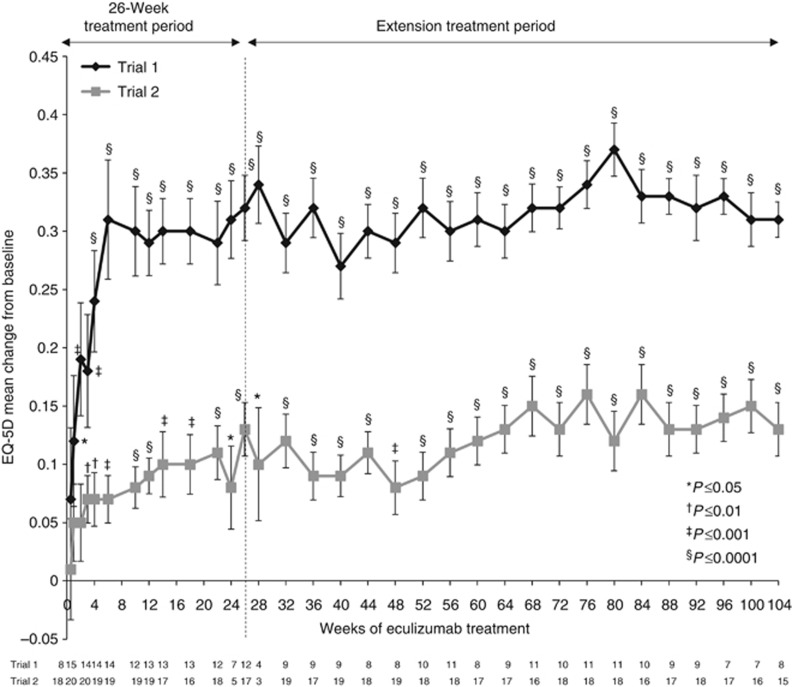
Health-related quality of life
Eculizumab significantly improved the HRQoL (health-related quality of life), as measured by changes from baseline on the EuroQoL Group 5-Dimension Self-Report Questionnaire (EQ-5D; Figure 6).22 Improvement began after week 1 in trial 1 (P=0.0398) and week 3 in trial 2 (P=0.0013), and was maintained over 2 years of treatment (P<0.05 compared with baseline).
Figure 6.
Least-squares mean change from baseline on patient HRQoL through 2 years of eculizumab treatment in trial 1 and trial 2 (P<0.0001 in both trials at the 2-year cutoff). Bars represent s.e. A change from baseline of 0.06 in EQ-5D score is considered clinically meaningful.24 EQ-5D, EuroQol 5-Dimension Questionnaire; HRQoL, health-related quality of life.
Safety
All 17 patients enrolled in trial 1 and 20 patients enrolled in trial 2 reported one or more adverse events (AEs). Through the 2-year data cutoff, 17 patients (100%) in trial 1 and 12 patients (60%) in trial 2 reported serious AEs. Table 6 lists AEs and serious AEs considered possibly, probably, or definitely associated with eculizumab, as identified by the investigator, through 2 years of treatment. AEs were reported with less frequency over time from week 26 to the 2-year update; no new or cumulative toxicities were observed. There were no cases of meningococcal infection in either trial. Among the six patients in trial 1 and two patients in trial 2 who discontinued eculizumab, there were no reported TMA manifestations within 8 weeks of follow-up. The presence of nonneutralizing human anti-human antibodies was confirmed in one patient in trial 1, who received a single dose of eculizumab and discontinued following diagnosis of systemic lupus erythematosus (an exclusion criterion). One patient in trial 2—who required PI to stop an intestinal hemorrhage through the introduction of clotting factors—initiated hemodialysis owing to severe renal failure, and died after 1.9 years of eculizumab treatment owing to complications from intestinal hemorrhage (as mentioned above). This was deemed unrelated to eculizumab, although the exact cause remains unknown.
Table 6. Adverse events considered possibly, probably, or definitely associated with eculizumab treatment, as identified by the investigator.
|
Trial 1 (N=17) |
Trial 2 (N=20) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Patients,
n
(%) |
Patients,
n
(%) |
|||||||||||
| Adverse event | 1 Day–6 months | 6–12 Months | 12–18 Months | 18+ Months | All | Greatest severity | 1 Day–6 months | 6–12 Months | 12–18 Months | 18+ Months | All | Greatest severity |
| Serious adverse eventsa | ||||||||||||
| Accelerated hypertension | 1 (6) | 1 (6) | — | — | 2 (12) | Moderate | — | — | — | — | — | — |
| Asymptomatic bacteriuria | — | 1 (6) | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Hypertension | 1 (6) | — | — | — | 1 (6) | Severe | — | — | — | — | — | — |
| Influenza | — | — | — | — | — | — | — | — | 1 (5) | — | 1 (5) | Severe |
| Peritonitis | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Severe |
| Venous sclerosis at infusion site | — | — | — | — | — | — | 1 (5) | 1 (5) | — | — | 2 (10) | Severe |
| Adverse events | ||||||||||||
| Abnormal blood clotting | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Mild |
| Alopecia | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Mild |
| Anemia | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Moderate |
| Asthenia | 1 (6) | 1 (6) | Moderate | — | — | — | — | —- | —- | |||
| Chest discomfort | — | — | — | — | — | — | — | — | 1 (5) | — | 1 (5) | Mild |
| Cough or productive cough | — | — | — | — | — | — | 1 (5) | — | 1 (5) | — | 2 (10) | Mild |
| Deafness bilateral | — | — | — | — | — | — | — | 1 (5) | — | — | 1 (5) | Moderate |
| Dermatitis | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Diarrhea | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Erythema | 1 (6) | — | — | — | 1 (6) | Moderate | — | — | — | — | — | — |
| Extravasation | — | — | — | — | —- | —- | 1 (5) | — | — | — | 1 (5) | Moderate |
| Fatigue | — | — | — | 1 (6) | 1 (6) | Moderate | — | — | — | — | — | — |
| Headache | 1 (6) | — | — | — | 1 (6) | Mild | 2 (10) | — | 1 (5) | — | 3 (15) | Moderate (1 patient) |
| Hematocrit decreased | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Hematuria | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Hemoglobin decreased | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Herpes zoster | — | 1 (6) | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Human polyomavirus (BK) infection | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Mild |
| Hypotension | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Moderate |
| Impetigo | 1 (6) | — | — | — | 1 (6) | Moderate | — | — | — | —- | —- | |
| Leukopenia | 2 (12) | — | — | — | 2 (12) | Mild | 1 (5) | 1 (5) | — | — | 2 (10) | Mild |
| Lymphopenia | — | — | — | — | — | — | 2 (10) | — | — | — | 2 (10) | Moderate (1 patient) |
| Menorrhagia | — | — | — | — | — | — | — | —- | —- | 1 (5) | 1 (5) | Mild |
| Nasal congestion | — | — | — | — | — | — | — | 1 (5) | — | — | 1 (5) | Mild |
| Nasopharyngitis | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Mild |
| Nausea | 2 (12) | — | — | — | 2 (12) | Mild | — | — | — | — | —- | —- |
| Pharyngolaryngeal pain | — | — | — | — | — | — | — | 1 (5) | — | — | 1 (5) | Moderate |
| Pruritus | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Mild |
| Pyrexia | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Q fever | — | — | — | — | — | — | — | 1 (5) | — | — | 1 (5) | Moderate |
| Rhinorrhea | — | — | — | — | — | — | 1 (5) | — | — | — | 1 (5) | Moderate |
| Tremor | 1 (6) | — | — | — | 1 (6) | Moderate | — | — | — | — | — | — |
| Urinary tract infection | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Vertigo | 1 (6) | — | — | — | 1 (6) | Mild | — | — | — | — | — | — |
| Vomiting | 2 (12) | — | — | 1 (6) | 3 (18) | Mild | — | — | — | — | — | — |
A serious adverse event was defined as any event that results in death, is immediately life threatening, requires hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect.
DISCUSSION
Eculizumab was proven to inhibit the complement-mediated TMA and improve outcomes for patients with aHUS at 26 weeks and 1 year.21 Here, a 2-year analysis conducted to examine longer-term outcomes demonstrated that clinical benefits achieved at earlier time points–including improvements in hematologic parameters, renal function, and HRQoL—are maintained with ongoing therapy. Terminal complement activity was inhibited over 2 years in patients who remained in the studies. In trial 1, the mean change from baseline in eGFR at year 2 was significantly higher than that achieved at 1 year, although the P-value was derived from an ad hoc pairwise analysis and not protocol defined. Thus, it can only be stated that when compared with baseline the gains in eGFR at week 26 were maintained at year 2. Moreover, since the 1-year update, additional patients met criteria for specific renal parameters (i.e., improvement in eGFR, serum creatinine, and/or chronic kidney disease stage). Eculizumab was generally safe, and no cumulative toxicity was observed over 2 years.
Before the availability of eculizumab, patients with aHUS could be expected to progress to renal failure, and were at ongoing risk of serious TMA-related morbidity and death at rates that varied by genotype.4, 6 In the current trials, eculizumab-treated patients had improvements in chronic kidney disease over 2 years, and earlier therapy initiation was significantly correlated with greater gain in eGFR at the 2-year cutoff in both trials. However, only patients in trial 1, who were treated earlier than patients in trial 2, showed ongoing, significant improvement in eGFR between years 1 and 2. This is consistent with a conclusion from the previous report21 that early treatment initiation may offer patients the best possible chance to recover renal function. The mechanism by which eculizumab leads to gains in renal function is not known, but it may involve kidney remodeling,26, 27 ongoing dissolution of thrombi, maintenance of controlled blood pressure, and/or normalization of endothelial cell structure and function.28 In addition, no patient with a renal transplant required a new graft while receiving eculizumab during the studies, although two patients with grafts withdrew owing to worsening renal function. This outcome is distinct from the expected 24% graft loss rate after 1 year in untreated patients.12
The use of eculizumab was also associated with several other important clinical benefits,21, 29 including the discontinuation of PE/PI and dialysis, and significant improvements in QoL. In addition to being of limited clinical benefit for patients with aHUS,4, 13 PE/PI must be administered up to several times per week.13 Dialysis also severely impairs QoL, due to patient dependence on the procedure and associated restrictions of daily living.30 In trial 1, most patients on dialysis at baseline were able to discontinue within ∼1 week of eculizumab initiation. In comparison with the natural history of the disease and supportive care, ongoing therapy with eculizumab leads to an improvement in the overall well-being for patients with aHUS.
Current guidelines recommend initiation of eculizumab for both pediatric and adult patients as soon as a diagnosis of aHUS is made.7, 31 Tests to rule out thrombotic thrombocytopenic purpura (caused by severe deficiency of ADAMTS13 activity) and Shiga-like toxin–producing Escherichia coli HUS1, 2, 32 are widely available, give rapid results,7 and are useful in the differential diagnosis of aHUS.33 Mutational analysis is available, but it may take months to complete and is not required to diagnose aHUS and commence treatment;7, 31 furthermore, mutations are currently identified in only 50–70% of patients.4, 6, 7 Although C3 consumption has been reported in a consanguineous family with DGKE mutations,11 the role of eculizumab has not been established in patients with this mutation; the identified family had good outcomes with PE/PI. In both of the current trials, outcomes with eculizumab were similar for patients with and without identified complement abnormalities throughout 2 years of study.
Current regulatory guidance notes the potential risk of TMA manifestations following the discontinuation of eculizumab.15, 16 During the limited 8 weeks of follow-up in the current studies, 0/3 (0%) of patients who discontinued eculizumab experienced a TMA event. However, one additional patient experienced a TMA complication following a respiratory infection 80 days post-discontinuation. This patient restarted eculizumab with resolution of hemolysis and gradual renal improvement. Individual case studies19, 34, 35, 36, 37, 38 and a recent observational study39 report TMA manifestations following eculizumab discontinuation in six of the 16 patients (38% three with CFH mutations, one with C3, one with no mutation identified, and one with CFH autoantibody). Although limited, evidence suggests that close follow-up of patients who discontinue eculizumab is necessary to prevent poor outcomes in case of recurrence of TMA.20 Consensus regarding the optimal length of treatment must be informed by current treatment guidelines31 and better understanding of patients' underlying risk of adverse TMA outcomes.
In conclusion, 2-year analyses of these trials demonstrated that longer-term eculizumab therapy maintained inhibition of complement activity, TMA, and improvements in hematologic parameters and renal function. Furthermore, eculizumab continued to prevent progression to end-stage renal disease in the majority of patients with aHUS. Targeting the underlying complement-mediated etiology represents a significant advancement in the treatment of aHUS, and the favorable safety profile of eculizumab is compatible with its longer-term use. Results from a long-term, observational trial of patients who previously participated in clinical studies with eculizumab and data from a global aHUS registry will provide further insights regarding the effects of ongoing eculizumab treatment on TMA and renal function.
METHODS
Study design and patient selection
This paper reports 2-year findings from the long-term extension phases of two open-label, single-arm, multicenter, controlled trials that evaluated the efficacy and safety of eculizumab in patients with aHUS. Details of the study design and patient enrollment criteria were published previously.21 Genetic testing was not required for study entry, but C3, CFH, CFI, and CD46 mutations, hybrid CFH, copy-number variation in CFH and CFHRs, and anti-CFH autoantibodies were identified at study initiation. Patients in trial 1 were required to have clinical evidence of progressing TMA (i.e., platelet count <150 × 109/l and decreased by ⩾25% compared with an average of three measures obtained before the most recent TMA presentation) despite ⩾4 PE/PI sessions in the week before screening. Patients were eligible for trial 2 if they had PE/PI more than once every 2 weeks but three or more times per week, and did not have decreases in platelet count >25% for ⩾8 weeks before the first dose of eculizumab. Key exclusion criteria for both trials were ADAMTS13 activity ⩽5%, as measured at a central laboratory,40 evidence of Shiga toxin–producing Escherichia coli infection, or prior eculizumab exposure. Patients received immunization and/or prophylaxis against Neisseria meningitidis, as described previously.21
Patients were eligible for the extension phase of either study after completing the initial 26-week treatment period. No patients were excluded, and each patient and primary investigator made the decision to continue in the extension phase or transfer to eculizumab obtained via commercial route. During the extension, patients received a maintenance dose of 1200 mg eculizumab every 2 weeks. Patients who discontinued were required to attend follow-up visits 1, 2, 4, and 8 weeks after the last eculizumab dose.
Assessments
For pharmacodynamic studies, blood samples were collected every 26 weeks during the extension period and at the last visit. Complement activity was quantified as the degree of in vitro hemolysis of chicken erythrocytes by patient serum, as determined by spectrophotometry.25
Efficacy end points were defined previously21 and are described in Table 2. During the extension study, hematologic (including changes from baseline in levels of LDH, hemoglobin, and haptoglobin) and renal parameters (including chronic kidney disease stage and use of dialysis) were assessed every 4 weeks and at the last study visit. Measures of renal function were assessed every 8 weeks and at the last visit of the extension phase. eGFR was calculated using the Schwartz formula41 for patients <18 years of age at screening and the Modification of Diet in Renal Disease equation42 for adults.
The presence of human anti-human antibodies was assessed with an electrochemiluminescence bridging assay using eculizumab conjugated to biotin and to SulfoTag (Meso Scale Diagnostics, LLC; Rockville, MD), which emits light on application of electrochemical stimulation initiated at the electrode surface of a streptavidin-coated assay plate. Equimolar quantities of the two conjugated eculizumab structures were added to the serum-containing samples (2% (v/v)) and incubated overnight at room temperature to allow anti-eculizumab bridging complexes to form. Samples were then transferred to a blocked assay plate and incubated at room temperature in the dark for 3 h, followed by washing and measurement of electrochemiluminescence with the Sector Imager 2400 (Meso Scale Diagnostics, LLC; normalized against control samples).
Statistical analyses
Methods for statistical analysis have been described previously21 and were performed by Alexion Pharmaceuticals (Cheshire, CT) using a SAS System (version 9.2). Baseline demographic and disease characteristics were summarized using descriptive statistics. Efficacy and safety end points were analyzed based on data obtained through the 2-year cutoff and were compared with baseline values, which were defined as the last available measurement taken before the first eculizumab dose. The number and percentage of patients who achieved end points were reported at week 26, year 1, and year 2. Wilcoxon signed-rank tests were used to compare pre- and post-treatment TMA intervention rates. For changes from baseline in platelet count, a last observation carried forward approach was used for imputation of missing values through the 2-year cutoff. Least-squares mean changes from baseline in platelet count, eGFR, and EQ-5D were analyzed using an analysis of variance model in which baseline was a covariate and time was considered a categorical variable. Paired t-tests were used to compare changes from baseline and to generate pairwise comparisons of changes in eGFR between week 26, year 1, and year 2. Changes from baseline in eGFR in subgroups defined by the median duration of the current TMA manifestation before eculizumab initiation were assessed using t-tests.
Acknowledgments
LAG is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Medical writing/editorial support was provided by Kristen W Quinn and Faith Reidenbach of Peloton Advantage, LLC, supported by Alexion Pharmaceuticals, and by John Kincaid, Erin Harvey, and Kenyon Ogburn, of Alexion Pharmaceuticals.
These studies were sponsored by Alexion Pharmaceuticals. CL has received lecture, consultancy, and travel honoraria from Alexion Pharmaceuticals, and was the Canadian coordinator for trials of eculizumab in patients with aHUS that were funded by Alexion Pharmaceuticals, and holds unrestricted research grants from Alexion Pharmaceuticals; LAG has received research and consulting support from Alexion Pharmaceuticals. SB received research funding, consultancy fees, and travel honoraria from Alexion Pharmaceuticals. CLB is an employee and stockholder of Alexion Pharmaceuticals. YD has received honoraria and educational support from Alexion Pharmaceuticals. CL has received lecture and travel honoraria from Alexion Pharmaceuticals, and consultancy fees from Hoffmann-La Roche and Novartis International AG. CL has received consultancy fees and travel honoraria from Alexion Pharmaceuticals and was the French coordinator of eculizumab trials in patients with aHUS that were funded by Alexion Pharmaceuticals. The remaining authors declared no competing interests.
Footnotes
Statement of Prior Presentation: Aspects of these data were presented at the ASN 2012 congress: (1) Legendre CM, et al. Eculizumab (ECU) in Atypical Hemolytic Uremic Syndrome (aHUS) Patients (pts) with Progressing TMA: Continued Improvements at 2-Year Follow-Up. J Am Soc Nephrol. 2012;23:88A–89A. (2) Licht C, et al. Eculizumab (ECU) Is Effective in Atypical Hemolytic Uremic Syndrome (aHUS) Patients (Pts) with a Long Disease Duration and CKD: 2-Year Data. J Am Soc Nephrol. 2012;23:89A–90A.
References
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Heinen S, Skerka C. Thrombotic microangiopathies: new insights and new challenges. Curr Opin Nephrol Hypertens. 2010;19:372–378. doi: 10.1097/MNH.0b013e32833aff4a. [DOI] [PubMed] [Google Scholar]
- Licht C, Pluthero FG, Li L, et al. Platelet-associated complement factor H in healthy persons and patients with atypical HUS. Blood. 2009;114:4538–4545. doi: 10.1182/blood-2009-03-205096. [DOI] [PubMed] [Google Scholar]
- Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azukaitis K, Loirat C, Malina M, et al. Macrovascular involvement in a child with atypical hemolytic uremic syndrome. Pediatr Nephrol. 2014;29:1273–1277. doi: 10.1007/s00467-013-2713-3. [DOI] [PubMed] [Google Scholar]
- Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Fakhouri F, Roumenina LT, et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- Delvaeye M, Noris M, De Vriese A, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu F, Maga T, Meyer NC, et al. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2014;25:55–64. doi: 10.1681/ASN.2013050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire M, Fremeaux-Bacchi V, Schaefer F, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet. 2013;45:531–536. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westland R, Bodria M, Carrea A, et al. Phenotypic expansion of DGKE-associated diseases. J Am Soc Nephrol. 2014;25:1408–1414. doi: 10.1681/ASN.2013080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Quintrec M, Zuber J, Moulin B, et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant. 2013;13:663–675. doi: 10.1111/ajt.12077. [DOI] [PubMed] [Google Scholar]
- Loirat C, Garnier A, Sellier-Leclerc AL, et al. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36:673–681. doi: 10.1055/s-0030-1262890. [DOI] [PubMed] [Google Scholar]
- Ariceta G, Besbas N, Johnson S, et al. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol. 2009;24:687–696. doi: 10.1007/s00467-008-0964-1. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration . Soliris [prescribing information] Alexion Pharmaceuticals, Inc.: Cheshire, CT, USA; 2014. [Google Scholar]
- European Medicines Agency . Soliris [summary of product characteristics] Alexion Europe SAS: Paris, France; 2014. [Google Scholar]
- Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- Barnett AN, Asgari E, Chowdhury P, et al. The use of eculizumab in renal transplantation. Clin Transplant. 2013;27:E216–E229. doi: 10.1111/ctr.12102. [DOI] [PubMed] [Google Scholar]
- Chatelet V, Fremeaux-Bacchi V, Lobbedez T, et al. Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant. 2009;9:2644–2645. doi: 10.1111/j.1600-6143.2009.02817.x. [DOI] [PubMed] [Google Scholar]
- Zuber J, Quintrec ML, Krid S, et al. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant. 2012;12:3337–3354. doi: 10.1111/j.1600-6143.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- Pickard AS, Neary MP, Cella D.Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer Health Qual Life Outcomes 2007570[Erratum in: Health Qual Life Outcomes. 2010;8:4]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinder CS, Rinder HM, Smith BR, et al. Blockade of C5a and C5b-9 generation inhibits leukocyte and platelet activation during extracorporeal circulation. J Clin Invest. 1995;96:1564–1572. doi: 10.1172/JCI118195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macconi D, Sangalli F, Bonomelli M, et al. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol. 2009;174:797–807. doi: 10.2353/ajpath.2009.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi A, Gagliardini E, Sangalli F, et al. ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int. 2006;69:1124–1130. doi: 10.1038/sj.ki.5000060. [DOI] [PubMed] [Google Scholar]
- Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014;29:333–342. doi: 10.1007/s00467-013-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GM. Eculizumab: a review of its use in atypical haemolytic uraemic syndrome. Drugs. 2013;73:2053–2066. doi: 10.1007/s40265-013-0147-7. [DOI] [PubMed] [Google Scholar]
- Theofilou P. Quality of life in patients undergoing hemodialysis or peritoneal dialysis treatment. J Clin Med Res. 2011;3:132–138. doi: 10.4021/jocmr552w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campistol JM, Arias M, Ariceta G, et al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2013;33:27–45. doi: 10.3265/Nefrologia.pre2012.Nov.11781. [DOI] [PubMed] [Google Scholar]
- Benz K, Amann K. Thrombotic microangiopathy: new insights. Curr Opin Nephrol Hypertens. 2010;19:242–247. doi: 10.1097/MNH.0b013e3283378f25. [DOI] [PubMed] [Google Scholar]
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- Carr R, Cataland SR. Relapse of aHUS after discontinuation of therapy with eculizumab in a patient with aHUS and factor H mutation. Ann Hematol. 2013;92:845–846. doi: 10.1007/s00277-012-1622-z. [DOI] [PubMed] [Google Scholar]
- Alachkar N, Bagnasco SM, Montgomery RA. Eculizumab for the treatment of two recurrences of atypical hemolytic uremic syndrome in a kidney allograft. Transpl Int. 2012;25:e93–e95. doi: 10.1111/j.1432-2277.2012.01497.x. [DOI] [PubMed] [Google Scholar]
- Cayci FS, Cakar N, Hancer VS, et al. Eculizumab therapy in a child with hemolytic uremic syndrome and CFI mutation. Pediatr Nephrol. 2012;27:2327–2331. doi: 10.1007/s00467-012-2283-9. [DOI] [PubMed] [Google Scholar]
- Pu JJ, Sido A. Successful discontinuation of eculizumab therapy in a patient with aHUS. Ann Hematol. 2014;93:1423–1425. doi: 10.1007/s00277-013-1972-1. [DOI] [PubMed] [Google Scholar]
- Gulleroglu K, Fidan K, Hancer VS, et al. Neurologic involvement in atypical hemolytic uremic syndrome and successful treatment with eculizumab. Pediatr Nephrol. 2013;28:827–830. doi: 10.1007/s00467-013-2416-9. [DOI] [PubMed] [Google Scholar]
- Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64:633–637. doi: 10.1053/j.ajkd.2014.01.434. [DOI] [PubMed] [Google Scholar]
- Kokame K, Nobe Y, Kokubo Y, et al. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- Seikaly MG, Loleh S, Rosenblum A, et al. Validation of the Center for Medicare and Medicaid Services algorithm for eligibility for dialysis. Pediatr Nephrol. 2004;19:893–897. doi: 10.1007/s00467-004-1488-y. [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]