Abstract
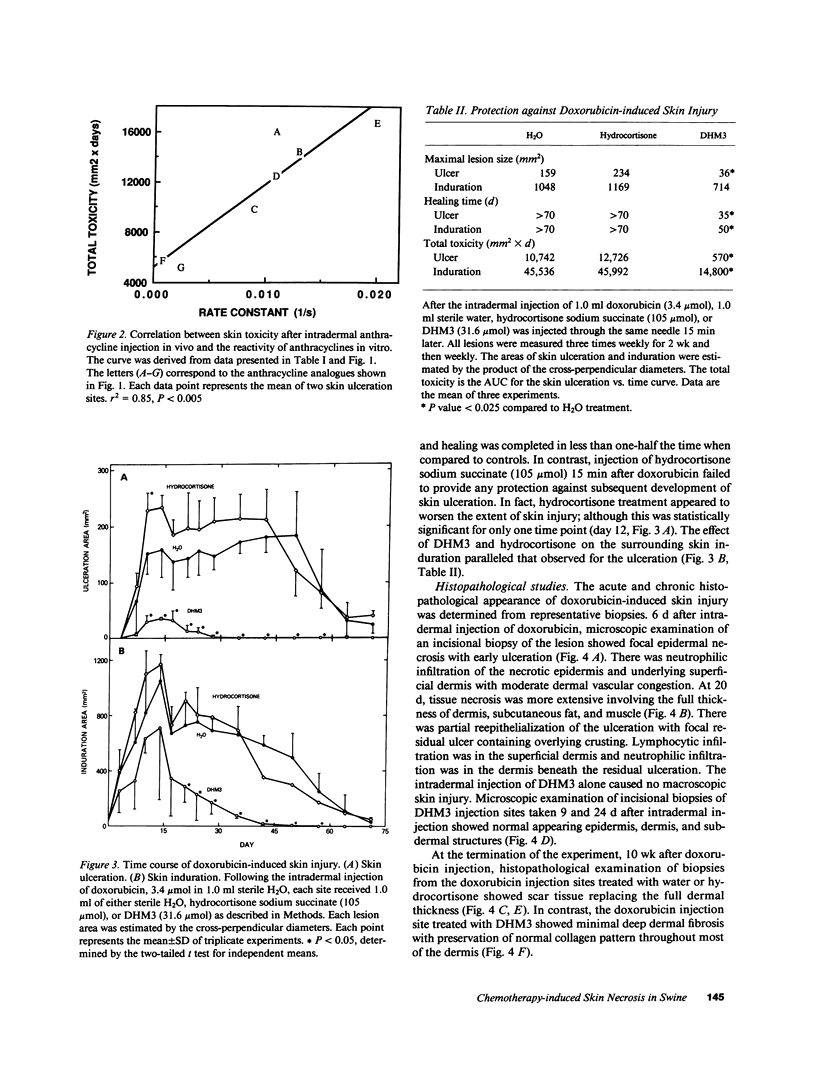
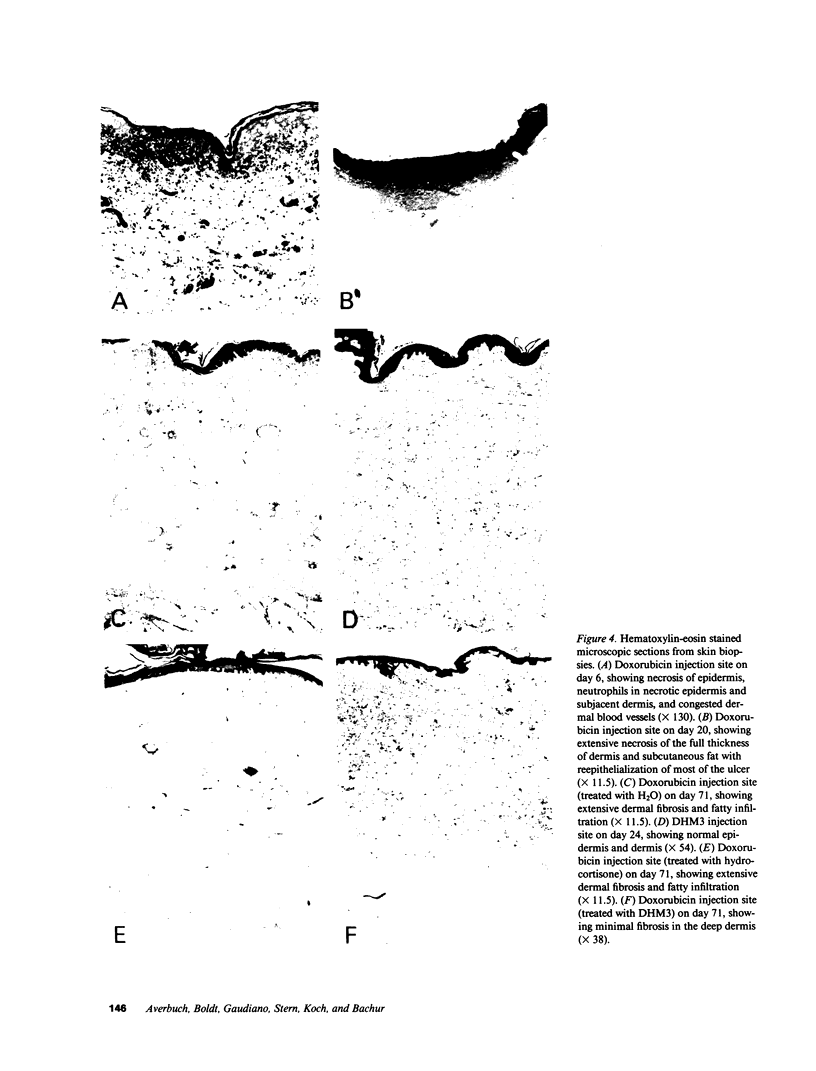
The reactivity of antitumor anthracycline and mitomycin C antibiotics with the oxomorpholinyl radical dimers, bi(3,5,5-trimethyl-2-oxomorpholin-3-yl) (TM3) and bi(3,5-dimethyl-5-hydroxymethyl-2-oxomorpholin-3-yl) (DHM3), was studied in vitro. The oxomorpholinyl radical reduced daunorubicin to a quinone methide intermediate that reacted with solvent to form 7-deoxydaunorubicinone. The solvolysis reaction followed first order kinetics, and the reactivity rate constants (k2) measured for seven anthracycline analogues ranged from 2 X 10(-2) s-1 to 8.0 X 10(-4) s-1. The chemical reactivity of each anthracycline quinone methide correlated with the total skin toxicity caused by the respective parent anthracycline following injection into swine skin. Microscopic examination of experimental lesions in swine skin resemble those observed in humans after inadvertant chemotherapy extravasation. Hydrocortisone sodium succinate was not effective for the treatment of doxorubicin-induced skin necrosis, whereas DHM3 was effective for the treatment of skin necrosis caused by all seven anthracyclines and by the quinone containing antibiotic, mitomycin C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella B. R., Fisher J. A chemical perspective on the anthracycline antitumor antibiotics. Environ Health Perspect. 1985 Dec;64:4–18. doi: 10.1289/ehp.85644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenta L. C., Manders E. K. Mitomycin C extravasation injuries. Cancer. 1983 Mar 15;51(6):1080–1082. doi: 10.1002/1097-0142(19830315)51:6<1080::aid-cncr2820510618>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Averbuch S. D., Gaudiano G., Koch T. H., Bachur N. R. Doxorubicin-induced skin necrosis in the swine model: protection with a novel radical dimer. J Clin Oncol. 1986 Jan;4(1):88–94. doi: 10.1200/JCO.1986.4.1.88. [DOI] [PubMed] [Google Scholar]
- Averbuch S. D., Gaudiano G., Koch T. H., Bachur N. R. Radical dimer rescue of toxicity and improved therapeutic index of adriamycin in tumor-bearing mice. Cancer Res. 1985 Dec;45(12 Pt 1):6200–6204. [PubMed] [Google Scholar]
- Bachur N. R., Gee M. V., Friedman R. D. Nuclear catalyzed antibiotic free radical formation. Cancer Res. 1982 Mar;42(3):1078–1081. [PubMed] [Google Scholar]
- Banks A. R., Jones T., Koch T. H., Friedman R. D., Bachur N. R. Prevention of adriamycin toxicity. Cancer Chemother Pharmacol. 1983;11(2):91–93. doi: 10.1007/BF00254252. [DOI] [PubMed] [Google Scholar]
- Barlock A. L., Howser D. M., Hubbard S. M. Nursing management of adriamycin extravasation. Am J Nurs. 1979 Jan;79(1):94–96. [PubMed] [Google Scholar]
- Bartek M. J., LaBudde J. A., Maibach H. I. Skin permeability in vivo: comparison in rat, rabbit, pig and man. J Invest Dermatol. 1972 Mar;58(3):114–123. doi: 10.1111/1523-1747.ep12538909. [DOI] [PubMed] [Google Scholar]
- Berlin V., Haseltine W. A. Reduction of adriamycin to a semiquinone-free radical by NADPH cytochrome P-450 reductase produces DNA cleavage in a reaction mediated by molecular oxygen. J Biol Chem. 1981 May 25;256(10):4747–4756. [PubMed] [Google Scholar]
- Dorr R. T., Alberts D. S., Chen H. S. The limited role of corticosteroids in ameliorating experimental doxorubicin skin toxicity in the mouse. Cancer Chemother Pharmacol. 1980;5(1):17–20. doi: 10.1007/BF00578557. [DOI] [PubMed] [Google Scholar]
- Fisher J., Abdella B. R., McLane K. E. Anthracycline antibiotic reduction by spinach ferredoxin-NADP+ reductase and ferredoxin. Biochemistry. 1985 Jul 2;24(14):3562–3571. doi: 10.1021/bi00335a026. [DOI] [PubMed] [Google Scholar]
- Harwood K. V., Aisner J. Treatment of chemotherapy extravasation: current status. Cancer Treat Rep. 1984 Jul-Aug;68(7-8):939–945. [PubMed] [Google Scholar]
- Ignoffo R. J., Friedman M. A. Therapy of local toxicities caused by extravasation of cancer chemotherapeutic drugs. Cancer Treat Rev. 1980 Mar;7(1):17–27. doi: 10.1016/s0305-7372(80)80023-5. [DOI] [PubMed] [Google Scholar]
- Larson D. L. Treatment of tissue extravasation by antitumor agents. Cancer. 1982 May 1;49(9):1796–1799. doi: 10.1002/1097-0142(19820501)49:9<1796::aid-cncr2820490911>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Larson D. L. What is the appropriate management of tissue extravasation by antitumor agents? Plast Reconstr Surg. 1985 Mar;75(3):397–405. doi: 10.1097/00006534-198503000-00017. [DOI] [PubMed] [Google Scholar]
- Linder R. M., Upton J., Osteen R. Management of extensive doxorubicin hydrochloride extravasation injuries. J Hand Surg Am. 1983 Jan;8(1):32–38. doi: 10.1016/s0363-5023(83)80048-3. [DOI] [PubMed] [Google Scholar]
- Loth T. S., Eversmann W. W., Jr Treatment methods for extravasations of chemotherapeutic agents: a comparative study. J Hand Surg Am. 1986 May;11(3):388–396. doi: 10.1016/s0363-5023(86)80147-2. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Chen H. H., Plambeck J. A., Acton E. M. Further studies on the generation of reactive oxygen species from activated anthracyclines and the relationship to cytotoxic action and cardiotoxic effects. Biochem Pharmacol. 1982 Feb 15;31(4):575–581. doi: 10.1016/0006-2952(82)90162-9. [DOI] [PubMed] [Google Scholar]
- MONTAGNA W., YUN J. S. THE SKIN OF THE DOMESTIC PIG. J Invest Dermatol. 1964 Jul;42:11–21. [PubMed] [Google Scholar]
- Mimnaugh E. G., Trush M. A., Ginsburg E., Gram T. E. Differential effects of anthracycline drugs on rat heart and liver microsomal reduced nicotinamide adenine dinucleotide phosphate-dependent lipid peroxidation. Cancer Res. 1982 Sep;42(9):3574–3582. [PubMed] [Google Scholar]
- Moore H. W. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977 Aug 5;197(4303):527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- Pan S. S., Iracki T., Bachur N. R. DNA alkylation by enzyme-activated mitomycin C. Mol Pharmacol. 1986 Jun;29(6):622–628. [PubMed] [Google Scholar]
- Rudolph R., Stein R. S., Pattillo R. A. Skin ulcers due to adriamycin. Cancer. 1976 Sep;38(3):1087–1094. doi: 10.1002/1097-0142(197609)38:3<1087::aid-cncr2820380308>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Leight G., Cone J., Schwarz S., Stuart L., Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976 Dec;22(6):559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- Siegel D. M., Giri S. N., Scheinholtz R. M., Schwartz L. W. Characteristics and effect of antiinflammatory drugs on adriamycin-induced inflammation in the mouse paw. Inflammation. 1980 Jun;4(2):233–247. doi: 10.1007/BF00914168. [DOI] [PubMed] [Google Scholar]
- Upton P. G., Yamaguchi K. T., Myers S., Kidwell T. P., Anderson R. J. Effects of antioxidants and hyperbaric oxygen in ameliorating experimental doxorubicin skin toxicity in the rat. Cancer Treat Rep. 1986 Apr;70(4):503–507. [PubMed] [Google Scholar]
- Wang J. J., Cortes E., Sinks L. F., Holland J. F. Therapeutic effect and toxicity of adriamycin in patients with neoplastic disease. Cancer. 1971 Oct;28(4):837–843. doi: 10.1002/1097-0142(1971)28:4<837::aid-cncr2820280406>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]