Abstract
Microglia, the resident immune cells of the brain, have recently been hypothesized to play a role both in neuronal diseases and age related neurogenic decline, and are theorized to be modulators of adult neurogenesis. Current methods for the isolation of microglia from cultured primary brain tissue result in relatively poor yield, requiring a large tissue sample or multiple specimens in order to obtain a sufficient number of microglia for cell and molecular analysis. We report here a method for the repetitive isolation of microglia from established glial monolayer cultures from which it is possible to expand the initial population of microglia roughly 10,000 fold. The expanded population expresses appropriate microglial morphology and phenotype markers, and demonstrates functionally normal phagocytosis, thus providing a high-yield assay for the investigation and analysis of microglia from a single initial dissection of primary tissue. Furthermore, this massive expansion is limited to microglia derived from the subventricular zone as the fold expansion of isolatable microglia was found to be up to 20 times greater than cultures from other brain regions, indicating unique properties for this persistently neurogenic region.
Keywords: neurogenesis, astrocytes, glial culture
INTRODUCTION
Microglia are believed to be derived from hematopoietic progenitors that infiltrate the brain during development (Cuadros et al., 1998), and to play a critical immunological role in the CNS, responding to inflammatory cues by shifting from a ramified, resting state to an activated, phagocytic amoeboid cell type (Kreutzberg et al., 1996). Microglia play neuroprotective and neurodegenerative roles during CNS diseases and injuries (Kim et al., 2005). In addition to their role in phagocytosis, activated microglia are also classified as antigen presenting cells due to their up-regulation of MHC II. The capacity for activated microglia to adopt a ramified morphology has been proposed to be partially induced by exposure to granulocyte macrophage colony stimulating factor (GM-CSF), presumably secreted by T-cells or reactive astrocytes (Aloisi et al., 2000). Being hematopoietic in origin, microglia share many characteristics with blood derived macrophages, such as expression of the pan-hematopoietic marker CD45 (Kim et al., 2005) and the macrophage-associated marker Beta2-Integrin (CD11b) (Giulian and Baker, 1986).
Dissociated neural tissue cultured from the subventricular zone (SVZ) of neonatal mice form a monolayer of cells containing astrocytes which possess the two unique characteristics consistent with stem cells: multipotency (as evidenced by the generation of neurospheres capable of multi-lineage differentiation) and self-renewal (as evidenced by the capacity for serial expansion) (Laywell et al., 2000). It was recently shown that, in addition to the generation of neurospheres, anchored SVZ astrocyte cultures are also capable of a unique form of inducible neurogenesis (Scheffler et al., 2005). Interestingly, only at low population doublings are SVZ astrocytes capable of generating neurons, with a nearly linear correlation between the number of microglia and the level of neurogenesis (Walton et al., 2006): the fewer microglia present, the fewer neurons induced from these cultures.
The unique association between microglia and astrocytes has been an area of intense scrutiny. Giulian and Baker (1985) discovered that microglia secrete factors that promote astroglia proliferation in vitro, while Ohno et al. (1990) later showed that GM-CSF is produced by cultured astrocytes, hinting at a potential symbiotic relationship between these two cell types in vitro. Two populations of microglia, amoeboid and ramified, have been shown to exist in primary astrocyte cultures: ramified/resting microglia existed within the monolayer in direct contact with the astrocytes while amoeboid/activated microglia were observed on the surface of the culture (Tanaka et al., 1999). Together these studies hint at a unique interaction between astrocytes and microglia, and since the SVZ contains a unique population of highly proliferative astrocytes, we hypothesized that this region was ideal for massive propagation of microglia.
Isolation and culture of microglia has been performed using primary brain dissociates, but always with relatively low yields and labor-intensive isolation procedures (Giulian and Baker, 1986). Typically, primary forebrain dissociates are cultured on adhesive plastics until a monolayer is generated, consisting largely of astrocytic cells with an overlying microglial population. Microglia are collected by vigorously agitating these cultures for 12 hours or more, and are purified based on differential attachment properties. There are a number of shortcomings associated with this system, such as the time-to-yield ratio (e.g. typically hours of shaking to obtain only 1×105 microglia) and the need to repeatedly sacrifice animals to obtain primary brain tissue. Methods, such as the addition of GM-CSF (Giulian and Ingeman, 1988; Giulian et al., 1995; Lee et al., 1994; Tomozawa et al., 1996) or macrophage colony stimulating factor (M-CSF) (Giulian and Ingeman, 1988; Tomozawa et al., 1996; Ponomarev et al., 2005), have been successfully employed to boost microglia yield, but these studies all involved the use of primary brain dissociates, and thus require large animal numbers, and potentially suffer from phenotypic variability due to mixed sources of tissue. More recently, Floden and Combs (2007) reported a method to repeatedly isolate microglia from an established monolayer of primary neonatal cortex without the use of exogenous mitogens. While this approach was promising, the microglia yield dropped steeply and progressively after the initial isolation.
Here we report a method not only to consistently isolate microglia in large numbers from a single SVZ culture, but also to take advantage of the proliferative nature of the SVZ astrocyte to subsequently isolate massive numbers of microglia from numerous serially expanded cultures -all derived from a single initial dissociation. This method is superior to other reported methods for several reasons: 1) the procedure is rapid and repeatable on the same culture; 2) nearly all cells isolated are functional microglia; 3) the yield is immense, with combined microglial yields roughly 10,000 times the starting population present in the original dissociated tissue; 4) it requires little initial tissue, allowing for large-scale microglia expansion from single biopsy or postmortem specimens; and 5) it allows for idiosyncratic analysis of single specimens from individual donors that could facilitate studies of microglial genetic and molecular phenotype, as well as their function and behavior in normal and disease states.
METHODS
Animals
C57BL/6 neonatal (2-3 days post-birth) or adult (4 months) mice were used as the model system, and were housed at the University of Florida’s Department of Animal Care Services, in compliance with IACUC regulations.
Generation of adherent neurogenic astrocyte cultures
SVZ tissue was obtained from C57BL/6 neonatal (postnatal day 2-3) mice. 2-3 brains were removed and the olfactory bulbs and cerebellum removed with a sterile blade. The tissue was further dissected to remove the cortical regions, with the remaining tissue comprised of primarily the regions surrounding the lateral ventricles. The tissue block was then minced with a sterile scalpel, and placed in ice-cold DMEM/F12 w/ HEPES and L-Glutamine (Gibco BRL, Carlsbad, CA; 11330-032) containing anti-biotic and anti-mycotic agents (Penicillin-Streptomycin, Gibco, 15140-122, and Fungizone Antimycotic, Gibco, 15295-017) for 15 minutes. The now sterile tissue was then pelleted by centrifugation at 400×g for 5 minutes, followed by removal of media and incubation in 0.25% Trypsin/EDTA solution (Atlanta Biologicals, Atlanta, GA; B81310) for 5 minutes. Trypsin activity was quenched by the addition of 1/5 volume fetal bovine serum (FBS: Atlanta Biological), after which the tissue was triturated to a single cell slurry by repeated pipetting through a series of descending diameter fire polished Pasteur pipettes. The slurry was washed in 5X volume DMEM/F12 and pelleted by centrifugation at 400×g for 5 minutes. Cells were re-suspended in neural growth medium (NGM) consisting of DMEM/F12 containing 5% FBS, N2 supplement (Gibco BRL, 17502-048), recombinant human epidermal growth factor (EGF) at a concentration of 20ng/mL (rhEGF, Sigma-Aldrich, St. Louis, MO; E9644), and basic fibroblast growth factor (FGF) at a concentration of 10ng/mL (bFGF, Sigma-Aldrich, F0291), plated onto tissue culture T-25 flasks and incubated for three days at 37°C in 5% CO2. Confluent cultures were trypsinzed for 5 minutes, the trypsin neutralized by addition of 5% FBS, and the cells collected and plated at a 1:3 density into T-75 tissue culture flasks in NGM (passage 1).
Passage 1 cultures were supplemented every 2-3 days with EGF/FGF until a confluent monolayer was established. Confluent monolayers were passaged at a 1:3 density as described above with a small proportion placed onto poly-ornithine coated glass coverslips for subsequent immunohistochemical analysis (see below). Passage 2 and beyond neurogenic astrocyte monolayers were induced to propagate microglia (see below). Induction was typically performed on two of the three T-75 cultures, with the third passaged at a 1:3 density to generate additional culture groups.
For the purposes of comparative analysis, brains from either neonatal (2-3 days post-birth) or adult (4 months old) C57BL/6 mice were dissected in such a fashion as to produce cultures derived from SVZ, cerebral cortex and cerebellum (see figure 6A for a schematic representation of the dissection). Neonatal cultures from all three regions typically became confluent within a few days at which time they were passaged and treated as described above. Adult cultures were supplemented with EGF/FGF every two days for two weeks until the SVZ-derived cultures had established a confluent monolayer, at which time the propagation of microglia was induced (see below). In contrast, the cerebellar and cerebral cortex derived cultures failed to generate confluent cultures.
Fig. 6.
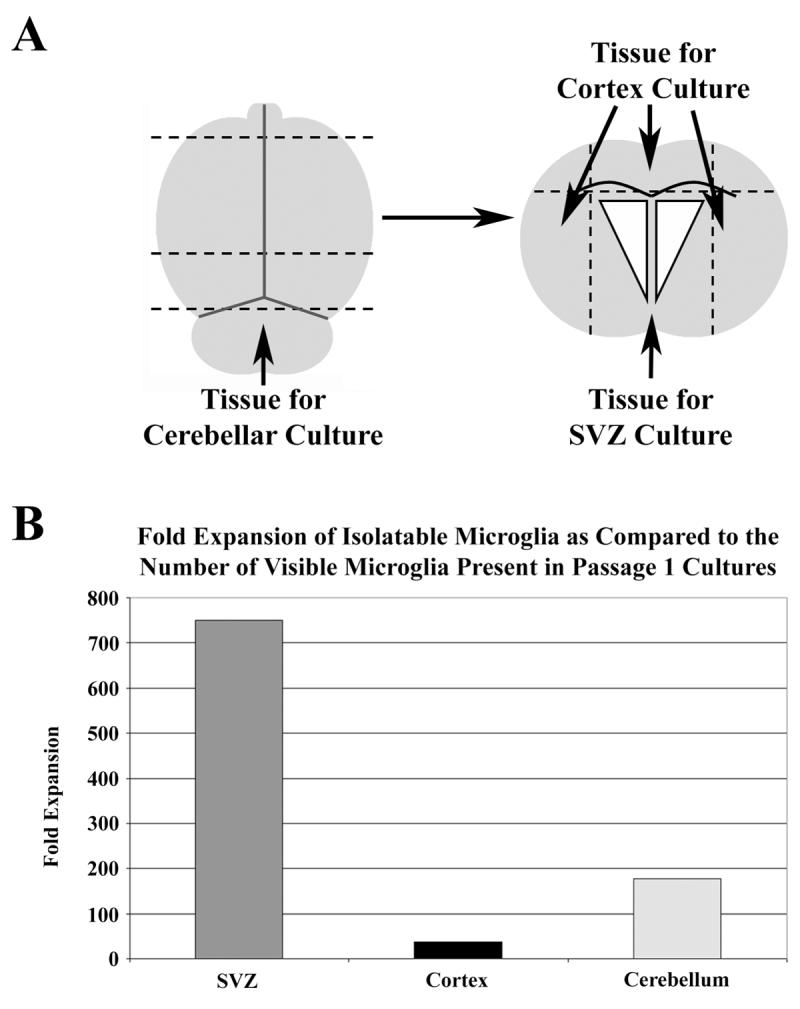
Comparative analysis of microglial expansion from different regions of the neonatal brain. Neonatal brains were harvested from postnatal day 3 mice, and their brains dissected as described in panel A (superior view), with dashed lines indicating incisions. The cerebellum was removed and cultured, while the forebrain region was further dissected so as to separate the cortex from the SVZ (coronal view). Panel B) Microglial yields from passage 1, 2, and 3 cultures (4 agitation isolations per passage) were combined and are presented as fold expansion as compared to the number of microglia present at passage 1 prior to induction of microglial proliferation.
Expansion of microglia from adherent neurogenic astrocyte cultures
Passage 1 and beyond neurogenic astrocyte monolayer cultures were allowed to grow to 100% confluency, after which the neural growth media was replaced with microglial proliferation media (MPM) consisting of DMEM/F12, 10% FBS, N2 supplement, and recombinant mouse granulocyte macrophage-colony stimulating factor (GM-CSF) at a concentration of 20ng/mL (rGM-CSF, Stem Cell Technologies Inc; #02735). MPM was replaced every three days until phase-bright microglia became visible in the adherent culture and floater cells became visible in the media. Propagated cultures were agitated at room temperature at 100rpm for 10 minutes, after which the media and the detached cells were collected for analysis. Fresh microglial expansion media was placed on the adherent cell populations which were again allowed to propagate in the humidified incubator until the next isolation, which typically occurred within 5 days, at which time the procedure was repeated.
Isolated cells were quantitated via phase bright microscopy with a hemacytometer and plated onto either tissue culture T-25 flasks or onto poly-ornithine coated glass coverslips for subsequent immunohistochemical analysis.
Immunohistochemical Analysis
The media was removed and the cells were fixed by incubation in 4% paraformaldehyde in PBS at room temperature for 15minutes then washed with PBS for 5 minutes. Cells were prepared for immunohistochemistry by first blocking at room temperature for 1 hour in PBS plus 0.01% Triton X-100 (PBSt) containing 10% fetal bovine serum. Primary antibodies were then applied to the sections overnight in PBSt plus 10%FBS with moderate agitation at 4°C. The antibodies used were either CD45 (rat monoclonal anti-CD45: Abcam, Cambridge, MA; ab3088-100), CD11b/Mac-1 (rat anti mouse CD11b: Becton Dickinson, Franklin Lakes, NJ: 550282), β-III tubulin (rabbit polyclonal to β-III Tubulin, Covance; Princeton, NJ; PRB-435P, dilution 1:5000) or glial fibrillary acidic protein (GFAP, rabbit polyclonal: Shandon Immunon, Milford MA; 490740, dilution 1:100). Residual primary antibody was removed by three 5 minute washes with PBS and secondary antibodies were applied at room temperature for 50 minutes in PBSt plus 10% FBS. The secondary antibodies used were either goat anti-rat IgG conjugated to Alexa Fluor 568 fluorochrome (Molecular Probes, Eugene, OR: A11077) or goat anti-rabbit IgG conjugated to Oregon Green 488 fluorochrome (Molecular Probes, O6381). Residual secondary antibodies were removed by three 5 minute washes in PBS. The cover-slips were placed onto glass slides and layered with Vectashield mounting medium plus DAPI (Burlingame, CA: H-1200) prior to cover-slipping.
Microscopy and Quantitative Analysis
Coverslips were analyzed and photographed by either fluorescence light microscopy using a Leica DMLB (Leica Microsystems AG, Wetzlar, Germany) or fluorescent confocal microscopy using a Leica TCS SP2 AOBS Spectral confocal microscope. Quantitation of stained cells was performed under fluorescence light microscopy by randomly sampling 4 fields of view at 20x magnification using the DAPI filter (excitation 360/40, suppression 470/40), allowing for the investigator to obtain a suitable field of view without knowing the immunohistochemical identity of the cells being quantitated. All DAPI nuclei were counted after which the filter was switched to either FITC (excitation 480/40, suppression 527/30) or rhodamine (excitation 515-60, suppression 590) and the stained cells quantitated allowing for the determination of percent contribution to the total cell number within that particular view. The values from all 4 fields were combined and averaged to generate an approximate estimation of the cellular distribution within that particular culture.
Phagocytic Assay
Adherent microglia derived from C57BL/6 neonatal SZV tissue were cultured in the presence of alexa-fluor-488 conjugated E. Coli particles (Invitrogen, catalog #E13231) at a concentration of 10-20 particles/cell for 4 hours. Microglia were then washed 3 times with sterile PBS before being fixed with 4% paraformaldehyde for 20 minutes at room temperature. Fixed cells were then washed once with PBS before initiation of the immunohistochemical protocol.
Cytogenetic Analysis
Established adherent neurogenic astrocyte cultures which had been induced to propagate microglia for various lengths of time were sent to the University of Florida Cytogenetics Laboratory where microglia were isolated from the cultures as described above. Conventional metaphase cell chromosomal studies (G-banding) were performed on 10 cells from each sample.
RESULTS
GM-CSF induces proliferation of microglia in SVZ cultures
We have previously shown that the SVZ of neonatal mice can be cultured as an adherent monolayer of neural cell types (Laywell et al., 2000). Early passage neonatal SVZ tissue cultured in the presence of EGF/FGF and 5% FBS (neural growth media, NGM; see methods) generates a heterogeneous layer containing astrocytes, microglia, and neurons (Fig. 1A-D), with a population of cells possessing an amoeboid morphology existing on the surface of the culture. The majority of these cells are immunopositive for the CD45 surface antigen consistent with the microglial phenotype (Supplementary Fig. 1). The few neurons observed in culture possess long processes, and are always in close proximity to microglia (Fig. 1D). Quantification reveals that nearly 40% of the total cells are microglia, 22% are astrocytes, 1.5% are neurons, and 36.5% are immunonegative for these markers (Fig. 1E). SVZ cultures adopt a markedly different morphology when exposed to microglial proliferation medium (MPM; see methods) containing 10% serum and 20ng/mL GM-CSF (Giulian and Ingeman, 1988). After 1 week in MPM, the previously observed population of microglia expands on the surface of the astrocytes (Fig. 1F), with a sub-population floating in the medium. Immunolabeling reveals that the predominant cell type in the culture are CD45+ microglia (Fig. 1G and H), while the proportion of GFAP+ astrocytes is reduced (Fig. 1, compare panels B and G). Neuronal processes are visibly shorter than those seen in control cultures (compare panels D and I). Quantification of the cell distribution reveals that now 54% are microglia (a 26% increase relative to controls), 15% are astrocytes, 1.0% are neurons, and 30% are negative for the antibodies used (Fig. 1J). Classifying the CD45+ microglia in these cultures as either amoeboid or ramified reveals that exposure to MPM results in a 25% increase in the number of amoeboid microglia, mirroring the relative percent increase (26%) in the total number of microglia.
Fig. 1.
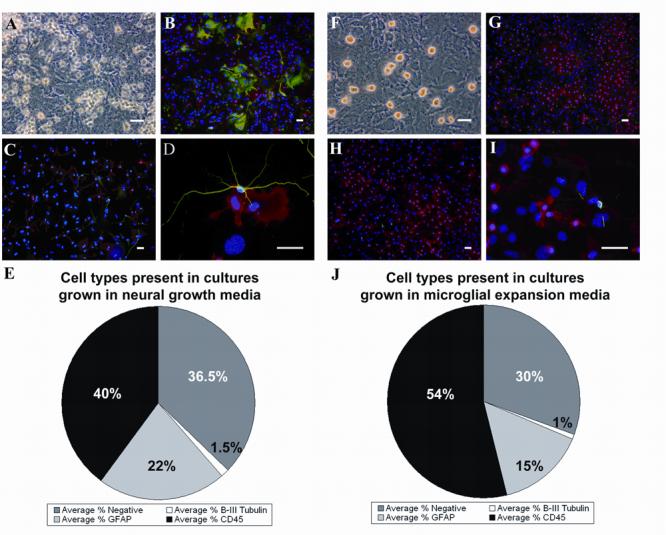
Exposure to MPM increases the number of microglia in passaged SVZ cultures. Primary cultures derived from the SVZ of neonatal mice (A-D) contain GFAP+ astrocytes (B, green), β-III tubulin+ neurons with long processes (C and C, green) and a significant population of CD45+ microglia (B-D, red). Quantitation of these three cell types is shown in panel E. Phase bright microglia are produced on the surface of the culture following conversion to MPM (F). GFAP+ astrocytes are less abundant (G, green), as are β-III tubulin+ neurons (H and I, green). CD45+ microglia visibly increase in number (G and H, red), which is depicted quantitatively in panel J. Blue in panels B-D and G-I = DAPI. Scale bars in all panels = 50μm.
Rapid, robust and repeatable isolation of expanded microglia
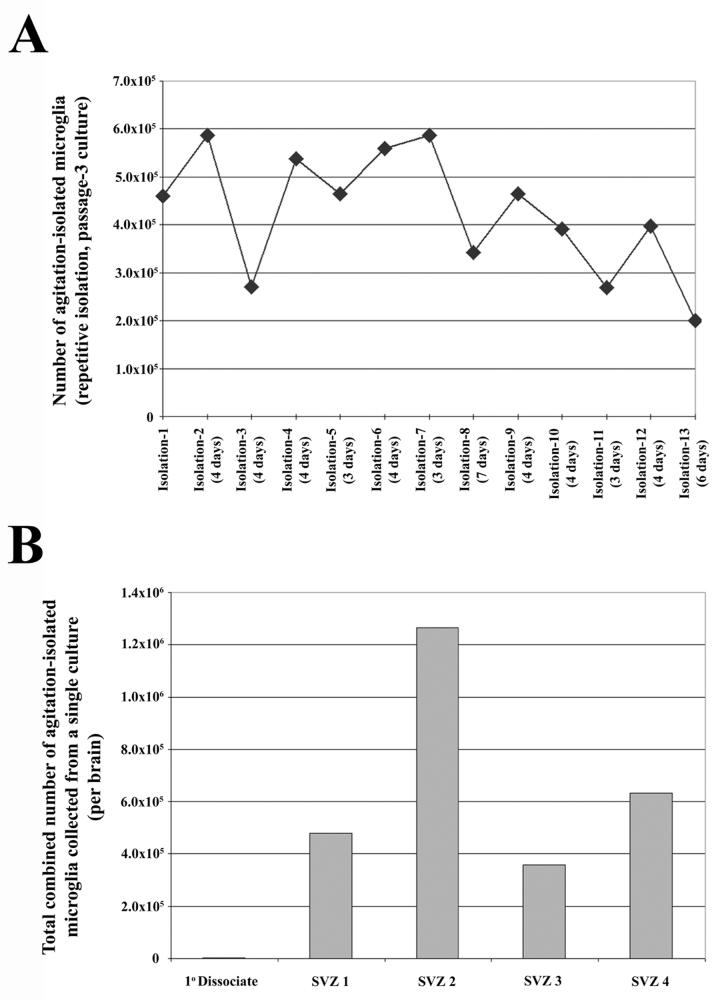
It is possible to isolate large numbers of expanded microglia by agitating the culture for 10 minutes at 100rpm at room temperature. Quantitative yield of microglia isolated varies from 7×104 to 3×106 from an adherent SVZ culture containing approximately 1×107 total cells. The microglial identity of the collected cells is confirmed by both morphology (Fig. 2A) and immunolabeling for CD45 (Fig. 2B & C) and CD11b (see supplementary Fig. 2). The isolations results in a 99% pure population of microglia, with only occasional astrocytes (Fig. 2 C & D) and no neurons detected. The expansion is robust, with microglia capable of being isolated every 4 days with an average yield of 3×105 cells. Furthermore, the isolation procedure is repeatable up to 13 isolations (the highest number tested) with similar yields occurring at each isolation (Fig. 3A), resulting in a combined average of roughly 1×106 microglia per culture. The phenotype of isolated microglia does not appear to change with each isolation, regardless of astrocyte passage number; the isolated population remains morphologically heterogeneous, yet maintains CD11b/CD45 immunopositivity, and is capable of proliferation and isolation in SVZ cultures from passage 1-6. The robustness of the expansion becomes more apparent when the initial number of microglia from the SVZ of a single dissociated neonatal brain is taken into account. We have found that roughly 400 adherent microglia are observed in these dissociates (data not shown), while on average, 6.84×105 microglia are isolated from a single culture derived from a single neonatal SVZ (Fig 3B), representing an average fold increase of 1700 from the initial population.
Fig. 2.
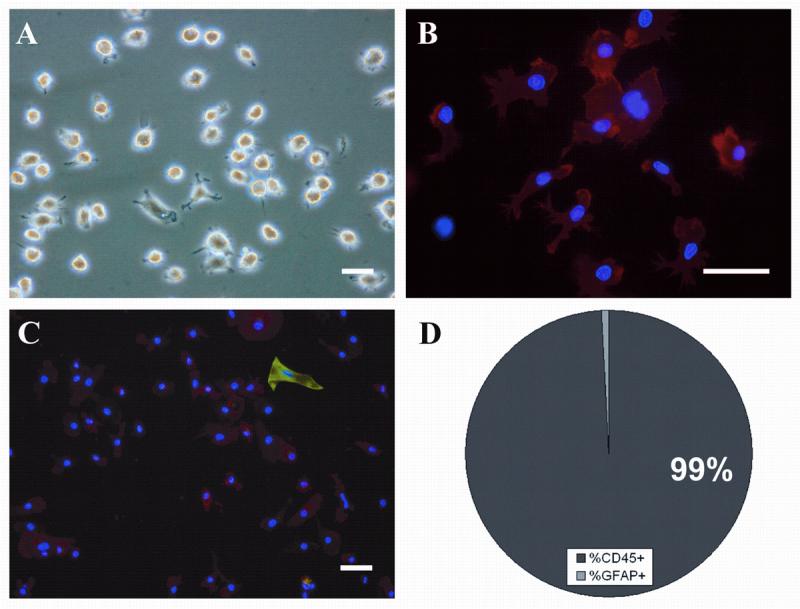
Microglia isolated from SVZ cultures grown in MPM are morphologically heterogeneous and devoid of contaminating cell types. 4 hours after attachment to treated coverslips, the majority of microglia possess amoeboid morphology (A). Following 24 hours of attachment and subsequent immunostaining for CD45, nearly all attached cells are positive, with both ramified and amoeboid cell types present (B, CD45/red, DAPI/blue). Only the occasional astrocytes are observed in collected cell populations (C, GFAP/green, CD45/red, DAPI/blue; and D). Scale bars in all panels = 50μm.
Fig. 3.
Large scale, repeatable isolation of microglia from SVZ cultures grown in MPM. (A) Time-line depicting the number of microglia isolated at each isolation from a single representative culture (passage-3). In this particular example, 13 isolations were performed over the course of 50 days (days between isolations listed with isolation number). (B) Graphical representation of the cumulative microglial yields from 4 single established cultures derived from the SVZ dissected from 4 independent brains compared to the initial number of microglia in a single primary dissociate. Each cumulative value is presented as the number of microglia per neonatal SVZ dissociate. The average cumulative number of microglia isolated per brain was 684,000 which, when compared to the initial microglial population in primary cultures (400 cells), represents a 1700 fold increase.
Isolated microglia are karyotypically and functionally normal
No clonal cytogenetic changes are detected among 10 metaphase cells examined from culture at three different passages (Fig 4A): Passage 3 SVZ Monolayer/Isolation 13 microglia (roughly 7 weeks in MPM); Passage 5 SVZ Monolayer/Isolation 6 microglia (roughly 3 weeks in MPM); Passage 6 SVZ Monolayer/Isolation 1 microglia (roughly 1 week in MPM). To test the functionality of the isolated microglia, adherent cells were cultured in the presence of alexa-fluor-488 conjugated E. Coli particles for 4 hours, and were found to be capable of phagocytosis (Fig. 4B and C). These findings demonstrate that expanded microglia are non-transformed and functional, independent of passage number or time of isolation.
Fig. 4.
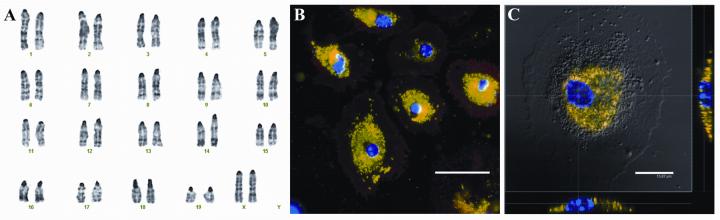
Microglia isolated from SVZ cultures grown in MPM are karyotypically normal and functionally phagocytic. (A) Representative metaphase chromosomal spread (G-banding) of microglia isolated from passage 3 SVZ cultures. (B) Isolated CD45+ microglia (red) are capable of phagocytosing fluorescently labeled E. Coli particles (green). (C) The presence of the particles within the microglia was confirmed by confocal microscopy and merged. This image represents the maximum projection of a 3-dimensional z-stack overlayed on top of the DIC micrograph of the same cell. Scale bars: B= 50μm; C= 15μm.
Massive expansion of microglia
SVZ astrocytes are highly expandable in vitro, making it possible to generate large numbers of cultures from a single dissection. This allows for millions of microglia to be isolated from a single dissection (see schematic in Fig. 5). We typically use at least two neonatal brains to initiate our cultures, and have found that primary SVZ dissociates from two neonatal brains yield an average of 800 adherent microglia. The initial passage of the resulting monolayer is performed at a 1:3 density, as are all subsequent passages. This allows for two flasks per passage to be induced to expand their microglial population while the third can be further passaged. Roughly 1×106 total microglia can be isolated from a single culture (typically from 4-5 isolations), resulting in 2×106 per passage and nearly 8×106 total when passages 2-5 are combined (the yield of microglia begins to diminish after passage 5). This expansion becomes more profound when this culture system is compared to those generated from cortical or cerebellar tissue. The brains of neonatal mice were dissected in such a fashion as to separate the SVZ, the cerebral cortex and the cerebellum (Fig. 6A). All three groups were cultured at equal densities and passaged upon reaching confluence. Induction of microglia was performed as described above on passage 2, 3, and 4 cultures. The SVZ and cerebellum cultures display a similar capacity for expansion, with cultures becoming confluent at near the same rate with a parallel capacity to survive serial passages. This was not found to be the case with the cortex cultures, which took nearly twice as long to generate confluent monolayers and were incapable of expansion past passage 3 (data not shown). With respect to microglial yield, the SVZ was found to be vastly superior, with fold expansion rates 20 fold and 4 times greater than the cortex and cerebellum cultures, respectively (Fig. 6B). This observation becomes more intriguing when it is analyzed in light of the fact that, in our hands, the naïve cortex cultures at passage 1 were found to contain nearly three times as many microglia as the SVZ before the induction of microglial proliferation, while the cerebellum contained nearly four times as many microglia (data not shown).
Fig. 5.
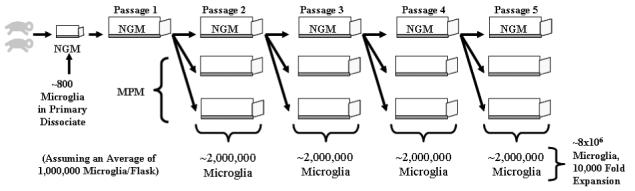
Summary of the protocol for massive expansion of microglia from dissociated neonatal SVZ tissue. The SVZ is dissected from the brains of 2-3 neonatal mice and cultured in NGM in a TC-25 flask until a confluent culture is established. Passage 1 is a 1:3 split into a TC-75 flask. By splitting all subsequent cultures at 1:3, two microglia production cultures (grown in MPM) are generated at each passage with one additional culture to be used for sequential expansion. The combined microglial yield from four separate passages yields an average of 8×106 cells, a 10,000-fold expansion from the initial microglial population.
Microglial expansion in adult cultures
In an effort to determine if the phenomenon was limited to the neonatal model, we repeated the previous experiment using the brains of 4 month old C57BL/6 mice as the tissue source. As before, the brains were dissected to produce separate cultures of SVZ, cortex and cerebellum. After two weeks in culture (NGM medium, growth factor supplemented every two days), the only region to produce near confluent cultures was the SVZ. As would be expected, after one week in MPM the SVZ cultures produced observable numbers of microglia on the surface of the culture which, when isolated, were found to be immunopositive for both CD45 and CD11b (Fig. 7A and B) and possess a morphology consistent with the microglia isolated from the neonatal cultures, yet produced a significantly lower number of isolatable microglia (roughly 10 times fewer) than the neonatal culture system. Of particular significance was the observation that neither the cerebellum nor the cerebral cortex derived cultures were found to form confluent cultures, nor were they capable of producing isolatable microglia in this model system.
Fig 7.
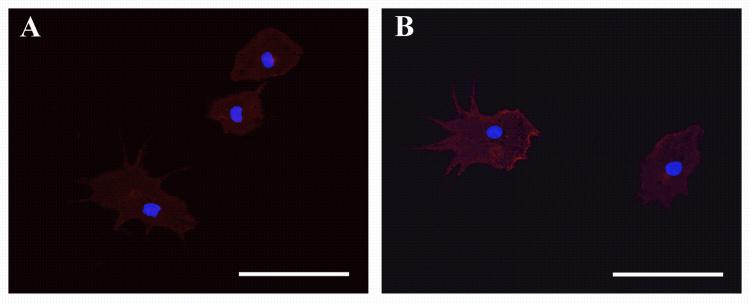
Adult SVZ cultures are capable of producing isolatable microglia in the presence of MPM. 4 hours after attachment to treated coverslips and subsequent immunostaining all attached cell types were found to be positive for CD11b (A) and CD45 (B), with both ramified and amoeboid cell types present. Blue in both panels is DAPI. Scale bars in both panels = 50μm.
DISCUSSION
A new method is described for the rapid, robust and, repeatable isolation of neurogenesis-associated microglia from dissociated neonatal mouse SVZ tissue. Since these cells are increasingly recognized as important players in potential translational neuroscience, large scale production of microglia may be important in transplantation therapies aimed at ameliorating age-related neurogenic (Walton et al., 2006) and cognitive decline as well as treating degenerative neurological diseases such as Alzheimer’s disease. Neonatal brain tissue is known to be more mitotically active than tissue obtained from adult brains, and also to posses a larger population of stem cells than more mature brains. The SVZ is one of the persistently neurogenic regions of the neonatal brain, and contains a population of NSCs which can be cultured in a variety of ways. One of these methods is to establish a monolayer of astrocytes within which the NSC is theorized to reside, allowing for the production not only of multipotent neurospheres but also the direct de novo generation of neurons. These “neurogenic astrocyte” cultures are highly proliferative, and allow for the simultaneous production of sequentially passaged cultures. We have found that the simultaneous addition of 20ng/mL of GM-CSF and 10% serum will induce the proliferation of microglia in SVZ cultures of varying passages, and that these newly expanded microglia can be isolated from the established monolayer through a short 10 minute agitation step. These microglia express appropriate surface antigens, are morphologically heterogeneous -with both amoeboid and ramified types present- and are functional as evidenced by their ability to phagocytose fluorescent bacterial particles. The expansion is extremely robust, with highly pure microglia being repeatedly isolated every 4 days in yields often exceeding 300,000 cells. It should be noted that the number of isolated microglia varied between dissection, sometimes as many as 3 fold, a phenomenon we attribute to the independent dissections as this variability was found to occur between cultures derived from the brains of age-matched neonatal mice. The phenotype of isolated microglia does not change with each isolation, regardless of passage number, and there is no alteration of the karyotype, regardless of the length of exposure to GM-CSF. This method allows for potentially tens of millions of microglia to be isolated from these cultures, all from a single dissection. These novel results possibly point to an as of yet an unidentified microglial progenitor cell type of the SVZ, and/or indicates that a special kind of microglial population exists in the SVZ as compared to the cerebellum and cerebral cortex. This is further supported by the observation that in the adult culture systems, only the SVZ was capable of expanding and forming a confluent culture, an ability attributed to the SVZ being a region of persistent neurogenesis, and thus maintaining some attributes of the developing brain, such as developmentally regulated extracellular matrix proteins and the capacity for extensive proliferation. We are unable to determine from these results whether the lack of microglial expansion in the cerebellar and cortical cultures is an intrinsic limitation of those regionally-specified microglia, or if the limited growth of astrocytes in these regions fail to support microglial expansion. Further studies are necessary to elucidate the complicated relationship between the “soil” (SVZ astrocytes) and the “seed” (microglia) in these regions.
Astrocytes and microglia are known to have unique interactions. Cortical microglia have been found to promote astroglial growth (Giulian and Baker, 1985), while astrocytes were later found to secrete GM-CSF (Ohno et al., 1990). In the SVZ, microglia have been found to play an indispensable role in the ability of neurogenic astrocytes to differentiate into neurons (Walton et al., 2006). Goings et al. (2006) discuss the phenomenon of persistently activated microglia in the SVZ (the reasons for which are unclear, but might include the presence of unique mitogens, higher cell death in the neuropoetic SVZ/RMS compared to surrounding structures, and the presence of a unique indigenous population of microglia), which are capable of migrating to adjacent areas of inflammation. It could be postulated that within the SVZ microglia and astrocytes mutually promote proliferation, and that this is translated in vitro through the steady production of microglia from an established monolayer of neurogenic astrocytes. In a study by Kuwabara et al. (2003), it is reported that there are two distinct microglial cell populations in 5 days in vitro (DIV) cultures and 13 DIV cultures, and that the 5 DIV microglia are proliferative and immature; is this the population that persists in passaged astrocytes? Finally, though unlikely, it has been theorized that astrocytes and microglia share a common progenitor (Fedoroff et al., 1997); could this be evident in the SVZ monolayers in which microglia are being continuously and persistently produced?
Most antecedent approaches used either whole forebrain or cerebral cortex to generate primary microglia, and usually involved variations of the protocol reported by Giulian and Baker (1986). In our hands, the SVZ is roughly 20 times more robust than the cerebral cortex with regard to the expansion and isolation of microglia. It stands to reason that other protocols may simply be relying on the advantage of the SVZ’s neurogenic microglial population when expanding and isolating microglia. Alternatively, the use of neonatal tissue is somewhat unique in terms of microglial expansion approaches, since most studies are conducted using adult tissue. Santambrogio, et al. (2001) reported that neonatal co-cultures plus GM-CSF yielded a ten-fold increase in microglia with a dendritic cell morphology, and concluded that neonatal microglia are developmentally plastic. The observed phenomenon of continuous production of microglia from SVZ cultures could be construed as controversial, given reports that microglia undergo telomeric shortening and senescence after 32 days in vitro (Flanary and Streit, 2004), but those studies were conducted with purified microglia, rather than the mixed glial culture reported here.
Aging has been shown to cause profound, deleterious morphological and functional changes in microglia (Conde and Streit, 2006), and it is well known that neurogenesis and memory decline with increasing age. Furthermore, microglia in aging brains have been implicated in contributing to the progression of neurological disorders such as Alzheimer’s disease (Deng et al., 2006). The dentate gyrus of the hippocampus is a region of persistent neurogenesis and plays a critical role in the formation of long term memories. Batista, et al. (2006) showed that the increased number of activated microglia in this region was directly related to the increase in neurogenesis in the dentate gyrus. With respect to Alzheimer’s Disease, microglia senescence has been implicated as one of the factors in the accumulation of amyloid-β plaques (Streit, 2002). Microglia are required for the removal of dead or dying neurons to allow for repair or remodeling of the injured area, but there is a coincidental persistence of disease state with the occurrence of age-related senescence of the microglia. Recent studies have indicated that not only are exogenous microglia capable of reducing the amount of amyloid-β particles in rats following intra-ventricular transplantation (Takata et al., 2007), but microglia derived from early postnatal brains are superior at phagocytosing amyloid-β particles when compared to microglia derived from adult brains (Floden and Combs, 2006). Finally, microglia have been proposed as therapeutic vehicles for delivery of retroviral therapies in the treatment of a variety of neurological diseases (Schloendorn et al., 2007), due to their innate ability to migrate and home to areas of neuro-inflammation. In addition to the aforementioned heterochronic benefits provided by the potential presence of neonatal microglia in aged brains, the method described here provides researchers with an exceedingly large number of microglia for subsequent modification with releasable proneurogenic factors prior to transplantation, leading to potentially beneficial outcomes in the treatment of a variety of neurological disorders.
Supplementary Material
ACKNOWLEDGEMENTS
D.A.S. is supported by NIH/NINDS R01 grants NS055165, NS37556, NS055165, NHLBI grant HL70143, and the McKnight Brain Research Foundation; E.D.L. is supported by NS056019 D.A.S. and E.D.L. own stock in RegenMed Inc, which may or may not receive royalties from the University of Florida as a result of the work presented herein.
REFERENCES
- Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional Maturation of Adult Mouse Resting Microglia into an APC Is Promoted by Granulocyte Macrophage Colony-Stimulating Factor and Interaction with Th1 Cells. J. Immunol. 2000;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. Microglia in the Aging Brain. J. Neuropathol. Exp. Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Navascués J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:73–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Deng XH, Bertini G, Xu YZ, Yan Z, Bentivoglio M. Cytokine-induced activation of glial cells in the mouse brain is enhanced at an advanced age. Neuroscience. 2006;141:645–661. doi: 10.1016/j.neuroscience.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Fedoroff S, Zhai R, Novak JP. Microglia and astroglia have a common progenitor cell. J Neurosci Res. 1997;50:477–486. doi: 10.1002/(SICI)1097-4547(19971101)50:3<477::AID-JNR14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45:75–88. doi: 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. J Neurosci. 2006;26:4644–4648. doi: 10.1523/JNEUROSCI.4822-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. J Neurosci Methods. 2007;164:218–224. doi: 10.1016/j.jneumeth.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Peptides released by amoeboid microglia regulate astroglial proliferation. J Cell Biol. 1985;101:2411–2415. doi: 10.1083/jcb.101.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of amoeboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Ingeman JE. Colony-stimulating factors as promoters of amoeboid microglia. J Neurosci. 1988;8:4707–4717. doi: 10.1523/JNEUROSCI.08-12-04707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995;15:7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goings GE, Kozlowski DA, Szele FG. Differential activation of microglia in neurogenic versus non-neurogenic regions of the forebrain. Glia. 2006;54:329–342. doi: 10.1002/glia.20381. [DOI] [PubMed] [Google Scholar]
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuwabara Y, Yokoyama A, Yang L, Toku K, Mori K, Takeda I, Shigekawa T, Zhang B, Maeda N, Sakanaka M, Tanaka J. Two Populations of Microglial Cells Isolated From Rat Primary Mixed Glial Cultures. J Neurosci Res. 2003;73:22–30. doi: 10.1002/jnr.10637. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland E, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Liu W, Brosnan CF, Dickson DW. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12:309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- Ohno K, Suzumura A, Sawada M, Marunouchi T. Production of granulocyte/macrophage colony-stimulating factor by cultured astrocytes. Biochem Biophys Res Commun. 1990;169:719–724. doi: 10.1016/0006-291x(90)90390-9. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Maresz K, Shriver LP, Dittel BN. Development of a culture system that supports adult microglial cell proliferation and maintenance in the resting state. J Immunol Methods. 2005;300:32–46. doi: 10.1016/j.jim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci USA. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloendorn J, Sethe S, Stolzing A. Cellular therapy using microglial cells. Rejuvenation Res. 2007;10:87–99. doi: 10.1089/rej.2006.0511. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as Neuroprotective, Immunocompetent Cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Takata K, Kitamura Y, Yanagisawa D, Morikawa S, Morita M, Inubushi T, Tsuchiya D, Chishiro S, Saeki M, Taniguchi T, Shimohama S, Tooyama I. Microglial transplantation increases amyloid-beta clearance in Alzheimer model rats. FEBS Lett. 2007;581:475–478. doi: 10.1016/j.febslet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Toku K, Sakanaka M, Maeda N. Morphological differentiation of microglial cells in culture: involvement of insoluble factors derived from astrocytes. Neurosci Res. 1999;34:207–215. doi: 10.1016/s0168-0102(99)00041-3. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y, Inoue T, Takahashi M, Adachi M, Satoh M. Apoptosis of cultured microglia by the deprivation of macrophage colony-stimulating factor. Neurosci Res. 1996;25:7–15. doi: 10.1016/0168-0102(96)01021-8. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.