Abstract
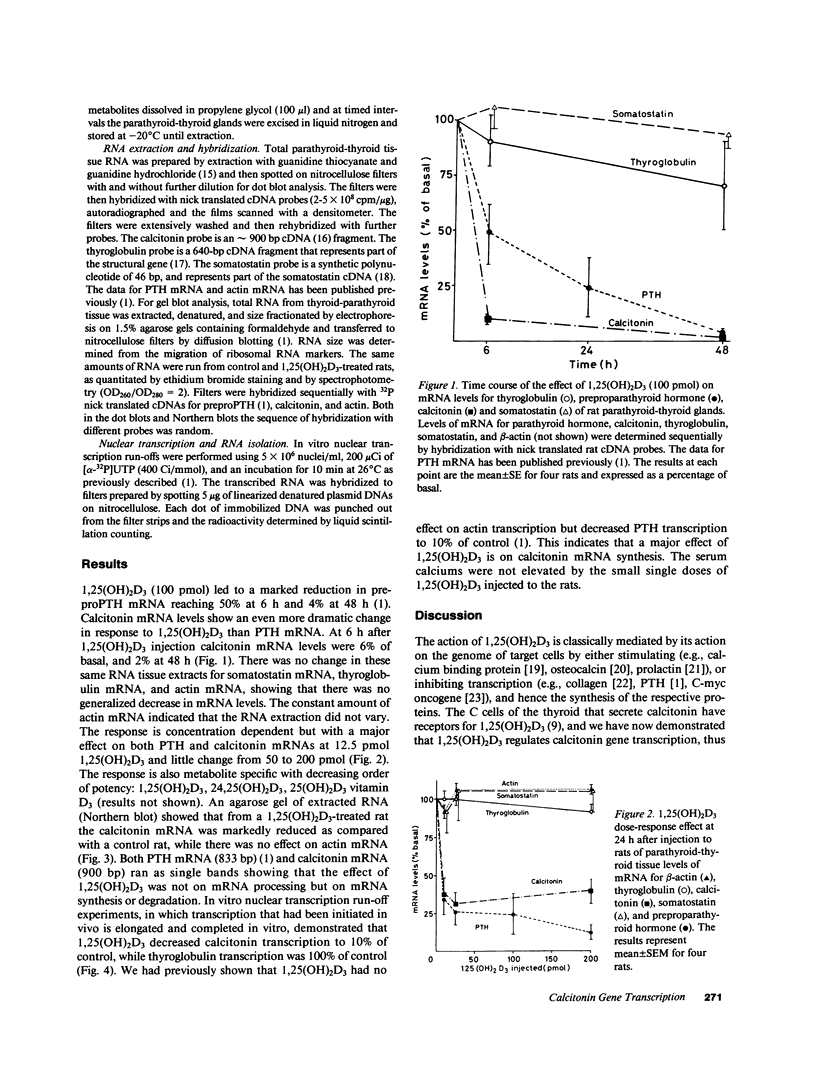
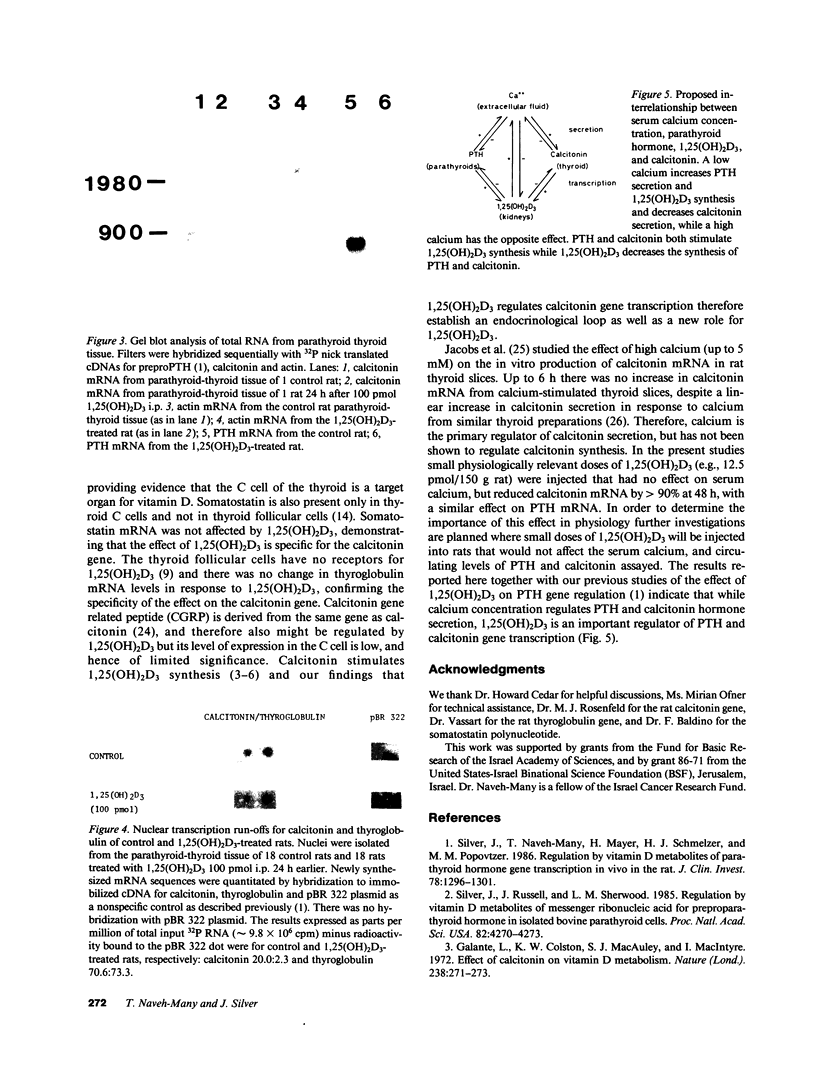
Calcitonin is secreted by the C cells of the thyroid in response to a raised serum calcium, and acts on bone to lower serum calcium. The C cells have specific receptors for the dihydroxymetabolite of vitamin D3, 1,25(OH)2D3. Moreover, calcitonin stimulates the synthesis of 1,25(OH)2D3 in the kidney. Parathyroid hormone (PTH), the third calciotrophic hormone, is also trophic to the renal synthesis of 1,25(OH)2D3, and in turn 1,25(OH)2D3 inhibits PTH gene transcription and synthesis. We report here the marked inhibition of calcitonin gene transcription by the injection of physiologically relevant doses of 1,25(OH)2D3 to normal rats that did not raise serum calcium. Calcitonin mRNA levels after 100 pmol 1,25(OH)2D3 decreased to 6% of basal at 6 h and 4% at 48 h, and a dose response showed a marked effect even after 12.5 pmol 1,25(OH)2D3, with no appreciably greater effect with larger doses (up to 200 pmol). Control genes, actin, thyroglobulin (thyroid follicular cells), somatostatin (thyroid C-cells) were not affected by 1,25(OH)2D3. Gel blots showed that 1,25(OH)2D3 decreased calcitonin mRNA levels without any change in its size. In vitro nuclear transcription showed that 1,25(OH)2D3-treated (100 pmol) rats' calcitonin transcription was 10% of control, while thyroglobulin and actin were 100%. We propose that calcium is the major regulator of PTH and calcitonin secretion, while 1,25(OH)2D3 is an important regulator of PTH and calcitonin gene transcription. We believe this to be the first demonstration of an effect of 1,25(OH)2D3 on the C cells thereby establishing a new target organ and site of action of vitamin D. Calcitonin is trophic to 1,25(OH)2D3 synthesis, which in turn inhibits calcitonin synthesis, which are the components of a new endocrinological feedback loop.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., David D. N., Rosenfeld M. G., Roos B. A., Evans R. M. Characterization of rat calcitonin mRNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4444–4448. doi: 10.1073/pnas.77.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentzen R., Baldino F., Jr, Davis L. G., Higgins G. A., Lin Y., Manning R. W., Wolfson B. In situ hybridization of putative somatostatin mRNA within hypothalamus of the rat using synthetic oligonucleotide probes. J Cell Biochem. 1985;27(4):415–422. doi: 10.1002/jcb.240270410. [DOI] [PubMed] [Google Scholar]
- Bell N. H., Queener S. Stimulation of calcitonin synthesis and release in vitro by calcium and dibutyryl cyclic AMP. Nature. 1974 Mar 22;248(446):343–344. doi: 10.1038/248343a0. [DOI] [PubMed] [Google Scholar]
- Brocas H., Christophe D., Van Heuverswijn B., Scherberg N., Vassart G. Molecular cloning of Pst I fragments from rat double stranded thyroglobulin complementary DNA. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1785–1792. doi: 10.1016/0006-291x(80)91381-9. [DOI] [PubMed] [Google Scholar]
- Charles M. A., Martial J., Zolock D., Morrissey R., Baxter J. D. Regulation of calcium-binding protein messenger RNA by 1,25-dihydroxycholecalciferol. Calcif Tissue Int. 1981;33(1):15–18. doi: 10.1007/BF02409407. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Desplan C., Benicourt C., Jullienne A., Segond N., Calmettes C., Moukhtar M. S., Milhaud G. Cell free translation of mRNA coding for human and murine calcitonin. FEBS Lett. 1980 Aug 11;117(1):89–92. doi: 10.1016/0014-5793(80)80919-7. [DOI] [PubMed] [Google Scholar]
- Freake H. C., MacIntyre I. Specific binding of 1,25-dihydroxycholecalciferol in human medullary thyroid carcinoma. Biochem J. 1982 Jul 15;206(1):181–184. doi: 10.1042/bj2060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante L., Colston K. W., MacAuley S. J., MacIntyre I. Effect of calcitonin on vitamin D metabolism. Nature. 1972 Aug 4;238(5362):271–273. doi: 10.1038/238271a0. [DOI] [PubMed] [Google Scholar]
- Heynen G., Cornet F., Franchimont P., Gaspar S., Plomteux G., Cession-Fossion G., Russel R. G., Kanis J. A. Comparison of acute effects of 1.25- and 24.25-dihydroxy-vitamin D3 in normal subjects. Acta Endocrinol (Copenh) 1981 Dec;98(4):619–624. doi: 10.1530/acta.0.0980619. [DOI] [PubMed] [Google Scholar]
- Horiuchi N., Takahashi H., Matsumoto T., Takahashi N., Shimazawa E., Suda T., Ogata E. Salmon calcitonin-induced stimulation of 1 alpha,25-dihydroxycholecalciferol synthesis in rats involving a mechanism independent of adenosine 3':5'-cyclic monophosphate. Biochem J. 1979 Nov 15;184(2):269–275. doi: 10.1042/bj1840269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. W., Simpson E., Penschow J., Hudson P., Coghlan J., Niall H. Characterization and localization of calcitonin messenger ribonucleic acid in rat thyroid. Endocrinology. 1983 Nov;113(5):1616–1622. doi: 10.1210/endo-113-5-1616. [DOI] [PubMed] [Google Scholar]
- Jaeger P., Jones W., Clemens T. L., Hayslett J. P. Evidence that calcitonin stimulates 1,25-dihydroxyvitamin D production and intestinal absorption of calcium in vivo. J Clin Invest. 1986 Aug;78(2):456–461. doi: 10.1172/JCI112597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature. 1981 May 28;291(5813):327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- Parsons J. A., Erlandsen S. L., Hegre O. D., McEvoy R. C., Elde R. P. Central and peripheral localization of somatostatin. Immunoenzyme immunocytochemical studies. J Histochem Cytochem. 1976 Jul;24(7):872–882. doi: 10.1177/24.7.60436. [DOI] [PubMed] [Google Scholar]
- Price P. A., Baukol S. A. 1,25-dihydroxyvitamin D3 increases serum levels of the vitamin K-dependent bone protein. Biochem Biophys Res Commun. 1981 Apr 15;99(3):928–935. doi: 10.1016/0006-291x(81)91252-3. [DOI] [PubMed] [Google Scholar]
- Raue F., Deutschle I., Küntzel C., Ziegler R. Reversible diminished calcitonin secretion in the rat during chronic hypercalcemia. Endocrinology. 1984 Dec;115(6):2362–2367. doi: 10.1210/endo-115-6-2362. [DOI] [PubMed] [Google Scholar]
- Raue F., Deutschle I., Ziegler R. Acute effect of 1,25-dihydroxy-vitamin D3 on calcitonin secretion in rats. Horm Metab Res. 1983 Apr;15(4):208–209. doi: 10.1055/s-2007-1018674. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Birnberg N. C., Mermod J. J., Murdoch G. H., Evans R. M. Calcitonin, prolactin, and growth hormone gene expression as model systems for the characterization of neuroendocrine regulation. Recent Prog Horm Res. 1983;39:305–351. doi: 10.1016/b978-0-12-571139-5.50012-4. [DOI] [PubMed] [Google Scholar]
- Segond N., Legendre B., Tahri E. H., Besnard P., Jullienne A., Moukhtar M. S., Garel J. M. Increased level of calcitonin mRNA after 1,25-dihydroxyvitamin D3 injection in the rat. FEBS Lett. 1985 May 20;184(2):268–272. doi: 10.1016/0014-5793(85)80620-7. [DOI] [PubMed] [Google Scholar]
- Silver J., Naveh-Many T., Mayer H., Schmelzer H. J., Popovtzer M. M. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986 Nov;78(5):1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Russell J., Sherwood L. M. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4270–4273. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R., Charman M., Lawson D. E. Stimulation of intestinal calcium-binding-protein mRNA synthesis in the nucleus of vitamin D-deficient chicks by 1,25-dihydroxycholecalciferol. Biochem J. 1978 Dec 1;175(3):1089–1094. doi: 10.1042/bj1751089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark J. D., Tashjian A. H., Jr Regulation of prolactin mRNA by 1,25-dihydroxyvitamin D3 in GH4C1 cells. J Biol Chem. 1983 Oct 25;258(20):12118–12121. [PubMed] [Google Scholar]
- Wolfe H. J., Delellis R. A. Familial medullary thyroid carcinoma and C cell hyperplasia. Clin Endocrinol Metab. 1981 Jul;10(2):351–365. doi: 10.1016/s0300-595x(81)80027-8. [DOI] [PubMed] [Google Scholar]