Abstract
Mesenchymal stromal cells (MSCs) are promising candidates for tissue engineering and regenerative medicine. The multipotent stem cell component of MSC isolates is able to differentiate into derivatives of the mesodermal lineage including adipocytes, osteocytes, chondrocytes, and myocytes. Many common pathways have been described in the regulation of adipogenesis and osteogenesis. However, stimulation of osteogenesis appears to suppress adipogenesis and vice-versa. Increasing evidence implicates a tight regulation of these processes by reactive oxygen species (ROS). ROS are short-lived oxygen-containing molecules that display high chemical reactivity toward DNA, RNA, proteins, and lipids. Mitochondrial complexes I and III, and the NADPH oxidase isoform NOX4 are major sources of ROS production during MSC differentiation. ROS are thought to interact with several pathways that affect the transcription machinery required for MSC differentiation including the Wnt, Hedgehog, and FOXO signaling cascades. On the other hand, elevated levels of ROS, defined as oxidative stress, lead to arrest of the MSC cell cycle and apoptosis. Tightly regulated levels of ROS are therefore critical for MSC terminal differentiation, although the precise sources, localization, levels and the exact species of ROS implicated remain to be determined. This review provides a detailed overview of the influence of ROS on adipogenic and osteogenic differentiation in MSCs.
Introduction
Reactive oxygen species (ROS) are oxygen-derived small molecules, which react readily with a variety of chemical structures such as proteins, lipids, sugars, and nucleic acids. Most ROS that have been described in living organisms include the superoxide anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), hydroxyl ion (OH−), and nitric oxide (NO). ROS are often termed free radicals; this does not apply to H2O2 and ONOO−, which are nonradical ROS.
Chemists first discovered free radicals and described their highly reactive nature. Biologists then investigated the role of free radicals in biological systems. In 1956 Harman, a radiation biologist, introduced his noteworthy observations on the role of ROS in the aging process that were similar to his findings on radiation damage [1]. Shortly afterward the concept that emerged was that ROS lead to cellular damage in aging [2].
Nowadays, it is increasingly recognized that ROS are involved in the regulation of cell function despite the fact that for many years they were considered to be harmful elements in biological systems. Indeed high levels of ROS cause cell damage by oxidation and nitration of macromolecules including DNA, RNA, proteins, and lipids. The concept that ROS are harmful was confirmed by the discovery of ROS detoxifying enzymes (eg, superoxide dismutase SOD, catalase, etc.), scavengers (eg, vitamin C and E) and the bactericidal activity of neutrophils, which is strongly dependent on the generation of large amounts of ROS in the phagosome. However, this concept was challenged following the description of a family of enzymes called NADPH oxidases (NOX-es) at the beginning of the 20th century. NOX enzymes generate ROS by oxidizing intracellular NADPH to NADP+ and the transfer of electrons through membranes to reduce molecular oxygen and generate the superoxide anion as a primary product.
It is currently believed that only unregulated levels of ROS are harmful, while regulated ROS production promotes essential signaling pathways, which regulate cell functions [3] such as cell proliferation, differentiation, survival, and apoptosis [4–6]. Redox regulation or controlled ROS generation is the net effect of a subtle balance between ROS generation and neutralization/utilization by cellular antioxidant systems. Thus oxidative stress represents an unbalanced situation in which ROS generation exceeds antioxidant systems leading to tissue damage.
The responses of adult human stem cells to different stress stimuli such as oxidative stress, heat shock, and γ-radiation have been widely studied in the context of tissue repair, tissue engineering, and transplantation [7]. Mesenchymal stromal cells (MSCs) are a heterogeneous population of cells that can be isolated from the mesenchyme or stroma of several tissues including bone marrow and adipose tissue [8–10]. Within this heterogeneous population is a subpopulation of cells with self-renewal and mutlipotent differentiation capacity that can be qualified as stem cells. Other sources include dental pulp, umbilical cord (Warton's jelly), cord blood, placenta, peripheral blood, and amniotic fluid [11–14]. There is an ongoing and intense debate regarding the precise origin, nature, and therapeutic potential of MSCs [15]. However, from an experimental perspective, MSCs display the following features: (1) following isolation, primary cultures of MSCs are plastic adherent and remain plastic adherent during subsequent propagation; (2) depending on their source, MSCs express several cell surface markers such as CD73, CD90, CD105, and CD44, but not CD31 or CD45, although there are many other markers that may be considered and may also be used to differentiate MSCs from different sources; (3) MSCs have the ability to differentiate into adipocytes, osteoblasts, and chondrocytes in vitro [16,17]; and (4) confirmation of the existence of the stem cell subpopulation requires in vitro clonogenic assays and demonstration that the cells have the ability to differentiate along the desired lineage in vivo [18].
MSCs from several sources are being assessed in a large number of clinical trials in a wide range of settings based on the following assumptions: (1) MSCs have the ability to home to sites of injury and inflammation; (2) MSCs have the ability to differentiate into cells of the mesodermal lineage; (3) MSCs secrete trophic factors that promote proliferation and differentiation of local progenitor cells [19]; (4) MSCs induce or increase neovascularization; (5) MSCs have immunomodulatory properties [20]; and (6) MSCs produce survival factors in ischemic tissues and have antioxidant properties [21]. However, despite the enormous effort that has thus far been invested into clinical trials, very few if any therapies have become part of routine clinical practice.
MSCs are known to have low levels of intracellular ROS and high levels of glutathione, a key antioxidant. They also constitutively express high levels of enzymes required to manage oxidative stress. For example, when compared with the pancreatic beta cell line INS-1, expression of SOD1, SOD2, CAT, and GPX1 was significantly higher in MSCs. These enzymes are able to scavenge peroxide and peroxynitrite (ONOO−). Thus, it has been proposed that the high antioxidant capacity of MSCs makes them ideal for the treatment of pathologies in which tissue damage is linked to oxidative stress [21].
In terms of redox regulation, numerous recent reports describe the importance of oxidants on MSC differentiation into adipocytes [22], osteocytes [23], chondrocytes [24], and myocytes [25] through activation of signaling cascades involved in differentiation [26–32]. Increased adipogenic fate suppresses the osteogenic lineage, while upregulation of osteogenic signaling attenuates adipogenic terminal differentiation. Many signaling pathways such as Wnt, FOXO, Hh, NEL-like protein 1 (NELL-1), insulin-like growth factor (IGF), and bone morphogenetic protein (BMP) determine MSC terminal fate. Among the pathways that favor either adipogenic or osteogenic differentiation via their activation or suppression, IGF and BMP have a dual effect. These pathways positively regulate both adipogenesis and osteogenesis [28,33–36].
There is evidence for the role for ROS in MSC survival, proliferation, and terminal differentiation, and ROS affect adipogenesis or osteogenesis by stimulating or inhibiting several MSC differentiation signaling cascades. Recently, the impact of ROS on MSC differentiation has generated a great deal of interest due to its potential application in the clinic (ie, diabetes [37], hypertension [38], atherosclerosis [39], carcinogenesis, and aging [40]. Understanding the impact of ROS on MSC terminal fate will increase our knowledge of the nature and behavior of these cells and how this may be harnessed for therapeutic purposes. This includes the use of ROS inhibitors/activators as pharmaceutical agents. This review focuses on the various potential sources of ROS in MSCs and how they might influence adipogenic and osteogenic differentiation. We will also summarize the studies that have applied exogenous ROS to MSCs and the studies that measure intra and extracellular ROS during MSC differentiation.
Cellular Sources of ROS
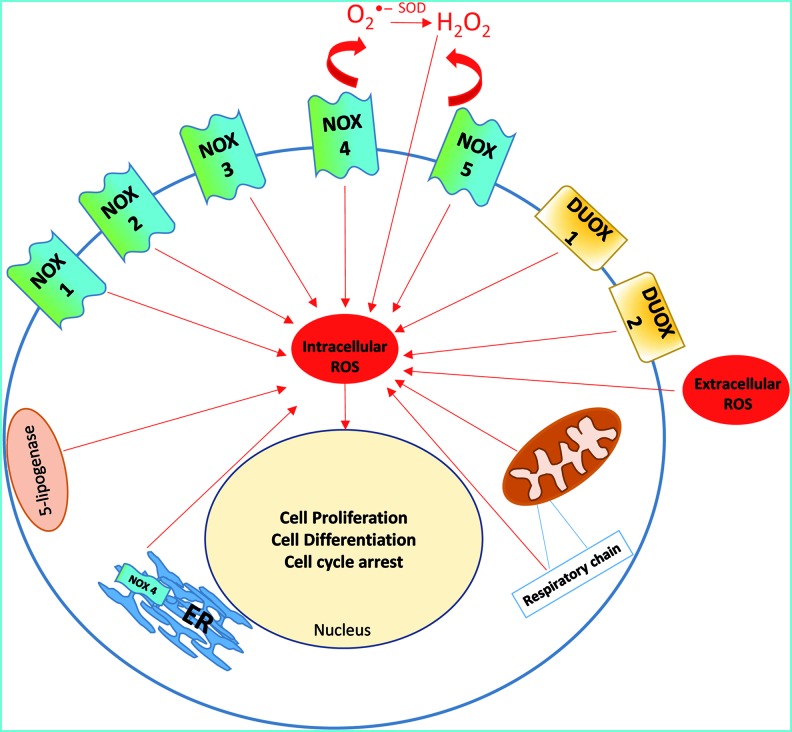
ROS generation can be physiological, pathological, and tissue specific and varies under different circumstances [41]. ROS can be generated in mitochondrial electron transport systems, by NADPH oxidases, xanthine oxidase, cytochrome P450, nitric oxide synthases, lipoxygenases, heme oxygenase, cyclooxygenases, myeloperoxidase, and monoamine oxidases [42,43]. ROS in mammalian cells can be localized in (1) mitochondria [44]; (2) peroxisomes [45]; (3) endoplasmic reticulum (ER) [46]; (4) cytosol (ie, NO synthases, lipoxygenases) [47,48]; (5) the plasma membrane (ie, NADPH oxidases, lipoxygenase) [49]; and (6) the extracellular space [50] (Fig. 1). It has been suggested that NADPH oxidases are localized on the ER and possibly mitochondria [51,52].
FIG. 1.
Sources of reactive oxygen species (ROS). ROS can be intracellulary generated by mitochondria and diverse NOX isoforms, peroxisome, endoplasmic reticulum (ER), xanthine oxidase, and lipogenase. It can also be applied from exogenous sources. Irrespective of its source, it may cause cell proliferation, differentiation, and/or cell cycle arrest, and this effect appears to be concentration dependent. Color images available online at www.liebertpub.com/scd
Mitochondria
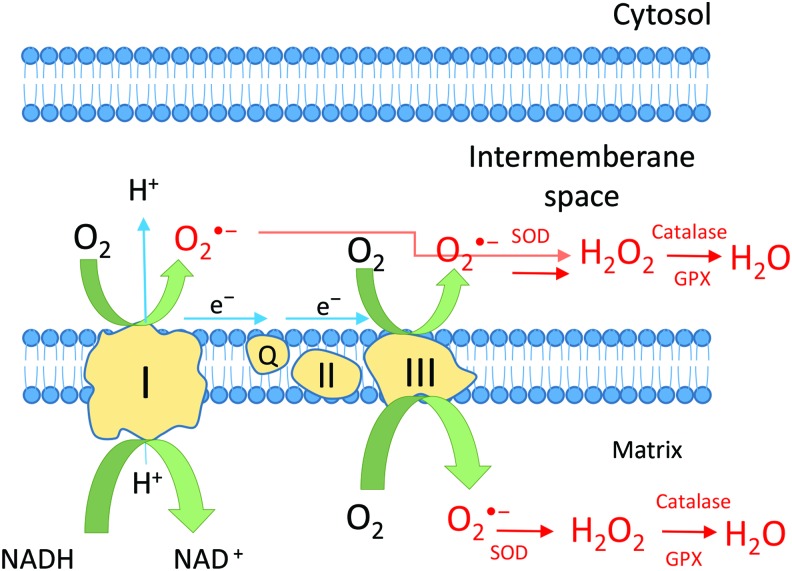
Mitochondria are the main source of ROS and the mitochondrial electron transport machinery is thought to be a primary generator of ROS. A small fraction of oxygen escapes from mitochondria during the generation of adenosine triphosphate (ATP) and water. This fraction is then implicated in the formation of ROS. O2•− is the first ROS produced by mitochondria. The main sources of mitochondrial O2•− are complex I [53] and complex III [54]. Complex I (NADH ubiquinone oxidoreductase) produces O2•− in the matrix, whereas complex III (co-enzyme Q, bc1 complex, uniquinone/cytochrome c reductase) induces O2•− production either in the matrix or the inter-membrane space (Fig. 2). O2•− is then transformed into a more stable form, H2O2, through the activity of Mn, Cu, and Zn-SOD in the inter-membrane space [55]. H2O2 may have different fates. It can be removed by antioxidants such as catalase and peroxidases [56] or converted to H2O and O2 by glutathione peroxidase (GPX) or can act as a signaling molecule in the cytosol in several pathways including the stress response, cell cycle, energy metabolism, and redox balance [57] (Fig. 2). Inhibition of complex I and III by rotenone and antimycin induces ROS generation by the respiratory chain machinery. Other potential sources of ROS within mitochondria could be α-ketoglutarate dehydrogenase [58] located in the matrix and monoamine oxidase [59] at the outer membrane. Recently, some investigators have suggested that NOX4 is localized in mitochondria, although this remains to be confirmed [60,61].
FIG. 2.
Mitochondrial ROS production. The production of the superoxide anion, O2•−, by complex I and complex III in the matrix or the inter-membrane space forms H2O2 through the activity of SOD catalase dismutation. H2O2 can then be converted to H2O and O2 by glutathione peroxidase (GPX) and catalase or may play a second messenger role in essential signaling pathways. Color images available online at www.liebertpub.com/scd
NADPH oxidases
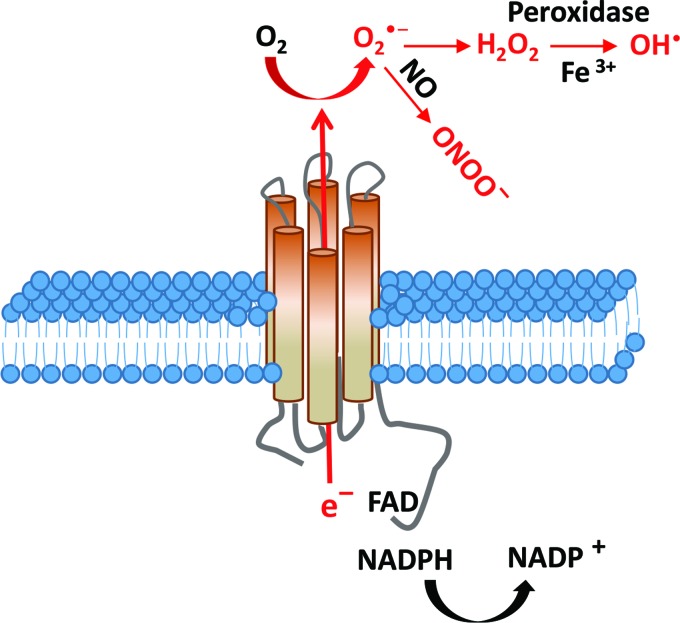
Nonmitochondrial ROS production was first described in neutrophils and macrophages during phagocytosis [62]. NOX consists of a family of seven isoforms that catalyze the reduction of oxygen to superoxide using the pyridine nucleotide NADPH as an electron donor and molecular oxygen as electron acceptor, with the secondary production of other ROS (Fig. 3). In phagocytes, NOX activity requires the cytosolic regulators p47phox, p40phox, p67phox, and the small GTPase RAC. An active complex is formed when the catalytic (gp91phox) and the regulatory subunits are assembled [5].
FIG. 3.
NADPH oxidases. NOX enzymes reduce oxygen to O2•− by using pyridine nucleotide NADPH as an electron donor and molecular oxygen as an electron acceptor. ROS will be generated as the secondary product. A part of O2•− can directly react with nitric oxide (NO•) to form a toxic peroxynitrite. It can also be dismutated by superoxide dismutase to form hydrogen peroxide to induce cell signaling cascades or directly react with Fe3+ to form hydroxyl radical. Color images available online at www.liebertpub.com/scd
While it has been known since the seventies [63] that phagocytes contain an NADPH oxidase activity, a number of studies performed in the nineties described low amounts of ROS in nonphagocytic tissues such as smooth muscle cells [64,65]. In 1999 the first nonphagocytic NOX open reading frame was described in the colon [66]. This isoform is nowadays called NOX1. Other NOX enzymes were then described in other tissues. Seven NOX isoforms are detected in most mammals: NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2. However, NOX5 is absent in mice and rats for reasons that are not understood. It has been reported that levels of NOX4 and NOX5 expression are higher than NOX1, NOX2, and NOX3 in adipose-derived MSCs [67]. Most NOX isoforms produce O2•− as a primary product. However, H2O2 is the dominant ROS detected for NOX4, DUOX1, and DUOX2 [68,69]. This is generally explained by the rapid dismutation of O2•− into H2O2.
Role of ROS in Regulating MSC Fate
There is a large difference in energy metabolism and cellular redox status between pluripotent stem cells and terminally differentiated (stem) cells. For example, in the proliferative phase (early passages), embryonic stem (ES) cells express high levels of glycolytic enzymes and mitochondria consume low amounts of oxygen. However, differentiated ES cells show a lower glycolytic flux, less than half of that predicted in proliferating ES cells [70]. In addition, the degree of stemness of adult stromal stem cells is linked to the intracellular distribution of mitochondria: stem cell differentiation competence could be defined by a perinuclear arrangement of mitochondria, a low ATP/cell content and a high rate of oxygen consumption, whereas lack of these characteristics was an indication of stem cell differentiation [71].
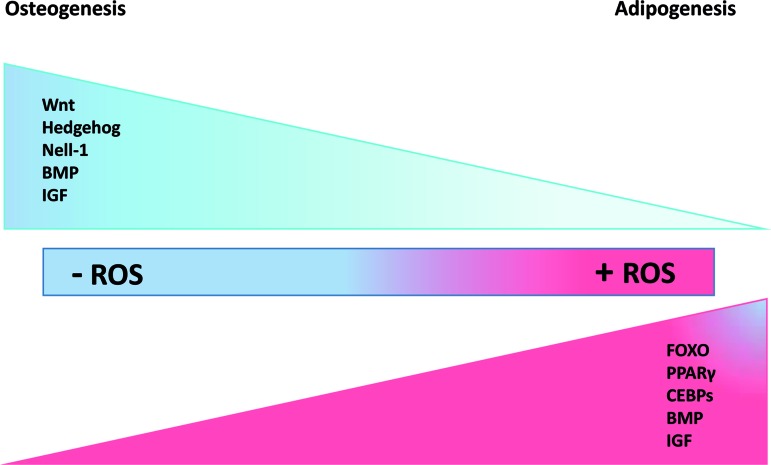
It is believed that MSCs derived from diverse sources (mostly from adipose tissue and bone marrow) implanted at a site of tissue injury are able to survive, proliferate, and differentiate into various cell types. However, several tissue regeneration-based studies have reported that the majority of engrafted MSCs die after several days and only a small percentage survive, which are hardly enough to replace lost tissue [72,73]. This low cell survival rate is due to local hypoxia. Eto et al. demonstrated that adipose-derived MSCs are very sensitive to oxygen concentrations and that only those cells implanted less than 300 μm from an oxygen source would survive, the others undergo apoptosis [74]. Therefore, most transplanted MSCs experience oxidative stress and the excessive ROS produced either by host tissues or by MSCs themselves is believed to account for cell cycle arrest and cell death. ROS can induce the activation of MAPK pathways such as JNK and p38MAPK and ERK along with activation of apoptotic proteins and suppression of antiapoptotic pathways [75]. On the other hand, many investigations have claimed that mitochondrial metabolism and ROS generation regulate MSC differentiation into adipocytes, chondrocytes, osteocytes, and neuronal cells [23,52,75,76]. ROS induce micro RNA-210 (miR-21) expression via PDGFR-b, Akt, and ERK pathways. Micro RNAs act primarily at the post-transcriptional level. Consequently, MSC proliferation and migration increase as a result of miR-210 expression. Micro RNAs suppress mRNA translation and/or promote degradation [77]. Additionally, NADPH oxidase complex induced ROS was reported to induce cell survival cascades through activation of PI3K/Akt pathways and inhibition of p38 MAPK [78]. Therefore, based on previous studies, there is evidence for a role for ROS in MSC survival, proliferation, and terminal differentiation. The impact of the oxidative environment on the regulation of osteogenesis and adipogenesis is described in the following sections (Fig. 4).
FIG. 4.
ROS control signaling cascades involved in osteogenesis/adipogenesis. Wnt/β-catenin, MAPK (NELL-1), and Hh signaling induce osteogenesis while FOXO, PPARγ, and CEBPs signaling stimulate adipogenesis. BMP and IGF signaling have a dual effect in inducing both of these terminal fates. Induction of osteogenesis is optimal in the absence of ROS while induction of adipogenesis is optimal in the presence of ROS. Color images available online at www.liebertpub.com/scd
Oxidative Stress and Antioxidant Regulation During Osteogenesis
Several studies have suggested a link between oxidative stress, osteogenic differentiation and bone formation. It is known that oxidative stress impairs skeletal integrity, and reduces osteogenic differentiation of murine preosteoblastic (MC3T3-E1) and bone marrow-derived stromal (M2-10B4) cell lines [79]. On the other hand, by using antioxidants such as pyro-lidinedithiocarbamate, a thiol-containing antioxidant and Trolox, a hydrophilic vitamin E analogue, osteogenic differentiation could be restored [79] suggesting that antioxidants may play a role in preventing age-related osteoporosis [80,81].
A study on mitochondrial metabolism revealed that osteogenic induction in human bone marrow-derived MSCs in vitro is associated with an upregulation of mtDNA copy number, protein subunits of respiratory enzymes, oxygen consumption rate and antioxidant enzymes, but a reduction in the levels of intracellular ROS [51]. The authors reported a dramatic reduction in intracellular levels of H2O2 and O2•− on the second day of osteogenic induction. In addition, they reported that 14 days after induction the protein levels of the antioxidant enzymes Mn-SOD and catalase were upregulated three and four fold, respectively.
When compared to untreated cells, addition of exogenous H2O2 (125–500 μM) to human bone marrow-derived MSCs reduced activity of alkaline phosphatase [82], a marker of osteogenic differentiation. Thus, excessive amounts of ROS prevent MSC osteogenic differentiation in these cells [51]. Furthermore, differentiation of murine osteoblast precursors into osteoblasts was abolished by exogenous H2O2 (50 μM H2O2 for 1 h) [83].
Animals studies have demonstrated that reduced osteoblast and bone formation, increased osteoblast and osteocyte apoptosis and decreased bone mineral density are due to increased oxidative stress in aged mice. Loss of sex steroids expedites the effects of aging by reducing defence mechanisms against oxidative stress [84].
Taken together, these observations suggest that ROS suppress osteoblast differentiation, and antioxidants could potentially rescue this phenomenon. Table 1 summarizes the effects of ROS on osteogenic differentiation.
Table 1.
Role of Reactive Oxygen Species in Osteogenic and Adipogenic Differentiation
| Source | Osteogenic differentiation | Adipogenic differentiation |
|---|---|---|
| Intracellular | ||
| Mitochondria | mtDNA copy number, protein subunits of respiratory enzymes and oxygen consumption rate are upregulated while intracellular H2O2 and O2•− are reduced after osteogenic induction [51] | Inhibition of the mitochondrial electron transport chain suppresses MSC adipogenic differentiation [76] |
| Oxidative stress in aged mice results in reduced osteoblast and bone formation, increased osteoblast and osteocyte apoptosis and decreased bone density [84] | ROS produced by mitochondrial complex III are required for activation of adipogenesis. Intracellular ROS increase after exposure of MSCs to an adipogenic cocktail [139] | |
| NOX4 mRNA expression is decreased while NOX2 mRNA expression is unchanged during adipogenic differentiation [92] | ||
| NAPDH oxidase | NOX4 knockout mice display higher bone density. NOX4 is involved in the transformation of osteoblasts to osteoclasts and is thus responsible for reduced bone density [112] | NOX4 increases lipid accumulation even in the absence of insulin. siRNA against NOX4 inhibit insulin-induced accumulation of lipid droplets in 3T3-L1 cells. Overexpression of NOX4 increases adipogenesis [22] |
| Elevated oxidative stress and consequently elevated NADPH oxidase in accumulated fat is related to obesity-associated metabolic syndrome in humans and mice [85] | ||
| NOX4 induces adipogenesis in adipose-derived MSCs [87] | ||
| Knock down of NOX4 inhibits MSC adipogenic differentiation even in the presence of an adipogenic cocktail [92] | ||
| Reduced expression of NOX4 is a hallmark of adipogenesis in 3T3-L1 cells [85,92,141] | ||
| Extracellular | H2O2 reduces Alp activity in osteogenic induced hMSC [51] | H2O2 induced oxidative stress induces MSC adipogenesis [22] |
| H2O2 abolishes osteogenesis in osteoblast progenitors [84] | Treating 3T3-L1 cells with H2O2 results in adipogenesis even in the absence of insulin [85] | |
| H2O2 induced oxidative stress reduces Gli protein levels thus preventing Hh signaling and reducing osteogenesis. The level of Alp mRNA expression is reduced [111] | H2O2 increase adipogenesis in 3T3-L1 cells in a dose-dependent manner [86] | |
| H2O2 inhibits expression of osteogenic differentiation markers in MC3T3-E1 and M2-10B4 cell lines. Alp activity is also reduced [79] | H2O2 diminishes expression of adipo-cytokines [87] | |
| eNOS rather than iNOS governs adipogenesis. NO stimulates rat preadipocyte differentiation [93] | ||
eNOS, endothelial nitric oxide synthases; iNOS, inducible nitric oxide synthases; MSC, mesenchymal stromal cell; ROS, reactive oxygen species.
Oxidative Stress and Antioxidant Regulation During Adipogenesis
Schröder et al. have suggested that stimulation of murine 3T3-L1 cells and human preadipocytes with exogenous H2O2 (30 μM, every other day) results in adipogenic differentiation even in the absence of insulin [85]. A dose-dependent role for H2O2 in regulating adipogenesis in 3T3-L1 preadipocytes has been observed, with higher doses of H2O2 (1 and 10 μM) increasing adipogenesis [86]. Additionally, mimicking oxidative stress by addition of exogenous H2O2 (100 μM) for 8 days was shown to induce adipogenesis in human adipose-derived MSCs [22]. In contrast, one study has reported that incubation with H2O2 (0.1–0.5 mM) diminished expression of adipo-cytokine mRNAs such as the fat-derived hormone adiponectin and the transcription factor PPARγ murine 3T3-L1 cells in a dose-dependent manner [87].
Recently, several studies have focused on antioxidant levels during adipogenesis in an oxidative stress environment [86,88–91]. Application of an antioxidant such as N-acetyl-l-cysteine (NAC) inhibited the expression of transcription factors such as C/EBPα (days 2 and 4) and PPARγ (day 4) in rat bone marrow MSCs and murine 10T1/2 cells [92]. In agreement with these findings, increased levels of ROS production in adipose tissue are accompanied by decreased expression of antioxidative enzymes such as Cu, Zn-SOD, and catalase. ROS production was significantly decreased by the antioxidants apocynin or NAC in fully differentiated 3T3-L1 adipocytes [87]. Recently, it was demonstrated that a concomitant increase in the expression of SOD3 mRNA and protein occurs with the differentiation of human bone marrow-derived MSCs into adipocytes [90] and during the early stages of adipogenesis in 3T3-L1 cells [89]. Using siRNA interference to knock down Mn-SOD, it was observed that the expression of late adipogenesis markers such as adiponectin and fatty acid-binding protein 4 (FABP4) was reduced, demonstrating that Mn-SOD knockdown impairs adipogenesis in 3T3-L1 cells [88]. Similary, upregulation of antioxidant enzymes such as SOD, catalase, and GPX have been observed during adipogenesis in human adipose-derived MSCs [22].
Several studies have looked at the role of other free radicals in adipogenesis. For example, a stimulatory role for endogenous nitric oxide (NO) on adipogenesis in preadipocytes derived from rat white adipose tissue has been reported [93]. A 50% increase in basal levels of NO was observed on the first two days after adipogenic differentiation. As inducible nitric oxide synthases (iNOS) inhibitors such as 1400W and aminoguanidine had a minor impact on differentiation and NO production [93], endothelial nitric oxide synthases (eNOS) rather than iNOS may be the major isoform of nitric oxide synthase that modulates adipogenesis [93].
Taken together, these findings demonstrate that an oxidized intracellular environment favors murine and human MSC and preadipocyte differentiation into mature adipocytes. ROS increases the expression of genes associated with adipogenesis. Additionally, adipocytes contain higher levels of intracellular ROS compared with progenitors. The adipogenic process increases the expression of antioxidants, an event that could become a hallmark of adipogenesis. Table 1 summarizes the effects of ROS on MSC adipogenic differentiation.
Regulation of Differentiation Toward an Osteogenic/Adipogenic Fate
Bone is produced by two mechanisms: (1) intramembranous ossification, the direct differentiation of mesenchymal progenitors into osteoblasts; and (2) endochondral ossification, bone formation via a cartilage anlagen, a mechanism initiated by the formation of MSC clusters [94]. Adipogenic differentiation occurs via two phases: (1) commitment of MSCs to a preadipocyte stage; and (2) terminal differentiation of preadipocytes into mature adipocytes [95].
The interplay of several extracellular signals such as hormones (glucocorticoid and parathyroid hormones) and ligands of the wingless/invected1 (Wnt), BMP, fibroblast growth factor (FGF), transforming growth factor β (TGFβ), and hedgehog signaling pathways are required for osteogenic differentiation [96,97].
The main signaling pathways that determine MSC terminal fate are reviewed in the following sections.
Evidence for a Potential Role for ROS in Inhibiting Bone Formation
WNT signaling
Wnt is a molecular switch for adipogenic/osteogenic differentiation. The Wnt canonical pathway (β-catenin dependent) is initiated through the binding of extracellular Wnt ligands to the frizzled seven pass transmembrane receptors (Frz). Consequently, intracellular signaling of the complex of axin, glycogen synthase kinase 3 (GSK3), and adenomatosis polyposis scientifica 5 coli (APC) protein will be inhibited. Upon Wnt signaling, β-catenin degradation is inhibited by the Axin/APC/GSK3 complex, which results in the translocation of β-catenin from the cytoplasm to the nucleus [29]. Nuclear β-catenin binds to the T-cell factor/lymphoid-enhancing factor (Tcf/Lef), which then forms a transcriptional effector for activating Wnt target genes [32] (Fig. 5).
FIG. 5.
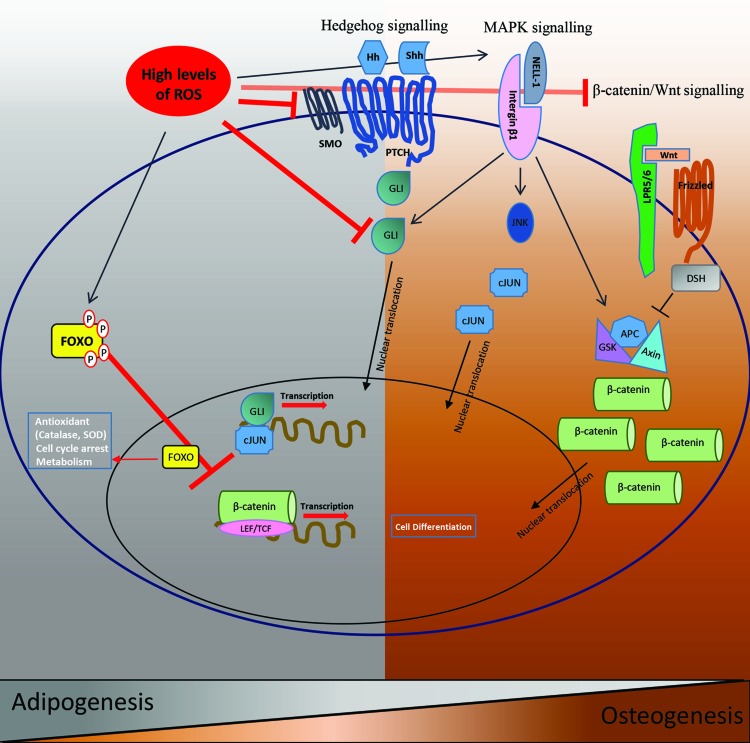
ROS suppress important osteogenic signaling pathways while they promote adipogenic signaling pathways. Wnt/β-catenin and Hh signaling cascades induce osteogenesis and this is inhibited in the presence of high levels of ROS, which favors adipogenesis. MAPK signaling induces osteogenesis and is stimulated by ROS. In response to oxidative stress, FOXOs are phosphorylated and translocate to the nucleus where they attenuate the transcription of osteogenic genes while inducing adipogenic differentiation. The active form FOXO also induces the regulation of antioxidant and cell cycle arrest genes. The expression of antioxidants also increases adipogenic differentiation. Color images available online at www.liebertpub.com/scd
Currently available data suggest that Wnt/β-catenin positively regulate osteoblast and osteoclast activity. In line with this notion, disruption of the Wnt/β-catenin pathway impairs osteogenesis [29]. β-catenin induces essential signals for osteogenic initiation [98] and conditional inactivation converts osteoblasts into chondrocytes and thus delays skeletal mineralization. Wnt/β-catenin signaling suppress adipogenesis and thus favors osteogenesis by reducing the expression of C/EBPα and PPARγ mRNAs; these molecules are key regulators of adipogenesis and suppressors of osteogenesis [32,99]. Recently, several studies have reported the adverse effect of oxidative stress on osteoblastogenesis [100]. Interestingly, the suppressive effect of H2O2 on Tcf-mediated transcription was abolished by overexpression of β-catenin. An in vivo study reported that ROS increases with increasing age, which in turn decreases the expression of Wnt target genes such as Axin2 and Opg in 31-month-old mice when compared with 4-month-old mice, and thus diminishes osteogenesis [83]. Taken together, these findings suggest that ROS inhibit the osteoinductive effect of Wnt signaling, although under normal circumstances this pathway positively stimulates osteogenesis.
FOXO signaling
Bone involution such as occurs during decreased bone formation and increased bone marrow adiposity is associated with increased oxidative stress and decreased growth factor production. This results in the activation of the FOXO family of transcription factors [101]. Indeed, the defence mechanism against oxidative stress is governed by the FOXO family of transcription factors [102–104]. The FOXO family consists of four members: FOXO1 (or Fkhr), FOXO3a (or Fkhrl1), FOXO4 (or Afx), and FOXO6 [105]. β-catenin is also required for FOXO-mediated transcriptional downstream effectors of the Wnt/β-catenin pathway [103]. In osteoblast progenitors and many other cell types, the association of β-catenin with FOXOs increases in the presence of oxidative stress [103]. In the absence of growth factors or in the presence of high levels of ROS, FOXO is activated. It then translocates to the nucleus and induces the transcription of a variety of target genes such as antioxidants (Fig. 5). It is known that FOXO represses osteogenic differentiation [101]. An in vivo investigation in both female and male C57BL/6 mice reported that FOXOs impair bone formation by antagonizing Wnt signaling [83].
Iyer et al. have demonstrated that mice deficient in FOXO1, -3, and -4 in osteoblast progenitors exhibit increased osteoblast number and a higher bone mass [101]. In line with this observation, treating murine osteoblastic cells with 100 μM H2O2 for 1 h enhances β-catenin and FOXO3 association. β-catenin in turn is essential for FOXO target gene stimulation by H2O2. FOXO transcription is promoted by H2O2 while Wnt/Tcf mediated transcription and osteoblast differentiation is reduced [83]. In response to oxidative stress, FOXOs induce cell cycle arrest and dormancy [104,106]. Under such conditions, FOXOs regulate transcription of antioxidant enzymes (eg, catalase, Mn-SOD) and also genes that play role in the cell cycle and cell longevity [102,107] (Fig. 5). Collectively, this suggests that activation of FOXO signaling by oxidative stress attenuates the osteogenic process.
Hedgehog signaling
At least three members of the Hedgehog signaling, Hh, family have been described in vertebrates: sonic hedgehog (Shh), Indian hedgehog (Ihh), and desert hedgehog (Dhh) [108]. Hedgehog signaling is activated by the binding of an Hh ligand to the receptor patched (PTCH), a 12 pass transmembrane protein that inhibits smoothened (Smo), a 7 pass transmembrane protein. This inhibition leads to translocation of the glioblastoma gene product [109] family of DNA-binding proteins to the nucleus where transcription of Hh target genes follows [29,110,111] (Fig. 5). Osteoinductive [111] and anti-adipogenic roles have been ascribed to Hh signaling (99, 135–140). Oxidative stress inhibits Hh-induced osteogenic differentiation in murine primary bone marrow-derived and other MSC cell lines [111] (Fig. 5). Addition of nonphysiological levels of H2O2 (0.5–1 mM) for 72 h suppressed Hh signaling thus inhibiting Hh-mediated osteogenic differentiation in bone marrow stromal cells [111]. The expression of osteogenic differentiation markers such as Alp, OSX, and BSP was significantly reduced, indicating that in MSCs, Shh-induced osteogenesis is inhibited by H2O2-induced oxidative stress (0.5–1 mM H2O2 for 48 h). Moreover, H2O2-induced oxidative stress impaired the proliferation of MSCs. Taken together, these data suggest that under normal conditions, Hh signaling induces osteogenesis. However, ROS inhibits the osteoinductive effect of Hh signaling.
NADPH oxidase
The impact of NOX isoforms on MSC osteogenic differentiation has been less well studied. NOX induced ROS has been implicated in bone disease. NOX4, as a constitutively active source of ROS, is involved in osteoclastogenesis. It has recently been reported that NOX4 knockout mice display higher bone density. Interestingly, a specific single nucleotide polymorphism (SNP) in the human NOX4 gene has been shown to contribute to the greater expression of bone turnover markers and reduced bone density in women. NOX4 expression could be thus responsible for reduced bone density [112]. Understanding the role of NOX isoforms in osteoblast formation is therefore essential in studies on bone loss and regeneration. Future studies should determine the levels of ROS required for osteogenic attenuation and should also clarify the role of the different sources of ROS in osteogenic signaling pathways.
Evidence for a Potential Role for ROS in Promoting Adipogenesis
WNT signaling
A large body of experimental evidence, both in vitro and in vivo, has demonstrated the inhibitory role of Wnt molecules during adipogenic differentiation of mesenchymal or preadipocyte cells [99,113–115]. It is known that Wnt inhibits the early stages of adipogenesis. Inhibiting the Wnt pathway stimulates the generation of adipocytes in 3T3-L1 preadipocytes [99]. From a pathophysiological perspective, several genetic studies have revealed that polymorphisms in genes of the WNT signaling pathway are linked to the development of obesity and type 2 diabetes in humans [113]. It has also been shown that Wnt signaling promotes MSC osteogenic, myogenic, and chondrogenic differentiation and abrogates adipogenic differentiation [116–119]. β-catenin activation suppressed PPARγ expression and impaired murine 3T3-L1 adipogenic differentiation [120]. Taken together, these findings indicate that Wnt has an adipo-repressive effect. However, in an oxidative environment, β-catenin diverts to FOXO instead of Tcf to suppress Wnt, and thus favors adipogenesis [83]. Further studies should reveal the role of ROS, their optimum level, and the role of different ROS generators in regulating the Wnt pathway.
FOXO signaling
FOXO negatively regulates adipogenesis. The expression and transcriptional activity of PPARγ, the master transcription factor for adipogenesis, is suppressed by FOXO1 [121]. Insulin induces phosphorylation of Akt, which then activates adipogenic transcription factors, specifically PPARγ. Akt promotes PPARγ expression by FOXO1 exclusion from the nucleus [122]. Akt tightly governs the function of FOXO proteins through Akt-mediated phosphorylation mechanisms [123,124]. FOXO plays a key role in maintaining cellular redox homeostasis. FOXO1 limits oxidative stress in human adipose-derived MSCs by upregulating antioxidant enzymes [22]. Additionally, mice lacking FOXO1, FOXO3, and FOXO4 showed decreased adiposity in aged bone marrow, although osteoblast number was increased and these mice had a greater bone mass in old age [101]. Using siRNAs against FOXO1 in murine 3T3-L1 preadipocytes, decreased lipid droplet formation was observed after adipogenic induction. Adipogenesis was more severely inhibited when cells were exposed to FOXO1 siRNA before induction of adipogenic differentiation. Downregulation of FOXO1 in 3T3-L1 cells resulted in a decrease in expression of the adipogenic transcription factors, PPARγ and C/EBPα [123]. However, another study in 3T3-F442A cells and murine embryonic fibroblasts suggested that FOXO1 prevents adipogenic differentiation [124]. Similarly, SIRT2, a cytoplasmic sirtuin, indirectly inhibits PPARγ by reducing FOXO1 acetylation and phosphorylation; this increased the amount of FOXO1 in the nucleus and consequently PPARγ transcription was repressed. These results propose a cell type and context-dependent role for FOXO expression in cellular signaling during adipogenesis [22]. Additionally, the exact role of FOXO, its regulation by phosphorylation and its effect on antioxidant expression requires further investigation.
Hedgehog signaling
An antiadipogenic role has been suggested for the Hh pathway upon its activation [31,125–130]. Hedgehog signaling inhibits adipogenic differentiation of murine 3T3-L1, NIH-3T3, and C3H10T1/2 cells, but when this pathway is inhibited adipogenesis is promoted. Hedgehog signaling induces antiadipogenic transcription factors (eg, Gata2 and Gilz) to repress adipogenesis. Consequently, the antiadipogenic factors downregulate PPARγ expression [127]. Under normal conditions, Hh signaling induces osteogenesis while inhibiting adipogenesis. However, oxidative stress inhibits Hh-induced osteogenic differentiation, and may thus favour adipogenesis. To our knowledge, there is no study reporting the role of oxidative stress in the regulation of adipogenic differentiation via Hh signaling. It would therefore be of interest to assess this possible relationship in future investigations.
Transcription factors
Several transcription factors such as CCAAT/enhancer-binding proteins (C/EBPs) and PPARγ play a key role adipogenesis [29]. Transcription factors such as C/EBPβ and C/EBPδ are the major adipogenesis regulators during early phases of differentiation. Thus, C/EBPδ is expressed during the early phase of adipogenesis and disappears in the late phase [131]. C/EBPα and PPARγ regulate terminal differentiation stages [132]. Coordinated activity between these two transcription factors functions as a positive feedback loop in which PPARγ activates the promoter of the gene encoding C/EBPα and vice versa. This induces the expression of adipocyte specific genes such as glucose transporter GLUT4 (also known as SLC2A4), lipoprotein lipase, fatty acid-binding protein 4 (FABP4, also known as aP2), adiponectin, and leptin [133–135]. Many factors affect the ability of PPARγ to influence the adipogenic process. Sirtuin 1(SIRT1), a histone/protein deacetylase, directly binds to PPARγ and impairs adipogenesis [136]. It also acts as a PPARγ co-repressor [137]. SIRT2, a cytoplasmic sirtuin, indirectly inhibits PPARγ. It has been shown that SIRT2 is downregulated during adipogenesis in murine 3T3-L1 cells [138].
Mitochondrial ROS
Mitochondrial ROS production appears to be critical in promoting the differentiation of human bone marrow-derived MSCs and an increase in ROS levels supports an unrestricted oxidative environment for launching signaling events leading to adipogenic differentiation [139]. Not only do ROS modulate adipogenic differentiation, but they also impact on MSC proliferation. Mitochondrial ROS was suggested to inhibit proliferation of murine 3T3-L1 preadipocytes [140]. Similarly, it was demonstrated that ROS produced by mitochondrial complex III is required for activation of adipogenic gene transcription in human bone marrow-derived MSCs. Intracellular H2O2 was increased after two days upon exposure of MSCs to adipogenic induction medium (containing indomethacin, dexamethasone, isobutylmethylxanthine, and insulin). However, mitochondrial targeted antioxidants (500 nM MitoCP) attenuated the amount of intracellular H2O2 and consequently impaired lipid accumulation during adipogenesis. In relation to this observation, protein levels of major adipogenic transcription factors such as C/EBPα and PPARγ2 were dramatically decreased. However, adipogenesis was rescued when cells were treated with D-galactose (0.5 mM) to deliberately generate exogenous H2O2 [139]. In agreement with these findings, it has recently been suggested that elevated mitochondrial activity is an essential requirement for human MSC adipogenic differentiation. siRNA-based knockdown of the mitochondrial transcription factor A (TFAM), which suppresses mitochondrial activity, inhibited adipogenic differentiation. Under hypoxic conditions or by inhibition of the mitochondrial electron transport chain, mitochondrial respiration was reduced. As a result, adipogenic differentiation was significantly suppressed [76]. Taken together, these findings indicate that superoxide generated by the mitochondrial electron transport chain is converted to H2O2, which initiates the PPARγ transcriptional machinery that regulates adipocyte differentiation.
NADPH oxidase
NADPH oxidase was demonstrated to be the central source of ROS in adipocyte precursors [87,141]. NOX4, for instance, is highly expressed in preadipocytes and is emerging as a hallmark of preadipocte proliferation and differentiation [85,92,141]. Recently, Schröder et al. reported that the effect of NOX4 expression on proliferation and differentiation of murine 3T3-L1 and human adipose-derived MSCs is mediated by the MEK/ERK pathway. They suggested that NOX4 controls proliferation by activating the ROS-dependent phosphorylation of ERK1/2. In addition, both knockout and siRNA studies demonstrate a role for NOX4 in governing MSCs differentiation. siRNA directed against NOX4 resulted in inhibition of ERK1/2 thus promoting proliferation and impairing adipogenic differentiation of 3T3-L1 preadipocytes. siRNA against NOX4 resulted in inhibition of insulin-induced accumulation of lipid droplets in 3T3-L1 cells [85]. Similarly, RNA interference knockdown of NOX4 inhibited the adipogenic differentiation of rat bone marrow-derived MSCs and murine 10T1/2 cells, even in the presence of an adipogenic cocktail. NOX4 expression was decreased; NOX2 expression was, however, constant after adipogenic differentiation [92]. On the other hand, in the experiments reported by Schröder et al., overexpression of NOX4 increased the accumulation of lipid droplets even in the absence of insulin, demonstrating that NOX4 is a direct mediator of insulin-induced differentiation in human preadipocytes [85]. Similarly, overexpression of NOX4 was shown to induce adipogenesis in human adipose-derived MSCs [22]. A further study demonstrated that the level of production of ROS such as H2O2 increased in adipose tissue of obese mice when compared to control mice and was accompanied by increased expression of NADPH oxidase (gp91phox and p22phox, and cytosolic components p47phox, p67phox, and p40phox) and a reduction in the levels of antioxidant enzymes. ROS production was significantly decreased by the NOX inhibitor diphenyleneiodonium in murine 3T3-L1 preadipocytes [87]. Collectively, these data suggest a positive role for the NOX4 isoform of NADPH oxidase in the differentiation of adipogenic progenitors. Generation of ROS by NADPH oxidase appears to be necessary for positive regulation of MSC proliferation and adipogenic differentiation. Targeting various NOX isoforms may elucidate their role in the effect of ROS on MSC differentiation.
Therapeutic Role of ROS
Oxidative stress plays a key role in the pathogenesis of many diseases [87] such as diabetes [37], hypertension [38], atherosclerosis [39], carcinogenesis, metabolic, cardiovascular, pulmonary, and neurological diseases [142]. Recently, NOX inhibitors that target NOX1 and NOX4 enzymes have been used in patients with diabetic nephropathy [142]. The role of exogenous or endogenous ROS on osteogenesis and adipogenesis in the clinical setting has been less intensely investigated. However, ROS is known to be a critical factor in aging [40]. Increased oxidative stress, mainly associated with ageing, has been implicated in the pathogenesis of age-related bone loss in humans and mice [83]. Recently, it was reported that NOX4 is involved in osteoclastogenesis. Subsequently increased human bone resorption has been linked to NOX4, as a constitutively producer of H2O2 [112]. SNP analysis of NOX4 in middle-aged woman has revealed a link to altered bone density and plasma markers for bone turnover. In addition, NOX4 is highly expressed in osteoporotic bone in humans [112]. Thus, application of NOX inhibitors should be considered in the context of osteoporosis treatment and possibly bone-related disorders.
Studies in humans have revealed that accumulation of adipose tissue in obese patients is associated with increased systemic oxidative stress, which might therefore present an interesting target for the development of new therapies for obesity-associated metabolic syndrome [87]. In addition, several studies have reported elevated systemic oxidative stress in obesity [143]. A greater understanding of ROS signaling and the consequences thereof might therefore open new therapeutic avenues for the treatment of obesity and its comorbid entities.
Conclusion
ROS have for many years been regarded as having a negative effect on cell function and survival. However, it is becoming increasingly recognised that ROS also mediate important physiological functions. The findings reviewed here demonstrate a pivotal role for ROS in MSC differentiation. Regardless of the sources of ROS, it has been shown that osteogenic and adipogenic differentiation is partly ROS dependent. We conclude that osteogenesis is blunted by elevated ROS while ROS positively induces adipogenesis in MSCs and other adipogenic progenitors. The activity of the ROS generating NOX4 isoform most likely increases adipogenesis. However, mitochondrial ROS also appears to be necessary for MSC adipogenic differentiation. Taken together, these findings highlight the need to further investigate the role of ROS in regulating MSC differentiation. Further studies should clarify the role of ROS in each signaling cascade, the role of different sources of ROS and their concentration, the period required for treating cells with exogenous ROS and finally the impact of various antioxidants exogenously applied and/or produced in stem cell-based studies and also in the pathogenesis and treatment of relevant diseases.
Acknowledgments
The authors wish to thank Dr. Vincent Jaquet, Prof. Karl-Heinz Krause, and Prof. Jean-Claude Martinou for editorial assistance. Fatemeh Atashi and Ali Modarressi are funded by Swiss National Science Foundation (SNF) (grant #310030-120751). Michal Pepper is funded by the South African Medical Research Council in terms of the MRC's Flagships Awards Project SAMRC-RFA-UFSP-01-2013/STEM CELLS.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Harman D. (1956). Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300 [DOI] [PubMed] [Google Scholar]
- 2.Beckman KB. and Ames BN. (1998). The free radical theory of aging matures. Physiol Rev 78:547–581 [DOI] [PubMed] [Google Scholar]
- 3.Guzik TJ. and Harrison DG. (2006). Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today 11:524–533 [DOI] [PubMed] [Google Scholar]
- 4.Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED. and Selemidis S. (2012). NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal 16:1229–1247 [DOI] [PubMed] [Google Scholar]
- 5.Bedard K. and Krause KH. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- 6.Storz P. (2011). Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal 14:593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto WR. and Wright NA. (2011). Mesenchymal stem cells: from experiment to clinic. Fibrogenesis Tissue Repair 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuan RS, Boland G. and Tuli R. (2003). Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther 5:32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romanov YA, Darevskaya AN, Merzlikina NV. and Buravkova LB. (2005). Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med 140:138–143 [DOI] [PubMed] [Google Scholar]
- 10.Herzog EL, Chai L. and Krause DS. (2003). Plasticity of marrow-derived stem cells. Blood 102:3483–3493 [DOI] [PubMed] [Google Scholar]
- 11.Hass R, Kasper C, Bohm S. and Jacobs R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M. and Aldahmash A. (2013). Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev 9:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ. and Goebel WS. (2008). Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods 14:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E. and Ramasamy R. (2012). Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells 4:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K. and Gimble JM. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan AI. and Bruder SP. (2001). Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7:259–264 [DOI] [PubMed] [Google Scholar]
- 17.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 18.Bianco P, Robey PG. and Simmons PJ. (2008). Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AH, Lee MS, Lee MK, Kwon CH, et al. (2010). Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation 89:509–517 [DOI] [PubMed] [Google Scholar]
- 20.Caplan AI. and Dennis JE. (2006). Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084 [DOI] [PubMed] [Google Scholar]
- 21.Valle-Prieto A. and Conget PA. (2010). Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19:1885–1893 [DOI] [PubMed] [Google Scholar]
- 22.Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC. and Liu GS. (2013). Differentiation of human adipose -derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 22:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A. and Towler DA. (2007). Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci 1117:40–50 [DOI] [PubMed] [Google Scholar]
- 24.Mateos J, De la Fuente A, Lesende-Rodriguez I, Fernandez-Pernas P, Arufe MC. and Blanco FJ. (2013). Lamin A deregulation in human mesenchymal stem cells promotes an impairment in their chondrogenic potential and imbalance in their response to oxidative stress. Stem Cell Res 11:1137–1148 [DOI] [PubMed] [Google Scholar]
- 25.Boopathy AV, Pendergrass KD, Che PL, Yoon YS. and Davis ME. (2013). Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res Ther 4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei L. and Tontonoz P. (2004). Fat's loss is bone's gain. J Clin Invest 113:805–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beresford JN, Bennett JH, Devlin C, Leboy PS. and Owen ME. (1992). Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102 (Pt 2):341–351 [DOI] [PubMed] [Google Scholar]
- 28.Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR. and Harris SE. (1998). Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James AW. (2013). Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo) 2013:684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James AW, Pan A, Chiang M, Zara JN, Zhang X, Ting K. and Soo C. (2011). A new function of Nell-1 protein in repressing adipogenic differentiation. Biochem Biophys Res Commun 411:126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, et al. (2001). Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci 114:2085–2094 [DOI] [PubMed] [Google Scholar]
- 32.Takada I, Kouzmenko AP. and Kato S. (2009). Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5:442–447 [DOI] [PubMed] [Google Scholar]
- 33.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, et al. (2009). A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18:545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yakar S, Kim H, Zhao H, Toyoshima Y, Pennisi P, Gavrilova O. and Leroith D. (2005). The growth hormone-insulin like growth factor axis revisited: lessons from IGF-1 and IGF-1 receptor gene targeting. Pediatr Nephrol 20:251–254 [DOI] [PubMed] [Google Scholar]
- 35.Wabitsch M, Hauner H, Heinze E. and Teller WM. (1995). The role of growth hormone/insulin-like growth factors in adipocyte differentiation. Metabolism 44:45–49 [DOI] [PubMed] [Google Scholar]
- 36.Smith PJ, Wise LS, Berkowitz R, Wan C. and Rubin CS. (1988). Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem 263:9402–9408 [PubMed] [Google Scholar]
- 37.Brownlee M. (2001). Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- 38.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T. and Inoue M. (1991). Does superoxide underlie the pathogenesis of hypertension?. Proc Natl Acad Sci U S A 88:10045–10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohara Y, Peterson TE. and Harrison DG. (1993). Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91:2546–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banfi G, Iorio EL. and Corsi MM. (2008). Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med 46:1550–1555 [DOI] [PubMed] [Google Scholar]
- 41.Brown GC. and Borutaite V. (2012). There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 12:1–4 [DOI] [PubMed] [Google Scholar]
- 42.Griendling KK, Sorescu D. and Ushio-Fukai M. (2000). NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86:494–501 [DOI] [PubMed] [Google Scholar]
- 43.Braunersreuther V. and Jaquet V. (2012). Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches. Curr Pharm Biotechnol 13:97–114 [DOI] [PubMed] [Google Scholar]
- 44.Starkov AA. (2008). The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147:37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boveris A, Oshino N. and Chance B. (1972). The cellular production of hydrogen peroxide. Biochem J 128:617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C. and Fass D. (2006). Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci U S A 103:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kukreja RC, Kontos HA, Hess ML. and Ellis EF. (1986). PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 59:612–619 [DOI] [PubMed] [Google Scholar]
- 48.Roy P, Roy SK, Mitra A. and Kulkarni AP. (1994). Superoxide generation by lipoxygenase in the presence of NADH and NADPH. Biochim Biophys Acta 1214:171–179 [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell VB. and Azzi A. (1996). High rates of extracellular superoxide generation by cultured human fibroblasts: involvement of a lipid-metabolizing enzyme. Biochem J 318 (Pt 3):805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H. and Harrison DG. (2003). Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285:H2290–H2297 [DOI] [PubMed] [Google Scholar]
- 51.Chen CT, Shih YR, Kuo TK, Lee OK. and Wei YH. (2008). Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26:960–968 [DOI] [PubMed] [Google Scholar]
- 52.Nayernia Z, Jaquet V. and Krause KH. (2014). New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal 20:2815–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dikalov S. (2011). Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51:1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tahara EB, Navarete FD. and Kowaltowski AJ. (2009). Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46:1283–1297 [DOI] [PubMed] [Google Scholar]
- 55.Okado-Matsumoto A. and Fridovich I. (2001). Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276:38388–38393 [DOI] [PubMed] [Google Scholar]
- 56.Holmgren A. (2000). Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2:811–820 [DOI] [PubMed] [Google Scholar]
- 57.Droge W. (2002). Free radicals in the physiological control of cell function. Physiol Rev 82:47–95 [DOI] [PubMed] [Google Scholar]
- 58.Murphy MP. (2009). How mitochondria produce reactive oxygen species. Biochem J 417:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finkel T. (2011). Signal transduction by reactive oxygen species. J Cell Biol 194:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Case AJ, Li S, Basu U, Tian J. and Zimmerman MC. (2013). Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305:H19–H28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Block K, Gorin Y. and Abboud HE. (2009). Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106:14385–14390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, Jelinek DF. and Kay NE. (2010). Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood 116:2984–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segal AW. and Jones OT. (1978). Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 276:515–517 [DOI] [PubMed] [Google Scholar]
- 64.Nishio E. and Watanabe Y. (1997). The involvement of reactive oxygen species and arachidonic acid in alpha 1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br J Pharmacol 121:665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhoades RA, Packer CS, Roepke DA, Jin N. and Meiss RA. (1990). Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol 68:1581–1589 [DOI] [PubMed] [Google Scholar]
- 66.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK. and Lambeth JD. (1999). Cell transformation by the superoxide-generating oxidase Mox1. Nature 401:79–82 [DOI] [PubMed] [Google Scholar]
- 67.Park SG, Kim JH, Xia Y. and Sung JH. (2011). Generation of reactive oxygen species in adipose-derived stem cells: friend or foe?. Expert Opin Ther Targets 15:1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacFie TS, Poulsom R, Parker A, Warnes G, Boitsova T, Nijhuis A, Suraweera N, Poehlmann A, Szary J, et al. (2014). DUOX2 and DUOXA2 form the predominant enzyme system capable of producing the reactive oxygen species H2O2 in active ulcerative colitis and are modulated by 5-aminosalicylic acid. Inflamm Bowel Dis 20:514–524 [DOI] [PubMed] [Google Scholar]
- 69.Yoshihara A, Hara T, Kawashima A, Akama T, Tanigawa K, Wu H, Sue M, Ishido Y, Hiroi N, et al. (2012). Regulation of dual oxidase (DUOX) expression and H2O2 production by thyroglobulin. Thyroid 22:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J. and Beach D. (2007). A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal 9:293–299 [DOI] [PubMed] [Google Scholar]
- 71.Lonergan T, Brenner C. and Bavister B. (2006). Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol 208:149–153 [DOI] [PubMed] [Google Scholar]
- 72.Pittenger MF. and Martin BJ. (2004). Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95:9–20 [DOI] [PubMed] [Google Scholar]
- 73.Liu SP, Ding DC, Wang HJ, Su CY, Lin SZ, Li H. and Shyu WC. (2010). Nonsenescent Hsp27-upregulated MSCs implantation promotes neuroplasticity in stroke model. Cell Transplant 19:1261–1279 [DOI] [PubMed] [Google Scholar]
- 74.Eto H, Kato H, Suga H, Aoi N, Doi K, Kunoand S. and Yoshimura K. (2012). The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg 129:1081–1092 [DOI] [PubMed] [Google Scholar]
- 75.Rodrigues M, Turner O, Stolz D, Griffith LG. and Wells A. (2012). Production of reactive oxygen species by multipotent stromal cells/mesenchymal stem cells upon exposure to fas ligand. Cell Transplant 21:2171–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Marsboom G, Toth PT. and Rehman J. (2013). Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One 8:e77077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JH, Park SG, Song SY, Kim JK. and Sung JH. (2013). Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis 4:e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Zeigler MM, Lam GK, Hunter MG, Eubank TD, Khramtsov VV, Tridandapani S, Sen CK. and Marsh CB. (2007). The role of the NADPH oxidase complex, p38 MAPK, and Akt in regulating human monocyte/macrophage survival. Am J Respir Cell Mol Biol 36:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mody N, Parhami F, Sarafian TA. and Demer LL. (2001). Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 31:509–519 [DOI] [PubMed] [Google Scholar]
- 80.Basu S, Michaelsson K, Olofsson H, Johansson S. and Melhus H. (2001). Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288:275–279 [DOI] [PubMed] [Google Scholar]
- 81.Shouhed D, Kha HT, Richardson JA, Amantea CM, Hahn TJ. and Parhami F. (2005). Osteogenic oxysterols inhibit the adverse effects of oxidative stress on osteogenic differentiation of marrow stromal cells. J Cell Biochem 95:1276–1283 [DOI] [PubMed] [Google Scholar]
- 82.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G. and Vinante F. (2005). HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 106:59–66 [DOI] [PubMed] [Google Scholar]
- 83.Almeida M, Han L, Martin-Millan M, O'Brien CA. and Manolagas SC. (2007). Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298–27305 [DOI] [PubMed] [Google Scholar]
- 84.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, et al. (2007). Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schröder K, Wandzioch K, Helmcke I. and Brandes RP. (2009). Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29:239–245 [DOI] [PubMed] [Google Scholar]
- 86.Turker I, Zhang YH, Zhang YM. and Rehman J. (2007). Oxidative stress as a regulator of adipogenesis. Faseb J 21:830.5 [Google Scholar]
- 87.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M. and Shimomura I. (2004). Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krautbauer S, Eisinger K, Hader Y, Neumeier M. and Buechler C. (2014). Manganese superoxide dismutase knock-down in 3T3-L1 preadipocytes impairs subsequent adipogenesis. Mol Cell Biochem 393:69–76 [DOI] [PubMed] [Google Scholar]
- 89.Adachi T, Toishi T, Wu H, Kamiya T. and Hara H. (2009). Expression of extracellular superoxide dismutase during adipose differentiation in 3T3-L1 cells. Redox Rep 14:34–40 [DOI] [PubMed] [Google Scholar]
- 90.Nightingale H, Kemp K, Gray E, Hares K, Mallam E, Scolding N. and Wilkins A. (2012). Changes in expression of the antioxidant enzyme SOD3 occur upon differentiation of human bone marrow-derived mesenchymal stem cells in vitro. Stem Cells Dev 21:2026–2035 [DOI] [PubMed] [Google Scholar]
- 91.Lee H, Lee YJ, Choi H, Ko EH. and Kim JW. (2009). Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 284:10601–10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanda Y, Hinata T, Kang SW. and Watanabe Y. (2011). Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci 89:250–258 [DOI] [PubMed] [Google Scholar]
- 93.Yan H, Aziz E, Shillabeer G, Wong A, Shanghavi D, Kermouni A, Abdel-Hafez M. and Lau DC. (2002). Nitric oxide promotes differentiation of rat white preadipocytes in culture. J Lipid Res 43:2123–2129 [DOI] [PubMed] [Google Scholar]
- 94.Kronenberg HM. (2003). Developmental regulation of the growth plate. Nature 423:332–336 [DOI] [PubMed] [Google Scholar]
- 95.Cristancho AG. and Lazar MA. (2011). Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 12:722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marie PJ. (2008). Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys 473:98–105 [DOI] [PubMed] [Google Scholar]
- 97.Komori T. (2011). Signaling networks in RUNX2-dependent bone development. J Cell Biochem 112:750–755 [DOI] [PubMed] [Google Scholar]
- 98.Rodda SJ. and McMahon AP. (2006). Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231–3244 [DOI] [PubMed] [Google Scholar]
- 99.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL. and MacDougald OA. (2000). Inhibition of adipogenesis by Wnt signaling. Science 289:950–953 [DOI] [PubMed] [Google Scholar]
- 100.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM. and Luo SQ. (2004). Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 314:197–207 [DOI] [PubMed] [Google Scholar]
- 101.Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, de Cabo R, Jilka RL, Weinstein RS, O'Brien CA, Manolagas SC. and Almeida M. (2013). FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest 123:3409–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van der Horst A. and Burgering BM. (2007). Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 8:440–450 [DOI] [PubMed] [Google Scholar]
- 103.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM. and Korswagen HC. (2005). Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 308:1181–1184 [DOI] [PubMed] [Google Scholar]
- 104.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH. and Burgering BM. (2002). Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–321 [DOI] [PubMed] [Google Scholar]
- 105.Katoh M. and Katoh M. (2004). Human FOX gene family (Review). Int J Oncol 25:1495–1500 [PubMed] [Google Scholar]
- 106.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015 [DOI] [PubMed] [Google Scholar]
- 107.Salih DA. and Brunet A. (2008). FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McMahon AP, Ingham PW. and Tabin CJ. (2003). Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53:1–114 [DOI] [PubMed] [Google Scholar]
- 109.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, et al. (2004). Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95:911–921 [DOI] [PubMed] [Google Scholar]
- 110.Beachy PA, Karhadkar SS. and Berman DM. (2004). Tissue repair and stem cell renewal in carcinogenesis. Nature 432:324–331 [DOI] [PubMed] [Google Scholar]
- 111.Kim WK, Meliton V, Bourquard N, Hahn TJ. and Parhami F. (2010). Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J Cell Biochem 111:1199–1209 [DOI] [PubMed] [Google Scholar]
- 112.Goettsch C, Babelova A, Trummer O, Erben RG, Rauner M, Rammelt S, Weissmann N, Weinberger V, Benkhoff S, et al. (2013). NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Invest 123:4731–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Christodoulides C, Lagathu C, Sethi JK. and Vidal-Puig A. (2009). Adipogenesis and WNT signalling. Trends Endocrinol Metab 20:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laudes M. (2011). Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 46:R65–R72 [DOI] [PubMed] [Google Scholar]
- 115.Prestwich TC. and MacDougald OA. (2007). Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou S, Eid K. and Glowacki J. (2004). Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res 19:463–470 [DOI] [PubMed] [Google Scholar]
- 117.Akimoto T, Ushida T, Miyaki S, Akaogi H, Tsuchiya K, Yan Z, Williams RS. and Tateishi T. (2005). Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem Biophys Res Commun 329:381–385 [DOI] [PubMed] [Google Scholar]
- 118.Kirton JP, Crofts NJ, George SJ, Brennan K. and Canfield AE. (2007). Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res 101:581–589 [DOI] [PubMed] [Google Scholar]
- 119.Shang YC, Wang SH, Xiong F, Zhao CP, Peng FN, Feng SW, Li MS, Li Y. and Zhang C. (2007). Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol Sin 28:1761–1774 [DOI] [PubMed] [Google Scholar]
- 120.Liu J. and Farmer SR. (2004). Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem 279:45020–45027 [DOI] [PubMed] [Google Scholar]
- 121.Dowell P, Otto TC, Adi S. and Lane MD. (2003). Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J Biol Chem 278:45485–45491 [DOI] [PubMed] [Google Scholar]
- 122.Wang F. and Tong Q. (2009). SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1's repressive interaction with PPARgamma. Mol Biol Cell 20:801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Munekata K. and Sakamoto K. (2009). Forkhead transcription factor Foxo1 is essential for adipocyte differentiation. In Vitro Cell Dev Biol Anim 45:642–651 [DOI] [PubMed] [Google Scholar]
- 124.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC. and Accili D. (2003). The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell 4:119–129 [DOI] [PubMed] [Google Scholar]
- 125.Buhman KK, Wang LC, Tang Y, Swietlicki EA, Kennedy S, Xie Y, Liu ZY, Burkly LC, Levin MS, Rubin DC. and Davidson NO. (2004). Inhibition of Hedgehog signaling protects adult mice from diet-induced weight gain. J Nutr 134:2979–2984 [DOI] [PubMed] [Google Scholar]
- 126.Cousin W, Fontaine C, Dani C. and Peraldi P. (2007). Hedgehog and adipogenesis: fat and fiction. Biochimie 89:1447–1453 [DOI] [PubMed] [Google Scholar]
- 127.Suh JM, Gao X, McKay J, McKay R, Salo Z. and Graff JM. (2006). Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab 3:25–34 [DOI] [PubMed] [Google Scholar]
- 128.Sweet HO, Bronson RT, Donahue LR. and Davisson MT. (1996). Mesenchymal dysplasia: a recessive mutation on chromosome 13 of the mouse. J Hered 87:87–95 [DOI] [PubMed] [Google Scholar]
- 129.van der Horst G, Farih-Sips H, Lowik CW. and Karperien M. (2003). Hedgehog stimulates only osteoblastic differentiation of undifferentiated KS483 cells. Bone 33:899–910 [DOI] [PubMed] [Google Scholar]
- 130.Wu X, Walker J, Zhang J, Ding S. and Schultz PG. (2004). Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem Biol 11:1229–1238 [DOI] [PubMed] [Google Scholar]
- 131.Lefterova MI. and Lazar MA. (2009). New developments in adipogenesis. Trends Endocrinol Metab 20:107–114 [DOI] [PubMed] [Google Scholar]
- 132.Shao D. and Lazar MA. (1997). Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem 272:21473–21478 [DOI] [PubMed] [Google Scholar]
- 133.Lowe CE, O'Rahilly S. and Rochford JJ. (2011). Adipogenesis at a glance. J Cell Sci 124:2681–2686 [DOI] [PubMed] [Google Scholar]
- 134.Tontonoz P. and Spiegelman BM. (2008). Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312 [DOI] [PubMed] [Google Scholar]
- 135.Liu GS, Chan EC, Higuchi M, Dusting GJ. and Jiang F. (2012). Redox mechanisms in regulation of adipocyte differentiation: beyond a general stress response. Cells 1:976–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiang S, Wang W, Miner J. and Fromm M. (2012). Cross regulation of sirtuin 1, AMPK, and PPARgamma in conjugated linoleic acid treated adipocytes. PLoS One 7:e48874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW. and Guarente L. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jing E, Gesta S. and Kahn CR. (2007). SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab 6:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B. and Chandel NS. (2011). Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carriere A, Fernandez Y, Rigoulet M, Penicaud L. and Casteilla L. (2003). Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett 550:163–167 [DOI] [PubMed] [Google Scholar]
- 141.Mouche S, Mkaddem SB, Wang W, Katic M, Tseng YH, Carnesecchi S, Steger K, Foti M, Meier CA, et al. (2007). Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim Biophys Acta 1773:1015–1027 [DOI] [PubMed] [Google Scholar]
- 142.http://genkyotex.com/genkyotex/
- 143.Picklo M, Claycombe KJ. and Meydani M. (2012). Adipose dysfunction, interaction of reactive oxygen species, and inflammation. Adv Nutr 3:734–735 [DOI] [PMC free article] [PubMed] [Google Scholar]