Abstract
Geographic and ethnic differences impart an immense influence on the genetic susceptibility to Type 2 diabetes (T2D) and diabetic nephropathy (DN). Transforming growth factor-beta1 (TGF-β1), a ubiquitously expressed pro-fibrotic cytokine plays a pivotal role in mediating the hypertrophic and fibrotic manifestations of DN. The present study is aimed to study the association of TGF-β1 g.869T>C (rs1800470) and g.-509C>T (rs1800469) polymorphism in T2D and end stage renal disease (ESRD) cases from the two geographically and ethnically different populations from North India. A total of 1313 samples comprising 776 samples from Punjab (204 with ESRD, 257 without ESRD, and 315 healthy controls) and 537 samples from Jammu and Kashmir (150 with ESRD, 187 without ESRD, and 200 controls) were genotyped for TGF-β1 (rs1800470 and rs1800469) using ARMS-PCR. The CC genotype of rs1800470 increased ESRD risk by 3.1–4.5-fold in both populations. However, for rs1800469, the TT genotype provided 5.5-fold risk towards ESRD cases from Jammu and Kashmir and no risk for the cases from Punjab. The haplotype C-T conferred nearly a 2–3-fold risk towards T2D and ESRD and diplotype CC-CT conferred a 4-fold risk towards ESRD. Our results conclude that TGF-β1 (rs1800470) may increase the risk of both ESRD and T2D in both populations, but TGF-β1 (rs1800469) provided risk for only ESRD in the population of Jammu and Kashmir. The present study is one of the large sample sized genetic association studies of T2D and ESRD from Indian population and adds to the scholarship on global health omics.
Introduction
Type 2 diabetes (T2D) is a common, chronic, and complex disorder that is rapidly growing globally (Stumvoll et al., 2005). Diabetic nephropathy (DN) is the leading cause of end stage renal disease (ESRD) and one of the main mechanisms by which diabetes results in increased mortality (Lui et al., 1999; Paniagua et al., 2007). T2D and its related complications are more common among Indians, and it tends to impose a significant health care burden and reduces the overall quality of life (Unnikrishnan et al., 2007). Moreover, because of the high cost of both dialysis and transplantation, only countries with robust economies can meet the challenges of treating ESRD patients (Coresh et al., 2007).
There is a steep rise in the prevalence of T2D and DN in the population of Punjab, and Jammu and Kashmir attributed to the changing lifestyle and consumption of high fat diet (Bhatti et al., 2007, Mahajan et al., 2013). The two populations have widely different geographical distribution and environmental conditions that influence the genetic susceptibility to lifestyle diseases such as T2D and DN. There is strong evidence that genetic susceptibility factors are associated with T2D and DN, and several single nucleotide polymorphisms (SNPs) have also been linked with its increased likelihood (McDonough et al., 2011, Wheeler and Barroso, 2011).
Transforming growth factor-beta1 (TGF-β1), a widely expressed pro-fibrotic cytokine, plays a pivotal role in mediating the hypertrophic and fibrotic manifestations of DN (Sharma and Ziyadeh, 1995). The multiple mediators in the diabetic milieu upregulate TGF-β1-induced glomerular fibrogenesis, which accompanies disease progression from incipient to overt nephropathy and finally results in renal function decline among diabetic cases (Katz et al., 2002). The presence of SNPs in certain loci of the TGF-β1 gene affect its regulation and expression levels, among these, g.-509C>T, g.915G>C (Arg25 Pro, codon 25), and g.869T>C (Leucine10Proline, codon 10) are the most frequently studied polymorphisms (Dixon et al., 2003; Park et al., 2005; Shah et al., 2006).
Numerous studies have examined the association of these SNPs with diabetes, obesity, and inflammatory diseases (Ahluwalia et al., 2009; Dixon et al., 2003; Kumar et al., 2007; Park et al., 2005; Vettor et al., 2005). The exonic (g.869T>C) and promoter (g.-509C>T) polymorphisms have been associated with DN traits (Coll et al., 2004; Khalil et al., 2005; Prasad et al., 2007). As the public health impact of DN is expected to grow in the years to come due to the increasing prevalence of diabetes (Vinod, 2012) and moreover keeping in view the role of genetic predisposition for the development of these diseases, the present study aimed to determine the association of TGF-β1 g.869T>C and g.-509C>T polymorphisms in T2D and ESRD cases derived from T2D in the two geographically and ethnically different populations of North India.
Material and Methodology
Study design and sampling
The present case-control association study has been approved by the Ethics Committee of Guru Nanak Dev University, Amritsar. The power of the study is more than 80%, and to accomplish the proposed objectives a total of 1313 blood samples were collected, comprising of 776 samples from Punjab and 537 samples from Jammu and Kashmir. Samples from Punjab comprised of 461 T2D cases (204 with ESRD and 257 without ESRD) and 315 healthy controls. Samples from Jammu and Kashmir constituted 337 T2D cases (150 with ESRD and 187 without ESRD) and 200 controls. A written informed consent was obtained from all individuals. T2D without ESRD cases were diagnosed according to the criteria given by American Diabetes Association, 2011; these cases were without any microvascular and macrovascular complication. Diagnosis for T2D with ESRD cases was made as per Levey et al., (2011). ESRD cases with T2D as a primary disease were only included to analyze the effect of T2D on renal complications. ESRD cases with other microvascular and macrovascular complications such as diabetic retinopathy, neuropathy, and cardiovascular diseases were excluded from the study. The healthy controls selected were gender matched and above the age of 40 years with no family history of T2D. The techniques used for anthropometric measurements and biochemical analysis has been defined earlier by Raina et al., (2014).
SNP selection and genetic analysis
TGF-β1 gene SNPs rs1800469 (g.-509C>T) and rs1800470 (g.869T>C) selected for the present study are recorded in the public dbSNP database and have been reported to influence the etiology of T2D and ESRD. Peripheral blood samples obtained from the study participants were collected in tubes containing EDTA. Total genomic DNA was isolated from the venous blood using inorganic method (Miller et al., 1988). Genotyping of TGF-β1 g.869T>C and g.-509C>T polymorphisms was based on amplification refractory mutation detection system-polymerase chain reaction (ARMS-PCR). Primer sequences for TGF-β1 g.869T>C polymorphism were as described in Perrey et al., (1999), and primers for TGF-β1 g.-509C>T polymorphism were designed by web-based allele specific primer (WASP) software (Wangkumhang et al., 2007). 10% of the samples were randomly chosen and re-analyzed to assess reliability of the genotyping.
Statistical analysis
Statistical analyses were performed using statistical package for social science program (version 16.0; SPSS Inc., Chicago, IL). The power of the study was calculated using the CaTS power calculator (Skol et al., 2006). The continuous variables are represented as mean±standard deviation (SD). Genotypes and allele frequencies (represented as percentages) were calculated by gene counting method. Genotypes were tested for the Hardy Weinberg Equilibrium (HWE) using chi square analysis. The distribution of genotype and allele frequencies in cases and controls were compared by using 3×2 and 2×2 chi-square contingency tables and the extent of association was determined by Odd's ratio (OR) at 95% confidence interval (CI). Binary logistic regression analysis was used for correction of confounding variables such as age, sex, BMI, and WHR. The continuous data were compared using Student's t-test. Levene's test for measuring equality of variances was used to obtain the significance values for corresponding difference in means. Haplotype frequencies and pairwise linkage disequilibrium (LD) for the two TGF-β1 (869 and -509) polymorphisms among both studied populations were estimated using Haploview software. All results were considered significant at p<0.05. The p-value given in t-test (Bonferroni correction) and in model analysis (confounding factors) is corrected.
Results
Comparison of clinical characteristics of studied populations
The comparison of various demographic, clinical, and biochemical parameters between the studied disease groups and controls among the populations of Punjab and Jammu and Kashmir is given in Table 1, while comparison of various parameters of cases and controls between the two studied population groups is shown in Table 2.
Table 1.
Demographic, Clinical, and Biochemical Characteristics in Two Studied Populations
| Punjab | Jammu and Kashmir | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2D Cases | T2D Cases | |||||||||||
| Variables | With ESRD (n=204) | Without ESRD (n=257) | Controlls (n=315) | pa | pb | pc | With ESRD (n=150) | Without ESRD (n=187) | Controls (n=200) | pa | pb | pc |
| Age (years) | 59.62±8.10 | 55.59±10.51 | 54.72±10.80 | 9.18×10−08* | 5.28 | 6.26×10−05* | 60.48±6.90 | 53.54±7.96 | 52.64±8.06 | 5.2×10−19* | 4.272 | 5.61×10−15* |
| BMI (Kg/m2) | 23.63±4.53 | 27.58±5.17 | 27.59±5.84 | 1.33×10−15* | 15.488 | 2.60×10−15* | 22.39±3.10 | 25.23±3.44 | 23.99±2.23 | 9.84×10−07* | 6.35×10−04* | 1.50×10−22* |
| WC (cm) | – | 102.25±12.62 | 96.83±15.78 | – | 1.38×10−04* | – | – | 89.19±6.66 | 89.11±6.82 | – | 14.56 | – |
| HC (cm) | – | 102.82±11.69 | 100.28±14.72 | – | 0.384 | – | – | 96.99±7.39 | 97.00±7.31 | – | 15.68 | – |
| WHR | – | 1.00±0.08 | 0.97±0.08 | – | 4.10×10−04* | – | – | 0.92±0.03 | 0.92±0.02 | – | 10.10 | – |
| SBP (mmHg) | 134.48±16.35 | 130.70±18.16 | 125.38±14.13 | 3.65×10−09* | 2.83×10−03* | 0.308 | 139.24±18.57 | 127.07±11.94 | 121.98±10.70 | 1.89×10−17* | 2.21×10−04* | 2.82×10−09* |
| DBP (mmHg) | 83.72±10.39 | 85.30±9.21 | 82.94±8.54 | 4.94 | 0.032* | 1.204 | 86.27±12.44 | 85.72±9.33 | 82.35±8.14 | 0.013* | 3.04×10−03* | 9.254 |
| RBS (mg/dl) | 176.42±58.64 | 241.05±88.04 | 123.36±23.59 | 6.66×10−81* | 9.41×10−45* | 8.06×10−14* | 188.81±60.79 | 231.23±52.94 | 140.21±14.73 | 1.40×10−14* | 1.27×10−37* | 1.39×10−07* |
| FBS (mg/dl) | 105.02±23.70 | 169.74±62.65 | 100.10±17.21 | 4.693 | 2.16×10−11* | 9.88×10−10* | 94.23±24.94 | 151.93±26.98 | 86.39±10.52 | 2.886 | 1.04×10−26* | 2.86×10−10* |
| Cholesterol (mg/dl) | 139.58±51.17 | 175.61±51.63 | 171.39±47.72 | 8.76×10−11* | 5.152 | 8.64×10−12* | 145.32±37.89 | 159.99±35.79 | 148.08±31.17 | 6.084 | 0.016* | 4.37×10−03* |
| Triglyceride (mg/dl) | 144.65±60.40 | 199.27±108.9 | 183.32±109.82 | 5.88×10−06* | 1.408 | 8.01×10−10* | 143.64±45.51 | 176.57±62.33 | 167.41±57.76 | 2.83×10−04* | 2.144 | 6.27×10−07* |
| HDL (mg/dl) | 43.00±12.86 | 40.72±10.62 | 43.14±11.49 | 11.765 | 0.176 | 0.602 | 41.67±8.72 | 40.95±9.65 | 46.09±8.83 | 5.79×10−05* | 1.52×10−06* | 6.636 |
| LDL (mg/dl) | 67.65±51.01 | 95.04±48.70 | 91.59±46.62 | 1.68×10−06* | 6.368 | 1.53×10−07* | 74.92±37.58 | 84.17±36.29 | 68.50±31.27 | 1.183 | 1.25×10−04* | 0.322 |
| VLDL (mg/dl) | 28.93±12.08 | 39.85±21.79 | 36.66±21.96 | 5.88×10−06* | 1.408 | 8.01×10−10* | 28.73±9.10 | 35.31±12.47 | 33.48±11.55 | 2.83×10−04* | 2.144* | 6.27×10−07* |
| Urea (mg/dl) | 113.77±29.57 | 48.38±16.57 | 42.19±14.82 | 6.92×10−63* | 5.06×10−04* | 2.58×10−58* | 121.44±37.61 | 40.82±16.75 | 28.35±6.48 | 2.74×10−65* | 3.12×10−17* | 3.98×10−60* |
| Creatinine (mg/dl) | 6.49±1.40 | 1.18±0.74 | 0.89±0.492 | 3.24×10−81* | 3.73×10−05* | 1.32×10−84* | 6.09±2.13 | 1.05±0.43 | 0.71±0.172 | 6.81×10−66* | 2.10×10−19* | 3.49×10−63* |
| Duration T2D (years) | 13.41±8.24 | 8.12±6.74 | – | – | – | 1.62×10−11* | 12.15±6.47 | 5.09±4.92 | – | – | – | 1.50×10−07* |
| Duration ESRD (years) | 1.06±1.05 | – | – | – | – | – | 0.76±0.65 | – | – | – | – | – |
pa value between T2D with ESRD cases and controls; pb value between T2D without ESRD and controls; pc value between T2D with ESRD and T2D without ESRD.
p<0.05 is considered statistically significant (after Bonferroni correction).
BMI, body mass index; DBP, diastolic blood pressure; FBS, fasting blood sugar; HC, hip circumference; HDL, high density lipoprotein; LDL, low density lipoprotein; RBS, random blood sugar; SBP, systolic blood pressure; VLDL, very low density lipoprotein; WC, waist circumference; WHR, waist-hip ratio.
Table 2.
Demographic, Clinical, and Biochemical Characteristics Between the Two Studied Populations
| T2D with ESRD | T2D without ESRD | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Punjab (n=204) | Jammu and Kashmir (n=150) | Punjab (n=257) | Jammu and Kashmir (n=187) | Punjab (n=315) | Jammu and Kashmir (n=200) | pa | pb | pc |
| Age (years) | 59.62±8.10 | 60.48±6.90 | 55.59±10.51 | 53.54±7.96 | 54.72±10.80 | 52.64±8.06 | 0.293 | 0.020* | 0.013* |
| BMI (Kg/m2) | 23.63±4.53 | 22.39±3.10 | 27.58±5.17 | 25.23±3.44 | 27.59±5.84 | 23.99±2.23 | 0.002* | 2.27×10−08* | 1.98×10−20* |
| WC (cm) | – | – | 102.25±12.62 | 89.19±6.66 | 96.83±15.78 | 89.11±6.82 | – | 1.95×10−36* | 3.09×10−13* |
| HC (cm) | – | – | 102.82±11.69 | 96.99±7.39 | 100.28±14.72 | 97.00±7.31 | – | 4.92×10−10* | 0.001* |
| WHR | – | – | 1.00±0.08 | 0.92±0.03 | 0.97±0.08 | 0.92±0.02 | – | 5.39×10−37* | 1.06×10−19* |
| SBP (mmHg) | 134.48±16.35 | 139.24±18.57 | 130.70±18.16 | 127.07±11.94 | 125.38±14.13 | 121.98±10.70 | 0.013* | 0.012* | 0.002* |
| DBP (mmHg) | 83.72±10.39 | 86.27±12.44 | 85.30±9.21 | 85.72±9.33 | 82.94±8.54 | 82.35±8.14 | 0.041* | 0.642 | 0.438 |
| RBS (mg/dl) | 176.42±58.64 | 188.81±60.79 | 241.05±88.04 | 231.23±52.94 | 123.36±23.59 | 140.21±14.73 | 0.076 | 0.221 | 1.15×10−15* |
| FBS (mg/dl) | 105.02±23.70 | 94.23±24.94 | 169.74±62.65 | 151.93±26.98 | 100.10±17.21 | 86.39±10.52 | 0.143 | 0.038* | 0.0003* |
| Cholesterol (mg/dl) | 139.58±51.17 | 145.32±37.89 | 175.61±51.63 | 159.99±35.79 | 171.39±47.72 | 148.08±31.17 | 0.247 | 0.0002* | 7.5×10−11* |
| Triglyceride (mg/dl) | 144.65±60.40 | 143.64±45.51 | 199.27±108.9 | 176.57±62.33 | 183.32±109.82 | 167.41±57.76 | 0.857 | 0.006* | 0.034* |
| HDL (mg/dl) | 43.00±12.86 | 41.67±8.72 | 40.72±10.62 | 40.95±9.65 | 43.14±11.49 | 46.09±8.83 | 0.247 | 0.816 | 0.002* |
| LDL (mg/dl) | 67.65±51.01 | 74.92±37.58 | 95.04±48.70 | 84.17±36.29 | 91.59±46.62 | 68.50±31.27 | 0.141 | 0.008* | 6.66×10−11* |
| VLDL (mg/dl) | 28.93±12.08 | 28.73±9.10 | 39.85±21.79 | 35.31±12.47 | 36.66±21.96 | 33.48±11.55 | 0.857 | 0.006* | 0.034* |
| Urea (mg/dl) | 113.77±29.57 | 121.44±37.61 | 48.38±16.57 | 40.82±16.75 | 31.19±14.82 | 28.35±6.48 | 0.056 | 1.12×10−05* | 0.012* |
| Creatinine (mg/dl) | 6.49±1.40 | 6.09±2.13 | 1.18±0.74 | 1.05±0.43 | 0.89±0.492 | 0.71±0.172 | 0.061 | 0.029* | 7.18×10−08* |
| Duration T2D (years) | 13.41±8.24 | 12.15±6.47 | 8.12±6.74 | 5.09±4.92 | – | – | 0.018* | 1.23×10−07* | – |
| Duration ESRD (years) | 1.06±1.05 | 0.76±0.65 | – | – | – | – | 0.001* | – | – |
pa value T2D with ESRD (Punjab vs. Jammu and Kashmir); pb value T2D without ESRD (Punjab vs. Jammu and Kashmir), pc value controls (Punjab vs. Jammu and Kashmir).
p<0.05 is considered statistically significant.
BMI: body mass index; DBP, diastolic blood pressure; FBS, fasting blood sugar; HC, hip circumference; HDL, high density lipoprotein; LDL, low density lipoprotein; RBS, random blood sugar; SBP, systolic blood pressure; VLDL, very low density lipoprotein; WC, waist circumference; WHR, waist-hip ratio.
Frequency distribution of TGF-β1 g.869T>C and g.-509C>T polymorphisms
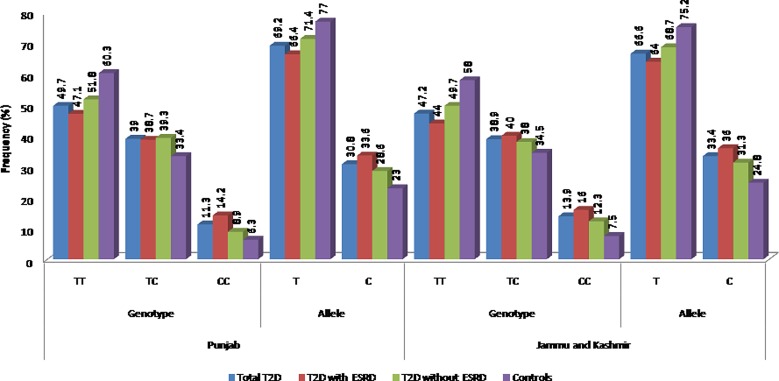
The comparison of genotype and allele frequencies of TGF-β g.869T>C polymorphism between the different disease groups and controls among the two studied populations is reported in Figure 1. The samples were categorized into total T2D cases, T2D cases with ESRD, T2D cases without ESRD, and controls. The genotypes in all the study groups were observed to be in HWE. The minor allele frequency (MAF) was significantly higher in cases than controls in both the population groups. However, in the case of genotype distribution, significant difference could be attributed for total T2D and T2D with ESRD cases, the same was not found among T2D without ESRD group in both studied populations. Further, model analysis revealed that the TC+CC genotype conferred 1.7-fold risk towards ESRD and 1.5-fold risk towards T2D development, and the CC genotype provide nearly 3-fold risk towards ESRD cases even after correcting for confounding factors in the population of Punjab. In the population of Jammu and Kashmir, in contrast to results in population of Punjab, TC+CC and CC genotype attributed risk towards only ESRD development and not towards T2D (Table 3).
FIG. 1.
Distribution of allele and genotype frequencies of TGF-β1 g.869T>C polymorphism in two studied populations.
Table 3.
Comparison of Allele and Genotype Frequencies of TGF-β1 g.869T>C and g.-509C>T Polymorphisms in Two Studied Populations
| TGF-β1 | Population of Punjab | Punjab of Jammu and Kashmir | ||||||
|---|---|---|---|---|---|---|---|---|
| g.869T>C | Total T2D vs Controls | T2D with ESRD vs Controls | T2D without ESRD vs Controls | T2D with ESRD vs T2D without ESRD | Total T2D vs Controls | T2D with ESRD vs Controls | T2D without ESRD vs Controls | T2D with ESRD vs T2D without ESRD |
| Genotypes | p=0.005* | p=0.001* | p=0.105 | p=0.191 | p=0.018* | p=0.009* | p=0.149 | p=0.476 |
| Alleles | p=0.0008* | p=0.0002* | p=0.031* | p=0.104 | p=0.003* | p=0.001* | p=0.043* | p=0.197 |
| 1.49 (1.18–1.89) | OR=1.69 (1.28–2.22) | OR=1.33 (1.03–1.75) | OR=1.27 (0.95–1.67) | OR=1.52 (1.15–2.0) | OR=1.72 (1.23–2.38) | OR=1.39 (1.01–1.89) | OR=1.23 (0.89–1.69) | |
| Dominant model (TC+CC vs TT) | p=0.017** | p=0.010** | p=0.017** | p=0.317 | p=0.115 | p=0.002** | p=0.103 | p=0.295 |
| OR=1.52 (1.08–2.14) | OR=1.69 (1.13–2.51) | OR=1.52 (1.08–2.14) | OR=1.20 (0.83–1.75) | OR=1.39 (0.93–2.08) | OR=2.26 (1.36–3.75) | OR=1.39 (0.93–2.08) | OR=1.27 (0.82–1.92) | |
| Recessive model (CC vs TT+TC) | p=0.168 | p=0.001** | p=0.241 | p=0.076 | p=0.109 | p=0.0001** | p=0.113 | p=0.330 |
| OR=1.57 (0.83–2.97) | OR=3.08 (1.55–6.09) | OR=1.45 (0.78–2.70) | OR=1.69 (0.94–3.03) | OR=1.78 (0.88–3.55) | OR=4.47 (1.99–10.05) | OR=1.72 (0.87–3.45) | OR=1.35 (0.74–2.5) | |
| Codominant model (TC vs TT+CC) | p=0.105 | p=0.210 | p=0.139 | p=0.900 | p=0.311 | p=0.291 | p=0.478 | p=0.704 |
| OR=1.28 (0.95–1.73) | OR=1.26 (0.88–1.82) | OR=1.29 (0.92–1.82) | OR=0.98 (0.67–1.42) | OR=1.21 (0.84–1.74) | OR=1.27 (0.82–1.96) | OR=1.16 (0.77–1.76) | OR=1.09 (0.70–1.69) | |
| TGF-β1 | Population of Punjab | Punjab of Jammu and Kashmir | ||||||
|---|---|---|---|---|---|---|---|---|
| g.-509C>T | Total T2D vs Controls | T2D with ESRD vs Controls | T2D without ESRD vs Controls | T2D with ESRD vs T2D without ESRD | Total T2D vs Controls | T2D with ESRD vs Controls | T2D without ESRD vs Controls | T2D with ESRD vs T2D without ESRD |
| Genotypes | p=0.292 | p=0.134 | p=0.748 | 0.463 | p=0.005* | p=0.002* | p=0.060 | p=0.403 |
| Alleles | p=0.099 | p=0.034* | p=0.430 | p=0.191 | p=0.001* | p=0.0005* | p=0.019* | p=0.223 |
| OR=1.23 (0.96–1.59) | OR=1.39 (1.02–1.89) | OR=1.12 (0.84–1.52) | OR=1.23 (0.90–1.69) | OR=1.75 (1.25–2.44) | OR=2.0 (1.35–2.94) | OR=1.59 (1.08–2.33) | OR=1.25 (0.87–1.82) | |
| Dominant model (CT+TT vs CC) | p=0.133 | p=0.058 | p=0.456 | p=0.254 | p=0.028* | p=0.017** | p=0.028** | p=0.363 |
| OR=1.27 (0.93–1.69) | OR=1.43 (0.99–2.04) | OR=1.14 (0.81–1.61) | OR=1.25 (0.85–1.82) | OR=1.64 (1.06–2.54) | OR=1.90 (1.12–3.21) | OR=1.64 (1.06–2.54) | OR=1.23 (0.79–1.92) | |
| Recessive model (TT vs CC+CT) | p=0.345 | p=0.201 | p=0.691 | p=0.393 | p=0.159 | p=0.024** | p=0.165 | p=0.223 |
| OR=1.35 (0.72–2.50) | OR=1.59 (0.78–3.23) | OR=1.16 (0.56–2.38) | OR=1.37 (0.66–2.86) | OR=2.68 (0.68–10.56) | OR=5.45 (1.25–23.82) | OR=2.56 (0.65–10.0) | OR=1.85 (0.68–5.0) | |
| Codominant model (CT vs CC+TT) | p=0.278 | p=0.188 | p=0.560 | p=0.463 | p=0.080 | p=0.143 | p=0.076 | p=0.710 |
| OR=1.19 (0.87–1.64) | OR=1.29 (0.88–1.90) | OR=1.12 (0.77–1.61) | OR=1.16 (0.78–1.73) | OR=1.49 (0.95–2.34) | OR=1.50 (0.87–2.57) | OR=1.50 (0.96–2.34) | OR=1.09 (0.69–1.72) | |
p<0.05 is considered significant, **p value corrected for age, sex, BMI, and WHR.
Figure 2 depicts the comparison of genotype and allele frequencies of TGF-β g.-509T>C promoter polymorphism. The group categorization was done in a similar manner as in TGF-β 869T>C polymorphism. The frequency of T allele and TT genotype was higher in cases than controls. However, significant difference could be attributed only for allele frequency distribution on comparing T2D with ESRD cases with controls. In the population of Punjab, no significant difference was observed under model analysis for any of the groups after correction. Similarly, in the population of Jammu and Kashmir, frequency of both T allele and the TT genotype was higher in T2D with ESRD cases as compared to other groups. A comparison of allelic and genotypic frequencies revealed statistically significant difference between cases and controls. Dominant model analysis revealed that the CT+TT genotype provided 1.6–1.9-fold risk towards ESRD and T2D development, respectively. Under the recessive model, the TT genotype attributed 5.5-fold risk towards ESRD and no risk towards T2D (Table 3).
FIG. 2.
Distribution of allele and genotype frequencies of TGF-β1 g.509C>T polymorphism in two studied populations.
Haplotype and linkage disequilibrium (LD) analysis
The distribution of haplotype frequency and measure of LD for both the studied populations is depicted in Table 4. The haplotype analysis was done to investigate whether a specific haplotype is associated with T2D or with ESRD. Based on measures of linkage disequilibrium (LD), it could be inferred that two SNPs of TGF-β1 (g.869T>C and g.-509C>T) were in slight LD among both T2D with and without ESRD cases from Punjab [D′=0.422, r2=112; D′=0.368, r2=0.087] and in T2D with ESRD cases from the Jammu and Kashmir population [D′=0.368, r2=0.075; D′=0.243, r2=0.032]. However controls from both populations were not in LD [D′=0.152, r2=0.018; D′=0.176, r2=0.015] (Fig. 3). After analyzing haplotype combinations, haplotype C-T conferred 1.6–2.2-fold risk in population of Punjab and 1.86–2.8-fold risk in population of Jammu and Kashmir towards development of T2D and ESRD. At the same time, haplotype T-C provided 1.5-fold protection towards ESRD cases from Punjab and 1.5–1.7-fold protection towards T2D and ESRD cases from Jammu and Kashmir. Further analysis after making diplotype combinations revealed that CC-CT combination increased the ESRD risk by nearly 4-fold in the population of Punjab, while in the population of Jammu and Kashmir, TT-CC combination conferred 1.5–2 fold protection towards T2D and ESRD (Table 5).
Table 4.
Distribution of Haplotype Frequency and Measure of LD, Observed in Comparison of Two Polymorphisms of the TGF-β1 Gene in Cases and Controls in the Studied Populations
| Punjab | Jammu and Kashmir | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency in cases vs Controls | Frequency in cases vs Controls | |||||||||||||
| Haplotypea | T2D with ESRD | T2D without ESRD | Controls | pa | OR (95% CI)a | pb | OR (95% CI)b | T2D with ESRD | T2D without ESRD | Controls | pa | OR (95% CI)a | pb | OR (95% CI)b |
| (TGF-β 869)T-C(TGF-β-509) | 0.567 | 0.618 | 0.654 | 0.005* | 0.69 (0.53–0.89) | 0.220 | 0.86 (0.68–1.10) | 0.542 | 0.584 | 0.672 | 0.0004* | 0.58(0.43–0.79) | 0.013* | 0.69(0.51–0.92) |
| (TGF-β 869)C-C(TGF-β-509) | 0.193 | 0.178 | 0.160 | 0.178 | 1.25 (0.90–1.73) | 0.459 | 1.12 (0.82–1.53) | 0.221 | 0.218 | 0.193 | 0.347 | 1.18(0.82–1.71) | 0.418 | 1.16(0.82–1.64) |
| (TGF-β 869)T-T(TGF-β-509) | 0.098 | 0.096 | 0.115 | 0.344 | 0.82 (0.55–1.23) | 0.259 | 0.82 (0.55–1.17) | 0.098 | 0.103 | 0.080 | 0.401 | 1.23(0.73–2.08) | 0.311 | 1.29(0.80–2.11) |
| (TGF-β 869)C-T(TGF-β-509) | 0.143 | 0.108 | 0.070 | 0.0004* | 2.23 (1.48–3.38) | 0.019* | 1.64 (1.08–2.49) | 0.139 | 0.095 | 0.055 | 0.0004* | 2.80(1.63–4.80) | 0.026* | 1.86(1.06–3.26) |
| LD measure | T2D with ESRD | T2D without ESRD | Controls | T2D with ESRD | T2D without ESRD | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g. +869T>C and g.-509 C>T | D′ | r2 | D′ | r2 | D′ | r2 | D′ | r2 | D′ | r2 | D′ | r2 |
| 0.422 | 0.112 | 0.368 | 0.087 | 0.152 | 0.018 | 0.368 | 0.075 | 0.243 | 0.032 | 0.176 | 0.015 | |
Order of SNPs in TGF-β1 haplotypes: 869T>C, -509 C>T; *p<0.05, OR- ODDs ratio, CI-Confidence interval.
pa (p value for T2D with ESRD vs Controls); pb (p-value for T2D without ESRD vs Controls).
ORa (odds ratio for T2D with ESRD vs Controls); ORb (odds ratio for T2D with ESRD vs Controls).
FIG. 3.
LD plot showing the position of the two TGF-β1 polymorphisms and pair-wise D′ values observed in the populations of Punjab and Jammu and Kashmir.
Table 5.
Risk of T2D and ESRD with Diplotype Combinations of TGF-β1 Gene in the Two Studied Populations
| Punjab | Jammu and Kashmir | |||||||
|---|---|---|---|---|---|---|---|---|
| T2D with ESRD vs Control | T2D without ESRD vs Control | T2D with ESRD vs Control | T2D without ESRD vs Control | |||||
| Diplotype combination | p | OR(95% CI) | p | OR(95% CI) | p | OR(95% CI) | p | OR(95% CI) |
| (TGF-β 869)TT/CC(TGF-β-509) | 0.070 | 0.71(049–1.03) | 0.321 | 0.84(0.60–1.18) | 0.002* | 0.49(0.31–0.76) | 0.043* | 0.66(0.44–0.99) |
| (TGF-β 869)TT/CT(TGF-β-509) | 0.184 | 0.69(0.40–1.19) | 0.358 | 0.79(0.48–1.30) | 0.399 | 1.32(0.69–2.50) | 0.578 | 1.19(0.64–2.21) |
| (TGF-β 869)TT/TT(TGF-β-509) | 0.303 | 0.44(0.09–2.12) | 0.101 | 0.17(0.02–1.41) | 0.740 | 0.66(0.06–7.40) | 0.946 | 1.07(0.15–7.68) |
| (TGF-β 869)TC/CC(TGF-β-509) | 0.738 | 0.93(0.59–1.45) | 0.051 | 1.14(0.76–1.71) | 0.886 | 1.04(0.63–1.70) | 0.720 | 0.92(0.57–1.47) |
| (TGF-β 869)TC/CT(TGF-β-509) | 0.097 | 1.54(0.93–2.58) | 0.440 | 1.22(0.7–2.02) | 0.186 | 1.55(0.81–2.95) | 0.238 | 1.45(0.78–2.70) |
| (TGF-β 869)TC/TT(TGF-β-509) | 0.284 | 1.83(0.61–5.53) | 0.242 | 1.87(0.66–5.32) | 0.838 | 1.34(0.08–21.53) | 0.310 | 3.24(0.33–31.47) |
| (TGF-β 869)CC/CC(TGF-β-509) | 0.547 | 1.30(0.55–3.07) | 0.473 | 0.71(0.27–1.82) | 0.720 | 1.20(0.45–3.18) | 0.541 | 1.33(0.54–3.28) |
| (TGF-β 869)CC/CT(TGF-β-509) | 0.012* | 3.88(1.34–11.17) | 0.062 | 2.77(0.95–8.09) | 0.277 | 1.82(0.62–5.37) | 0.253 | 1.83(0.65–5.13) |
| (TGF-β 869)CC/TT(TGF-β-509) | 0.060 | 3.70(0.94–14.46) | 0.325 | 2.06(0.49–8.72) | – | – | – | |
p<0.05, OR- ODDs ratio, CI-Confidence interval.
Discussion
In this study we have evaluated that g.869T>C and g.-509C>T polymorphisms of TGF-β1 are associated with T2D and progression to ESRD among T2D cases. The present report is the first study conducted in the population of Punjab and Jammu and Kashmir evaluating the association of these polymorphisms with the risk of developing ESRD in T2D patients. Most of the previously reported studies have compared T2D and DN with each other rather with healthy controls, which can give inconclusive association and moreover, the DN cases included were of all stages (from glomerular hyperfiltration to ESRD) (Prasad et al., 2007; Valladares-Salgado et al., 2010). So the present study enrolled only the last stage DN cases with ESRD and compared them with both T2D and healthy controls to get irrefutable results.
The TGF-β1 polymorphisms (g.869T>C and g.-509 C>T) are often associated with circulating concentration of TGF-β1 protein (Wong et al., 2003). The g.869T>C polymorphism at exon 1 position encoding the signal peptide results in the replacement of a leucine with proline at amino acid position 10 in the signal sequence (Li et al., 1999). It is speculated that this Leucine to Proline change (missense mutation) results in modifications in amino acid composition of the peptide, which affects its polarity and leads to differing rates of protein export (Wood et al., 2000). The C allele (proline) of this polymorphism was responsible 2.8-fold higher secretion of TGF-β1 and was also associated with high serum concentrations of TGF-β1. The g.-509C>T promoter SNP of the TGF-β1 gene results in increased plasma levels of TGF-β1, due to transcriptional suppression by activator protein-1 (AP1) binding T allele of -509 TGF- β1. Increased TGF-β1 levels are often associated with the -509T allele because of the loss of negative regulation by AP1 (Shah et al., 2006).
The analysis of TGF-β1 g.869T>C and g.-509C>T polymorphisms in the present studied populations revealed that MAF of TGF-β1 g.869T>C polymorphism was slightly higher in the population of Jammu and Kashmir (24.8%) as compared to that in the population of Punjab (23.0%). In contrast for g.-509C>T promoter polymorphism, MAF was higher in the population of Punjab (18.6%) in comparison to the population of Jammu and Kashmir (13.5%). The disparity was observed for both SNPs in different populations shown in Table 6. This difference observed in the MAF could be attributed to ethnic heterogeneity and population diversity (Cross et al., 2010).
Table 6.
Comparison of Minor Allele Frequency (MAF) of TGF-β1 g.869T>C and g.-509C>T Polymorphism in Different Populations
| Population | MAF (%) | Associated disease | Association status | Reference |
|---|---|---|---|---|
| TGF-β1 g.869T>C | ||||
| Punjab | 23.0 | T2D and ESRD | Present | Present study |
| Jammu and Kashmir | 24.8 | T2D and ESRD | Present | Present study |
| Netherland | 34.5 | Multiple Sclerosis | Present | Schrijver et al. 2004 |
| Ireland | 26.4 | Type 1 diabetes | Absent | Mcknight et al. 2007 |
| North-Indian (Uttar Pradesh) | 38.5 | ESRD | Present | Mittal and Manchanda, 2007 |
| China | 53.2 | Silicosis | Absent | Wu et al. 2008 |
| Italy | 47.0 | IgA nephropathy | Present | Brezzi et al. 2009 |
| South-Indian (Hyderabad) | 16.4 | Myopia | Present | Sandhya et al. 2011 |
| Turkey | 1.0 | Obesity | Absent | Kanra et al. 2011 |
| Egypt | 16.8 | T2D with and without DN | Present | El-Sherbini et al. 2013 |
| Korea | 49.0 | Ossification of the posterior longitudinal ligament (OPLL) | Absent | Han et al. 2013 |
| TGF-β1g.-509C>T | ||||
| Punjab | 18.6 | T2D and ESRD | Present | Present study |
| Jammu and Kashmir | 13.5 | T2D and ESRD | Present | Present study |
| United kingdom | 28.4 | Asthma | Present | Pulleyn et al. 2001 |
| France | 13.0 | Alzheimer's disease | Absent | Araria-Goumidi et al. 2002 |
| German | 32.5 | Coronary heart disease | Present | Koch et al. 2006 |
| North Indian (Punjab) | 39.9 | ESRD | Present | Kumar et al. 2007 |
| Ireland | 18.9 | Type 1 diabetes | Absent | Mcknight et al. 2007 |
| China | 47.2 | Pulmonary tuberculosis | Absent | Wu et al. 2008 |
| Italy | 50.5 | IgA nephropathy | Absent | Brezzi et al. 2009 |
| Turkey | 67.7 | Obesity | Absent | Kanra et al. 2011 |
| Iran | 51.9 | Periodontitis | Present | Arab et al. 2012 |
| Korea | 48.8 | Ossification of the posterior longitudinal ligament (OPLL) | Absent | Han et al. 2013 |
In the present study, 869 C allele and the CC genotype provided a risk for development of T2D and ESRD in both population groups. Further genetic model analysis also revealed that the CC genotype provided a higher risk as compared to the TC+CC genotype combination for ESRD manifestation. Similar trends were reported by a study on an Egyptian population where frequency of C-allele was higher in cases (both total T2D and T2D with ESRD) as compared to controls, and the TC genotype was providing 2–2.7-fold risk towards T2D and ESRD development (El-Sherbini et al., 2013). TGF-β1 g.869T>C polymorphism was observed to provide risk towards DN in Mexican (Valladares-salgado et al., 2010) and Chinese populations (Wong et al., 2003) and towards ESRD in a North Indian population (Mittal and Manchanda, 2007). Contrarily, reports on German and Spanish populations concluded that 869T allele rather than C allele was associated with ESRD susceptibility (Babel et al., 2006; Coll et al., 2004).
For TGF-β1 g.-509C>T polymorphism, the only significant difference observed in the population of Punjab was for alleles, when ESRD cases were compared with controls, where the -509T allele provided significant risk for the development of ESRD. However, in the population of Jammu and Kashmir, the -509T allele provided risk for the development of both T2D and ESRD and the -509TT genotype conferred nearly 5-fold risk towards ESRD progression among T2D cases. Similar results were reported by another study on Punjab population where -509T allele and the TT genotype frequency was higher in ESRD cases as compared to controls; however, the significant difference could be attributed only for genotypes (Kumar et al., 2007). Though a study on Asian Indians revealed higher frequency of T allele among chronic renal insufficiency patients, yet no significant difference was attained (Prasad et al., 2007). Similarly, a study on an Irish population also reported no significant association of -509 alleles with disease susceptibility (McKnight et al., 2007). These contrasting reports for both the polymorphisms emphasize the need to carry out additional studies in other populations. Moreover, there is a paucity of studies that have compared the role of these polymorphisms in T2D and DN.
Most recent studies have investigated the effect of haplotypes and diplotypes in order to obtain a more comprehensive analysis of the role of polymorphisms in the susceptibility to T2D and DN (El-Sherbini et al., 2013; Valladares-Salgado et al., 2010). The joint effect of TGF-β1 (869 and -509) polymorphisms towards T2D and DN has also not yet been studied. The haplotype C-T was observed to confer nearly a 2–3-fold risk towards T2D and ESRD whereas haplotype T-C provided nearly a 2-fold protection towards development of ESRD and T2D in the studied populations. Further, diplotype analysis revealed that diplotype CC-CT conferred a 3.26-fold risk towards ESRD and TT-CC provide a 2-fold protection for T2D and ESRD in the population of Punjab and Jammu and Kashmir, respectively.
The demographic and anthropometric distributions in the two populations showed that anthropometric variables among controls from Punjab population were significantly higher as compared to Jammu and Kashmir. This difference could be attributed to the increasing prevalence of obesity in Punjab, even in healthy control individuals. This observation was in line with the National Family Health Survey-3 Report, which suggested higher prevalence of generalized as well as central obesity in the population of Punjab than Jammu and Kashmir (Arnold et al., 2009). Because of the detrimental condition of ESRD cases, WC, HC, and WHR could not be computed. Both populations documented the higher percentage of males affected with ESRD (67% in Punjab; 68% in Jammu and Kashmir), which suggested the strong effect of gender on the progression to ESRD. Men progress to ESRD faster than postmenopausal women and these gender differences could be attributed to nephroprotective effects of female hormones, especially estrogen (Gluhovschi et al., 2012; Yanes et al., 2008).
Conclusion
The findings of the present study suggest that the presence of C-allele (869 T>C) is associated with a nearly 3–4-fold risk towards ESRD in both populations. However, the presence of T-allele (-509 C>T) of TGF-β1 gene conferred nearly a 5-fold risk towards ESRD cases from Jammu and Kashmir, but no significant association was observed in cases from Punjab. These variations could be due to geographical and ethnic differences present in the two populations, further emphasizing the role of environment in multifactorial diseases such as T2D and DN. Our study is the first one to analyze the combinatorial effect of TGF-β1 (869 and -509) polymorphisms towards ESRD and T2D development by suggesting the association of TGF-β1 haplotype and diplotype combinations with disease susceptibility. These findings are important as both diseases are prominent sources of morbidity and mortality in the Indian population. The present study has provided the preliminary data for TGF-β1 polymorphisms in both studied populations, and further expression studies can help in validating the role of these TGF-β1 SNPs with regard to T2D and ESRD.
Acknowledgments
The financial assistance to Priyanka Raina by the DST-INSPIRE program is acknowledged. The financial assistance under the scheme of “Centre with Potential for Excellence in Particular Area” through Grant No. F.8-2/2008 (NS/PE) (UGC,India) and “University with Potential for Excellence” Grant No.F.14-2/2008 (NS/PE) to Guru Nanak Dev University, Amritsar is humbly acknowledged. The assistance of Dr. S.K Bali (Head, Nephrology Department, Government Medical College, Jammu), Dr. Mohit Nagpal (Nephrologist, Fortis Escorts Hospital, Amritsar), and Dr. P.S Mokha (Head, Mokha Hospital, Amritsar) in sample collection is also humbly acknowledged.
Author Disclosure Statement
The authors declare they have no competing financial interests.
References
- Ahluwalia TS, Khullar M, Ahuja M, et al. (2009). Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS One 4, e5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. (ADA) (2011). Diagnosis and classification of diabetes mellitus. Diabetes Care 34, s62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold F, Parasuraman S, Arokiasamy P, and Kothari M. (2009). Nutrition in India, National family health survey-3. Retrieved on July 12, 2013. http://www.nfhsindia.org/nfhs3.pdf
- Babel N, Gabdrakhmanova L, Hammer MH, Schoenemann C, Skrypnikov V, Poliak N, Volk HD, and Reinke P. (2006). Predictive value of cytokine gene polymorphisms for the development of end-stage renal disease. J Nephrol 19, 802–807 [PubMed] [Google Scholar]
- Bhatti JS, Bhatti GK, Joshi A, et al. (2007). Identification of the risk factors for the high prevalence of type 2 diabetes and its complications in a Punjabi population: North Indian Diabetes Study: A case-control study. Intl J Diabetes Devel Countries 27, 108–115 [Google Scholar]
- Coll E, Cormand B, Campos B, González-Núñez D, Iñigo P, Botey A, and Poch E. (2004). Association of TGF-beta1 polymorphisms with chronic renal disease. J Nephrol 17, 794–799 [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, et al. (2007). Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 [DOI] [PubMed] [Google Scholar]
- Cross DS, Ivacic LC, Stefanski EL, and McCarty CA. (2010). Population based allele frequencies of disease associated polymorphisms in the Personalized Medicine Research Project. BMC Genetics 11, 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, Bhathal PS, Jonsson JR, Dixon AF, Powell EE, and O'Brien PE. (2003). Pro-fibrotic polymorphisms predictive of advanced liver fibrosis in the severely obese. J Hepatol 39, 967–971 [DOI] [PubMed] [Google Scholar]
- El-Sherbini SM, Shahen SM, Mosaad YM, Abdelgawad MS, and Talaat RM. (2013). Gene polymorphism of transforming growth factor-β1 in Egyptian patients with type 2 diabetes and diabetic nephropathy. Acta Biochim Biophys Sinica 45, 330–338 [DOI] [PubMed] [Google Scholar]
- Gluhovschi GH, Gluhovschi A, Anastasiu D, Petrica L, Gluhovschi C, and Velciov S. (2012). Chronic kidney disease and the involvement of estrogen hormones in its pathogenesis and progression. Roman J Int Med 50, 135–144 [PubMed] [Google Scholar]
- Katz A, Caramori ML, Sisson-Ross S, Groppoli T, Basgen JM, and Mauer M. (2002). An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Intl 61, 2058–2066 [DOI] [PubMed] [Google Scholar]
- Khalil MS, El Nahas AM, and Blakemore AI. (2005). Transforming growth factor-beta1 SNPs: Genetic and phenotypic correlations in progressive kidney insufficiency. Nephron Exper Nephrol 101, e31–41 [DOI] [PubMed] [Google Scholar]
- Kumar K, Gupta V, Singh A, Mohindru K, Choudhary M, and Sehajpal PK. (2007). Transforming growth factor-β1 C-509T promoter polymorphism in end stage renal disease. J Biotechnol 1, 29–36 [Google Scholar]
- Levey AS, De Jong PE, Coresh J, et al. (2011). The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Intl 80, 17–28 [DOI] [PubMed] [Google Scholar]
- Lui SF, Ho YW, Chau KF, Leung CB, and Choy BY. (1999). Renal Registry 1995–1999 Hong Kong. J Nephrol 1, 53–60 [Google Scholar]
- Mahajan A, Sharma S, Dhar MK, and Bamezai RNK. (2013). Risk factors of type 2 diabetes in population of Jammu and Kashmir, India. J Biomed Res 27, 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough CW, Palmer ND, Hicks PJ, et al. (2011). A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Intl 79, 563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight AJ, Savage DA, Patterson CC, Sadlier D, and Maxwell AP. (2007). Resequencing of genes for transforming growth factor beta1 (TGFB1) type 1 and 2 receptors (TGFBR1, TGFBR2), and association analysis of variants with diabetic nephropathy. BMC Med Genetics 23, 8–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, and Polesky HF. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal RD, and Manchanda PK. (2007). Is low-frequency distribution of TGF-beta genotype associated with increased risk for end-stage renal disease? DNA Cell Biol 26, 172–177 [DOI] [PubMed] [Google Scholar]
- Paniagua R, Ramos A, Fabian R, Lagunas J, and Amato D. (2007). Chronic kidney disease and dialysis in Mexico. Perit Dialysis Intl 27, 405–409 [PubMed] [Google Scholar]
- Park KS, Shin HD, Park BL, et al. (2005). Genetic polymorphisms in the transforming growth factor beta-induced gene associated with BMI. Human Mutat 25, 322. [DOI] [PubMed] [Google Scholar]
- Perrey C, Turner SJ, Pravica V, Howell WM, and Hutchinson IV. (1999). ARMS-PCR methodologies to determine IL-10, TNF-a, TNF-b and TGFb1 gene polymorphisms. Transplant Immunol 7, 127–128 [DOI] [PubMed] [Google Scholar]
- Prasad P, Tiwari AK, Kumar KM, Ammini AC, Gupta A, Gupta R, and Thelma BK. (2007). Association of TGF beta1, TNF alpha, CCR2 and CCR5 gene polymorphisms in type-2 diabetes and renal insufficiency among Asian Indians. BMC Med Genetics 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina P, Matharoo K, and Bhanwer AJS. (2014). Monocyte chemoattractant protein-1 (MCP-1) g.-2518A>G polymorphism and susceptibility to type 2 diabetes (T2D) and end stage renal disease (ESRD) in the North-West Indian population of Punjab. Ann Human Biol doi: 10.3109/03014460.2014.94193 [DOI] [PubMed] [Google Scholar]
- Shah R, Hurley CK, and Posch PE. (2006). A molecular mechanism for the differential regulation of TGF-beta1 expression due to the common SNP -509C-T (c. −1347C>T). Human Genetics 120, 461–469 [DOI] [PubMed] [Google Scholar]
- Shah R, Rahaman B, Hurley CK, and Posch PE. (2006). Allelic diversity in the TGFB1 regulatory region: Characterization of novel functional single nucleotide polymorphisms. Human Genetics 119, 61–74 [DOI] [PubMed] [Google Scholar]
- Sharma K, and Ziyadeh FN. (1995) Hyperglycemia and diabetic kidney disease: The case for transforming growth factor-beta as a key mediator. Diabetes 44, 1139–1146 [DOI] [PubMed] [Google Scholar]
- Skol AD, Scott LJ, Abecasis GR, and Boehnke M. (2006). Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nature Genetics 38, 209–213 [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Goldstein BJ, and van Haeften TW. (2005). Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 365, 1333–1346 [DOI] [PubMed] [Google Scholar]
- Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, and Mohan V. (2007) Prevalence and risk factors of diabetic nephropathy in an urban South Indian population the Chennai Urban Rural Epidemiology Study (CURES 45). Diabetes Care 30, 2019–2024 [DOI] [PubMed] [Google Scholar]
- Valladares-Salgado A, Angeles-Martínez J, Rosas M, et al. (2010). Association of polymorphisms within the transforming growth factor-β1 gene with diabetic nephropathy and serum cholesterol and triglyceride concentrations. Nephrology 15, 644–648 [DOI] [PubMed] [Google Scholar]
- Vettor R, Milan G, Rossato M, and Federspil G. (2005). Adipocytokines and insulin resistance. Aliment Pharmacol Therapeut 22, 3–10 [DOI] [PubMed] [Google Scholar]
- Vinod PB. (2012). Pathophysiology of diabetic nephropathy. Clinical Queries: Nephrology 102, 121–126 [Google Scholar]
- Wangkumhang P, Chaichoompu K, Ngamphiw C, et al. (2007). WASP: A Web-based allele-specific PCR assay designing tool for detecting SNPs and mutations. BMC Genomics 8, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, and Barroso I. (2011). Genome-wide association studies and type 2 diabetes. Brief Funct Genomics 10, 52–60 [DOI] [PubMed] [Google Scholar]
- Wong TYH, Poon P, Chow KM, Szeto CC, Cheung MK, and Li PKY. (2003). Association of transforming growth factor-beta (TGF-bold beta) T869C (Leu 10Pro) gene polymorphisms with type 2 diabetic nephropathy in Chinese. Kidney Intl 63, 1831–1835 [DOI] [PubMed] [Google Scholar]
- Wood NA, Thomson SC, Smith RM, and Bidwell JL. (2000). Identification of human TGF-beta1 signal (leader) sequence polymorphisms by PCR-RFLP. J Immunol Methods 234, 117–122 [DOI] [PubMed] [Google Scholar]
- Yanes LL, Sartori-Valinotti JC, and Reckelhoff JF. (2008). Sex steroids and renal disease lessons from animal studies. Hypertension 51, 976–981 [DOI] [PubMed] [Google Scholar]