Abstract
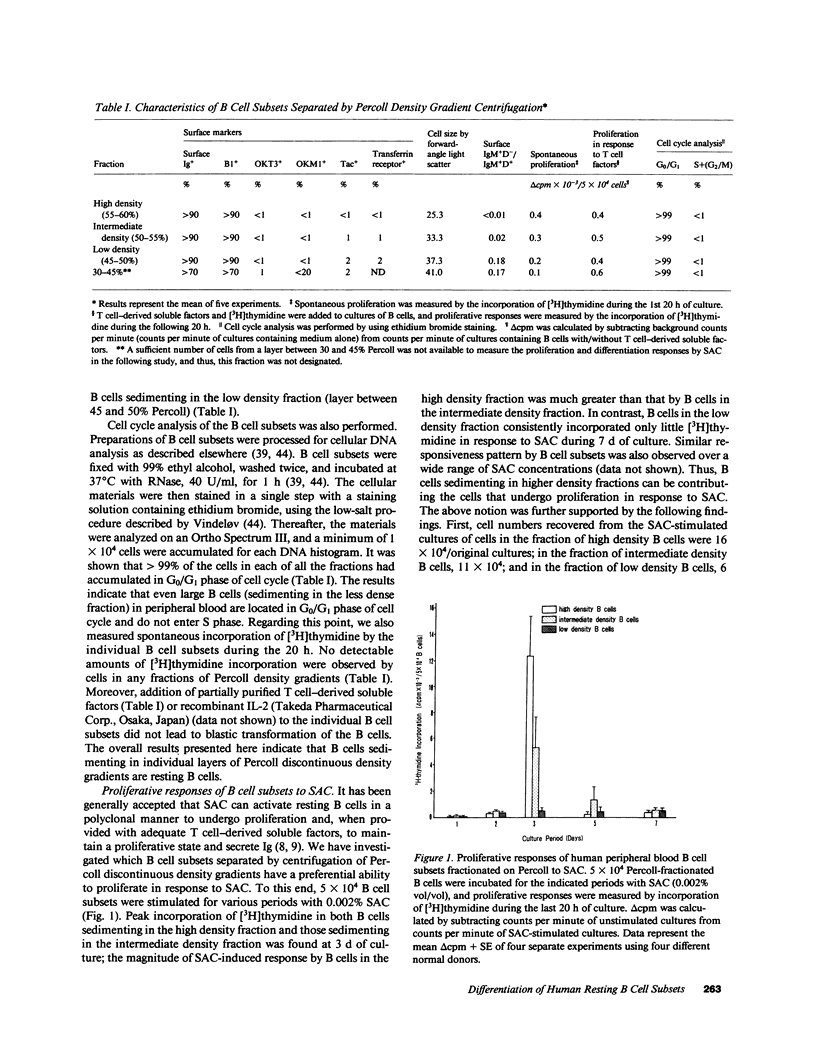
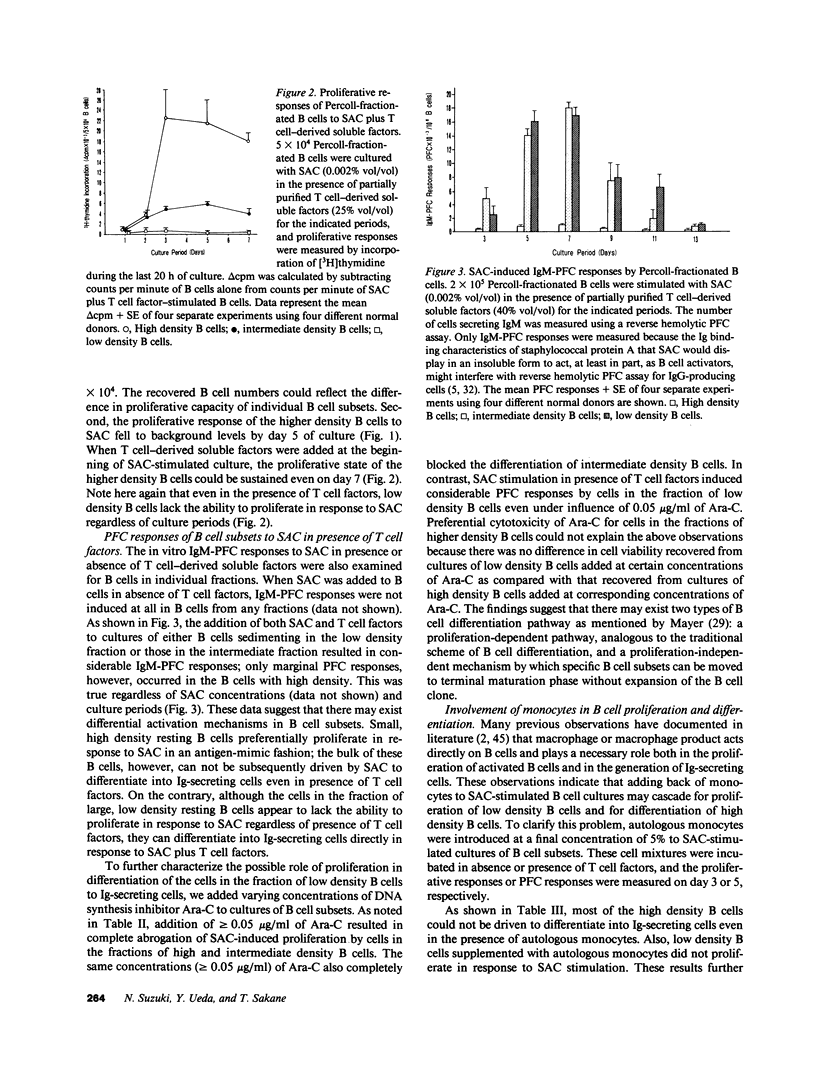
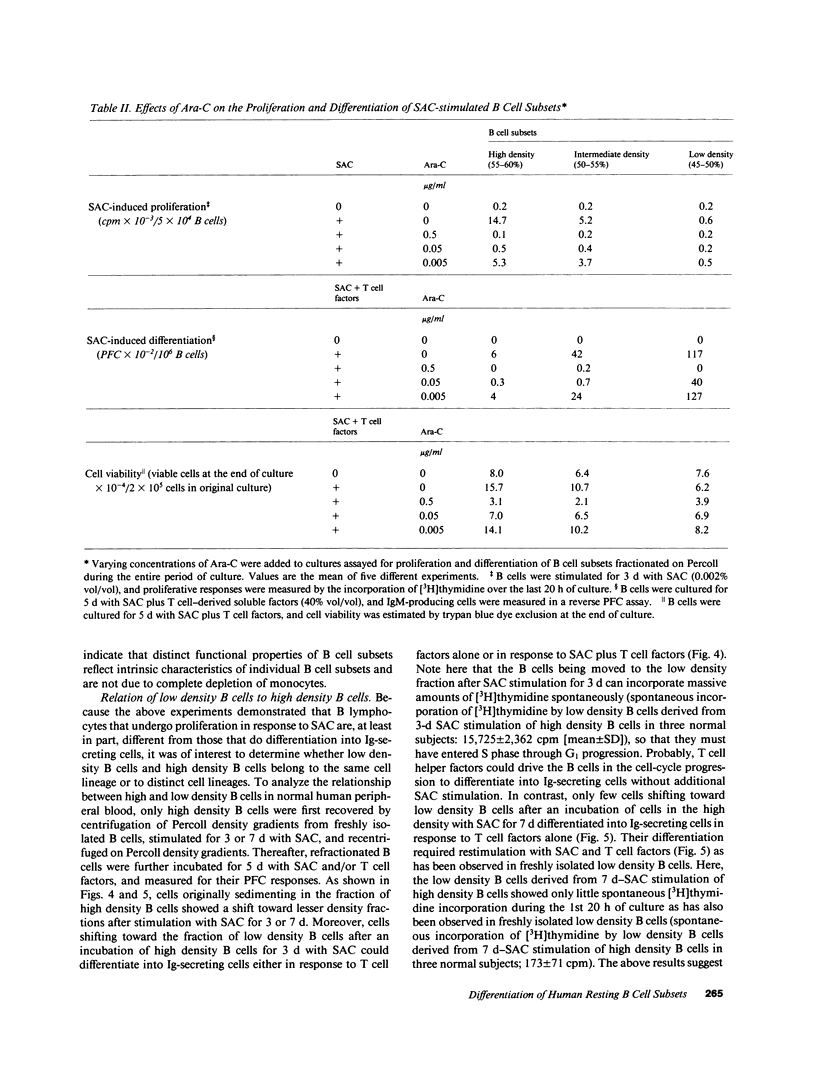
We have investigated differential mechanism for differentiation of human peripheral blood resting B cells to Ig-secreting cells. Purified resting B cells were further fractionated into subsets by discontinuous density gradients of Percoll, and proliferation and differentiation responses to Staphylococcus aureus Cowan I (SAC) and/or T cell-derived soluble factors were studied. High density resting B cells were stimulated to proliferate vigorously in response to SAC, but were poorly differentiated by SAC in presence of T cell factors. In contrast, low density resting B cells failed to proliferate in response to SAC and/or T cell factors; these cells could, however, be induced by stimulation with SAC plus T cell factors to become cells actively secreting Ig. These results indicate that there may exist heterogeneity in the human resting B cells: one subset of resting B cells (B cells with low density) can differentiate directly into Ig-secreting cells without the need for proliferation, and another subset (B cells with high density) can proliferate actively without subsequent differentiation into Ig-secreting cells. To address whether these resting B cell subsets belong to the same lineage, only high density B cells recovered from circulating resting B cells were first stimulated for 7 d with SAC, refractionated on Percoll gradients, and differentiation response of the refractionated B cells to SAC and T cell factors was examined. B cells shifting toward low density fraction were located in the resting status and could differentiate in response to SAC plus T cell factors. These results indicate that some of B cells with high density belong to the same cell lineage as those with low density and they must first proliferate before differentiation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Melchers F. T cell-dependent activation of resting B cells: requirement for both nonspecific unrestricted and antigen-specific Ia-restricted soluble factors. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2497–2501. doi: 10.1073/pnas.78.4.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgois A., Kitajima K., Hunter I. R., Askonas B. A. Surface immunoglobulins of lipopolysaccharide-stimulated spleen cells. The behavior of IgM, IgD and IgG. Eur J Immunol. 1977 Mar;7(3):151–153. doi: 10.1002/eji.1830070307. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Anderson K. C., Freedman A. S., Fisher D. C., Slaughenhoupt B., Schlossman S. F., Nadler L. M. Studies of in vitro activation and differentiation of human B lymphocytes. I. Phenotypic and functional characterization of the B cell population responding to anti-Ig antibody. J Immunol. 1985 Mar;134(3):1516–1523. [PubMed] [Google Scholar]
- Chen W. Y., Muñoz J., Fudenberg H. H., Tung E., Virella G. Polyclonal activation of human peripheral blood B lymphocytes by formaldehyde-fixed Salmonella paratyphi B. I. Immunoglobulin production without DNA synthesis. J Exp Med. 1981 Feb 1;153(2):365–374. doi: 10.1084/jem.153.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Ziff M., Vitetta E. S. Characterization of a B cell defect in the NZB mouse manifested by an increased ratio of surface IgM to IgD. J Immunol. 1978 Sep;121(3):973–977. [PubMed] [Google Scholar]
- Dagg M. K., Levitt D. Human B-lymphocyte subpopulations. I. Differentiation of density-separated B lymphocytes. Clin Immunol Immunopathol. 1981 Oct;21(1):39–49. doi: 10.1016/0090-1229(81)90193-8. [DOI] [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Kunkel H. G., Halper J. P., Harris S. R. Induction of in vitro differentiation and immunoglobulin synthesis of human leukemic B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1570–1578. doi: 10.1084/jem.148.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson J., Dooley N. J., Koski I. R., Blaese R. M. Immunoglobulin production induced in vitro by glucocorticoid hormones: T cell-dependent stimulation of immunoglobulin production without B cell proliferation in cultures of human peripheral blood lymphocytes. J Clin Invest. 1981 Dec;68(6):1539–1547. doi: 10.1172/JCI110408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Uhr J. W., Vitetta E. S. Induction of proliferation and differentiation of neoplastic B cells by anti-immunoglobulin and T-cell factors. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2507–2511. doi: 10.1073/pnas.78.4.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Lawton A. R. Response of human B cells to Staphylococcus aureus Cowan I: T-independent proliferation and T-dependent differentiation to immunoglobulin secretion involve subsets separable by rosetting with mouse erythrocytes. J Immunol. 1984 Oct;133(4):1891–1895. [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. The role of B cell proliferation in the generation of immunoglobulin-secreting cells in man. J Immunol. 1983 Jun;130(6):2597–2604. [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. The roles of T cell factors in activation, cell cycle progression, and differentiation of human B cells. J Immunol. 1985 Mar;134(3):1690–1701. [PubMed] [Google Scholar]
- Kishimoto T., Yoshizaki K., Kimoto M., Okada M., Kuritani T., Kikutani H., Shimizu K., Nakagawa T., Nakagawa N., Miki Y. B cell growth and differentiation factors and mechanism of B cell activation. Immunol Rev. 1984 Apr;78:97–118. doi: 10.1111/j.1600-065x.1984.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human B cell differentiation. II. Pokeweed mitogen-responsive B cells belong to a surface immunoglobulin D-negative subpopulation. J Exp Med. 1982 May 1;155(5):1561–1566. doi: 10.1084/jem.155.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama S., Kubagawa H., Cooper M. D. Activation of human B cells and inhibition of their terminal differentiation by monoclonal anti-mu antibodies. J Immunol. 1985 Jul;135(1):192–199. [PubMed] [Google Scholar]
- Mayer L. Terminal maturation of resting B cells by proliferation-independent B cells differentiation factors. J Exp Med. 1986 Aug 1;164(2):383–392. doi: 10.1084/jem.164.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Factors controlling the B-cell cycle. Annu Rev Immunol. 1986;4:13–36. doi: 10.1146/annurev.iy.04.040186.000305. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J., Lernhardt W., Schreier M. H. H-2-unrestricted polyclonal maturation without replication of small B cells induced by antigen-activated T cell help factors. Eur J Immunol. 1980 Sep;10(9):679–685. doi: 10.1002/eji.1830100905. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. T cell activation of antigen-specific antibody responses by large B cells is MHC restricted. J Immunol. 1986 Mar 15;136(6):2090–2094. [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Longo D. L., Volkman D. J., Smith K. A., Fauci A. S. Interleukin 2 receptors on human B cells. Implications for the role of interleukin 2 in human B cell function. J Exp Med. 1985 Jan 1;161(1):181–197. doi: 10.1084/jem.161.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kishimoto T., Miki Y., Kuritani T., Kaieda T., Yoshizaki K., Yamamura Y. T cell-replacing factor- (TRF) induced IgG secretion in a human B blastoid cell line and demonstration of acceptors for TRF. J Immunol. 1981 Aug;127(2):412–416. [PubMed] [Google Scholar]
- Neckers L. M., Yenokida G., James S. P. The role of the transferrin receptor in human B lymphocyte activation. J Immunol. 1984 Nov;133(5):2437–2441. [PubMed] [Google Scholar]
- Parker D. C. Separable helper factors support B cell proliferation and maturation to Ig secretion. J Immunol. 1982 Aug;129(2):469–474. [PubMed] [Google Scholar]
- Richard Y., Leprince C., Treton D., Galanaud P. Functional heterogeneity of nonresting B cells in human blood. Eur J Immunol. 1986 Oct;16(10):1303–1308. doi: 10.1002/eji.1830161019. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Maggi E., Del Prete G., Ricci M. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981 Oct;127(4):1307–1313. [PubMed] [Google Scholar]
- Ruscetti F. W., Gallo R. C. Human T-lymphocyte growth factor: regulation of growth and function of T lymphocytes. Blood. 1981 Mar;57(3):379–394. [PubMed] [Google Scholar]
- Sakane T., Ueda Y., Suzuki N., Niwa Y., Hoshino T., Tsunematsu T. OKT4+ and OKT8+ T lymphocytes produce soluble factors that can modulate growth and differentiation of human B cells. Clin Exp Immunol. 1985 Oct;62(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D. B cell maturation factor: effects on various cell populations. J Immunol. 1984 Feb;132(2):845–850. [PubMed] [Google Scholar]
- Simpson L. G., Isakson P. C. Role of DNA synthesis in secretion of immunoglobulin from murine B cells stimulated by T cell derived lymphokines. J Immunol. 1986 Sep 15;137(6):1797–1802. [PubMed] [Google Scholar]
- Suzuki N., Sakane T., Ueda Y., Murakawa Y., Tsunematsu T. Implications for the role of cognate interactions in in vitro human B cell activation by Staphylococcus aureus Cowan I and pokeweed mitogen. J Clin Invest. 1986 Jan;77(1):294–300. doi: 10.1172/JCI112290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Sakane T., Ueda Y., Tsunematsu T. Abnormal B cell function in patients with Behçet's disease. Arthritis Rheum. 1986 Feb;29(2):212–219. doi: 10.1002/art.1780290209. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Howard M., Kappler J., Marrack P., Watson J., Booth R., Wetzel G. D., Dutton R. W. Evidence for two distinct classes of murine B cell growth factors with activities in different functional assays. J Exp Med. 1983 Sep 1;158(3):822–835. doi: 10.1084/jem.158.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Scher I., Schaefer M. E., Lindsten T., Finkelman F. D., Mond J. J. Size-dependent B lymphocyte subpopulations: relationship of cell volume to surface phenotype, cell cycle, proliferative response, and requirements for antibody production to TNP-Ficoll and TNP-BA. J Immunol. 1984 Nov;133(5):2333–2342. [PubMed] [Google Scholar]
- Tomita S., Ambrus J. L., Jr, Volkman D. J., Longo D. L., Mitsuya H., Reitz M. S., Jr, Fauci A. S. Human T cell leukemia/lymphoma virus I infection and subsequent cloning of normal human B cells. Direct responsiveness of cloned cells to recombinant interleukin 2 by differentiation in the absence of enhanced proliferation. J Exp Med. 1985 Jul 1;162(1):393–398. doi: 10.1084/jem.162.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindelov L. L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch B Cell Pathol. 1977 Aug 10;24(3):227–242. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Polyclonal B-cell activators in the study of the regulation of immunoglobulin synthesis in the human system. Adv Immunol. 1982;32:1–63. doi: 10.1016/s0065-2776(08)60720-8. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K., Nakagawa T., Kaieda T., Muraguchi A., Yamamura Y., Kishimoto T. Induction of proliferation and Ig production in human B leukemic cells by anti-immunoglobulins and T cell factors. J Immunol. 1982 Mar;128(3):1296–1301. [PubMed] [Google Scholar]



