Abstract
Although antidepressants are generally effective in the treatment of major depressive disorder (MDD), it can still take weeks before patients feel the full antidepressant effects. Despite the efficacy of standard treatments, approximately two-thirds of patients with MDD fail to respond to pharmacotherapy. Therefore, the identification of blood biomarkers that can predict the treatment response to antidepressants would be highly useful in order to improve this situation. This article discusses inflammatory molecules as predictive biomarkers for antidepressant responses to several classes of antidepressants, including the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine.
Keywords: antidepressant, biomarker, cytokine, inflammation, ketamine, predictor, response
Introduction
Major depressive disorder (MDD) is the most prevalent psychiatric disorder, and is also amongst the most severe and debilitating. The World Health Organization estimates that more than 350 million individuals of all ages suffer from depression [1]. Although antidepressants are generally effective in the treatment of MDD, it can still take weeks before patients feel the full antidepressant effects. Despite the efficacy of standard treatments, approximately two-thirds of patients with MDD fail to respond to pharmacotherapy. Furthermore, there is a high rate of relapse, and MDD patients have a high risk of attempting suicide. Therefore, the identification of biomarkers that can predict the treatment response to antidepressants would be very useful to improve this situation [2,3,4].
Accumulating evidence suggests that inflammatory processes play a role in the pathophysiology of MDD [5,6,7]. When bacterial endotoxin lipopolysaccharide (LPS) is administered to rodents, depression-like behaviors are induced after the induction of inflammation [8,9,10,11]. Furthermore, antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs), could prevent depression-like behavior and alternations in serum pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), caused by LPS administration [12]. A meta-analysis showed the higher serum TNF-α levels in drug-free MDD patients compared with those in healthy controls [13]. A study using postmortem brain samples showed elevated gene expression of pro-inflammatory cytokines in the frontal cortex of people with a history of MDD [14]. Taken together, it is likely that both peripheral and central inflammation are associated with MDD and that anti-inflammatory drugs could ameliorate depressive symptoms in MDD patients.
C-reactive protein (CRP) is an annular ring-shaped, pentameric protein found in the blood plasma, and CRP levels were increased in response to inflammation. Meta-analyses showed that MDD is associated with increased CRP levels [15,16]. Recently, Uher et al. [17] reported an inflammatory biomarker as a differential predictor of clinical outcome in depression therapy. In a multicenter open-label randomized trial, the authors measured CRP, a commonly detectable biomarker of systemic inflammation, in patients with MDD who were randomly allocated to either 12 weeks of treatment with escitalopram (a SSRI, n = 115) or nortriptyline (a norepinephrine (NE) reuptake inhibitor (NRI), n = 126). Serum CRP levels at baseline differentially predicted treatment outcomes with both antidepressants. Patients with low levels of CRP (<1 mg/L) showed improvement on the Montegomery-Åsperg Depression Rating Scale (MADRS), with scores three points higher after escitalopram treatment, compared with nortriptyline. In contrast, patients with higher CRP levels scored three points higher on MADRS after nortriptyline, compared with escitalopram [17].
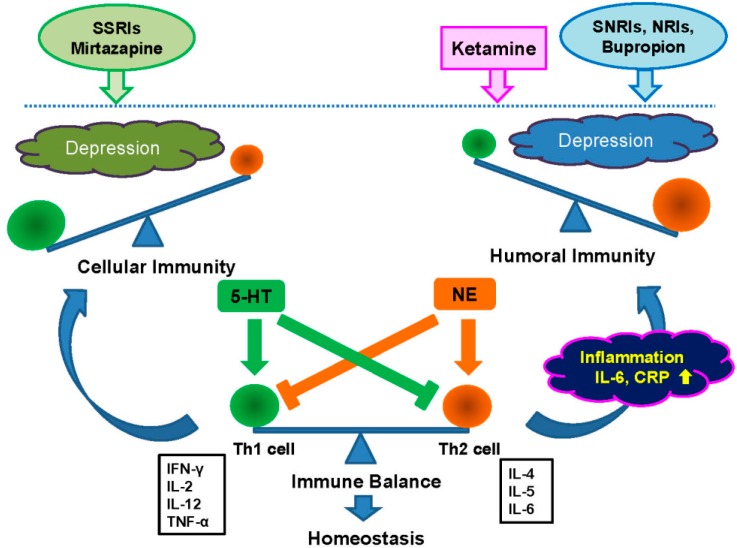
NE and 5-HT are known to confer differential effects on inflammation, and mediate a T helper 1 (Th1) shift and a T helper 2 (Th2) shift, respectively. Furthermore, 5-HT inhibits the production of Th2 cytokines such as interleukin 6 (IL-6), whereas NE inhibits production of Th1 pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) (Figure 1) [18]. The SSRIs (e.g., paroxetine, sertraline, fluoxetine, escitalopram) cause a Th1 shift. The 5-HT and NE reuptake inhibitors (SNRIs; venlafaxine, duloxetine), and NRIs (reboxetine) cause a Th2 shift [18]. Furthermore, bupropion (a NE and dopamine reuptake inhibitor) and mirtazapine (NaSSA: NE and Specific Serotonergic Antidepressant) may induce Th2 and Th1 shift, respectively. Thus, the antidepressants that affect 5-HT and NE distinctly affect immunity: while NRIs suppress Th1-type cytokines and shift the balance toward humoral immunity. The SSRIs reduce the production of Th2-type cytokines and shift the balance toward cellular immune response (Figure 1) [17,18]. It would therefore be of great interest to examine whether serum levels of IL-6 and TNF-α could serve as reliable biomarkers for a clinical response to these two antidepressants (escitalopram and nortriptyline) in this cohort sample.
Figure 1.
The balance between Th1 (cellular) and Th2 (humoral) response to the adaptive immune system. The immune system, composed of Th1-mediated cellular immunity and Th2-mediated humoral immunity, is essential to maintain health. Both Th1 and Th2 immunity are tightly controlled, but abnormalaties of the “immune balance” between Th1 and Th2 immunity is implicated in the pathophysiology of MDD. Th2 shift causes inflammation and increase in CRP protein and pro-inflammatory cytokines (e.g., IL-6), resulting in depressive symptom. Th1 shift also causes depressive symptom. 5-HT and NE are known to confer differential effects on inflammation. 5-HT and NE mediate a Th1 shift and a Th2 shift, respectively. Furthermore, 5-HT inhibits the production of Th2 cytokines such as IL-6, whereas NE inhibits production of Th1 pro-inflammatory cytokines, including TNF-α [18]. The SSRIs (e.g., paroxetine, sertraline, fluoxetine, escitalopram) cause a Th1 shift. The SNRIs (venlafaxine, duloxetine), and NRIs (nortriptyline, reboxetine) cause a Th2 shift [18]. Bupropion and mirtazapine may induce Th2 and Th1 shift, respectively [18]. In addition, the NMDA receptor antagonist ketamine may cause a Th2 shift. Thus, the regulation of the “immune balance” between Th1 and Th2 immunity is critical for therapy of MDD.
The N-methyl-d-aspartate receptor antagonist, ketamine, is the most attractive antidepressant therapy for patients with treatment-resistant MDD [19,20,21,22,23,24,25,26,27,28]. A single subanesthetic dose (0.5 mg/kg) of ketamine produces a rapid antidepressant effect in two-thirds of these treatment resistant MDD patients, which can last for over a week [20,21]. However, biomarkers able to differentiate between responding and non-responding patients have yet to be identified. In contrast, ketamine has the potential to elicit psychotomimetic and dissociative side effects and abuse liability, both of which could limit its use in clinical settings [24,25]. Identifying novel biomarkers capable of predicting the response to ketamine will be invaluable for selecting suitable patients for this therapy [29]. Very recently, we found that, at baseline, serum levels of IL-6 in the ketamine responder group were significantly higher than those of the control and non-responder groups [30]. In contrast, serum levels of IL-6 did not differ between control and non-responder groups. In addition, serum levels of TNF-α remained the same after ketamine infusion. These findings suggest serum IL-6 (not TNF-α) as a useful predictor for clinical outcome in patients with treatment-resistant MDD undergoing ketamine therapy [30]. The NMDA receptor antagonists such as ketamine may suppress Th1-type cytokines and shift the balance toward humoral immunity.
The current strategy for enhancing treatment outcomes in MDD relies on standardized sequential treatment algorithms and measurement-based care, but this approach is largely trial and error [2]. Incorporating reliable biomarkers into treatment algorithms could speed recovery from depression, by shortening or eliminating lengthy and ineffective clinical trials. In the future, blood levels of inflammatory molecules could serve as predictive biomarkers for antidepressant responses to several classes of antidepressants, including SSRIs, SNRIs, NRIs, NaSSAs, and NMDA receptor antagonists.
Acknowledgments
This study was supported by grants from the Health and Labour Sciences Research Grants, Japan (H26-seishin-ippan-015), the Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (24116006).
Author Contributions
Kenji Hashimoto wrote the article.
Conflicts of Interest
Kenji Hashimoto has served as a scientific consultant to Astellas, Dainippon-Sumitomo and Taisho, received research support from Abbvie, Dainippon-Sumitomo, Mochida, Otsuka, and Taisho, and is an inventor on a field patent application on “The use of R-ketamine in the treatment of psychiatric diseases” by Chiba University.
References
- 1.World Health Organization (WHO) Depression. Fact sheet No. 369/October 2012. [(accessed on 10 March 2015)]. Available online: http://www.who.int/mediacentre/factsheets/fs369/en/index.html.
- 2.Leuchter A.F., Cook I.A., Hamilton S.P., Narr K.L., Toga A., Hunter A.M., Faull K., Whitelegge J., Andrews A.M., Loo J., et al. Biomarkers to predict antidepressant response. Curr. Psychiatry Rep. 2010;12:553–562. doi: 10.1007/s11920-010-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins-de-Souza D., Harris L.W., Guest P.C., Turck C.W., Bahn S. The role of proteomics in depression research. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260:499–506. doi: 10.1007/s00406-009-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitenstein B., Scheuer S., Holsboer F. Are there meaningful biomarkers of treatment response for depression. Drug Discov. Today. 2014;19:539–561. doi: 10.1016/j.drudis.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res. Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raison C.L., Lowry C.A., Rook G.A. Inflammation, sanitation, and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelly K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor J.C., Lawson M.A., André C., Moreau M., Lestage J., Castanon N., Kelley K.W., Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma M., Ren Q., Zhang J.C., Hashimoto K. Effects of brilliant blue G on serum tumor necrosis factor-α levels and depression-like behavior in mice after lipopolysaccharide administration. Clin. Psychopharmacol. Neurosci. 2014;12:31–36. doi: 10.9758/cpn.2014.12.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J.C., Wu J., Fujita Y., Yao W., Ren Q., Yang C., Li S.X., Shirayama Y., Hashimoto K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2015;18:1–12. doi: 10.1016/j.neuropharm.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohgi Y., Futamura T., Kikuchi T., Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2013;103:856–859. doi: 10.1016/j.pbb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Shelton R.C., Claiborne J., Sidoryk-Wegrzynowicz M., Reddy R., Aschner M., Lewis D.A., Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 16.Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Uher R., Tansey K.E., Dew T., Maier W., Mors O., Hauser J., Dernovsek M.Z., Henigsberg N., Souery D., Farmer A., McGuffin P. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry. 2014;171:1278–1286. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- 18.Martino M., Rocchi G., Escelsior A., Fornaro M. Immunomodulation mechanism of antidepressants: Interactions between serotonin/norepinephrine balance and Th1/Th2 balance. Curr. Neuropharmacol. 2012;10:97–123. doi: 10.2174/157015912800604542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev. Neurother. 2011;11:33–36. doi: 10.1586/ern.10.176. [DOI] [PubMed] [Google Scholar]
- 20.Aan Het Rot M., Zarate C.A., Jr., Charney D.S., Mathew S.J. Ketamine for depression: Where do we go from here. Biol. Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krystal J.H., Sanacora G., Duman R.S. Rapid-acting glutamtergic antidepressants: The path to ketamine and beyond. Biol. Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarate C.A., Jr., Mathews D.C., Furey M.L. Human biomarkers of rapid antidepressant effects. Biol. Psychiatry. 2013;73:1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto K., Malchow B., Falkai P., Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K. The R-stereoisomer of ketamine as an alternative for ketamine for treatment-resistant major depression. Clin. Psychopharmacol. Neurosci. 2014;12:72–73. doi: 10.9758/cpn.2014.12.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C., Hashimoto K. Rapid antidepressant effects and abuse liability of ketamine. Psychopharmacology (Berl.) 2014;231:2041–2042. doi: 10.1007/s00213-014-3543-0. [DOI] [PubMed] [Google Scholar]
- 26.Hillhouse T.M., Porter J.H. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015;23:1–21. doi: 10.1037/a0038550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Wilde K.E., Levitch C.F., Murrough J.W., Mathew S.J., Iosifescu D.V. The promise of ketamine for treatment-resistant depression: Current evidence and future directions. Ann. N. Y. Acad. Sci. 2015 doi: 10.1111/nyas.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdallah C.G., Averill L.A., Krystal J.H. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann. N. Y. Acad. Sci. 2015 doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto K. Blood D-serine levels as a predictive biomarker for the rapid antidepressant effects of the NMDA receptor antagonist ketamine. Psychopharmacology (Berl.) 2014;231:4081–4082. doi: 10.1007/s00213-014-3735-7. [DOI] [PubMed] [Google Scholar]
- 30.Yang J.J., Wang N., Yang C., Shi J.Y., Yu H.Y., Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol. Psychiatry. 2015;77:e19–e20. doi: 10.1016/j.biopsych.2014.06.021. [DOI] [PubMed] [Google Scholar]