Abstract
The objective of this study was to explore the effect of the character of chitosans used, and the regeneration conditions employed on, the yield and physicochemical characteristics of regenerated products. Different concentrations of acetic acid were used to dissolve chitosans of 61.7% and 94.9% degree of deacetylation (DD), and weight-average molecular weight (Mw) of 176 and 97 kDa, respectively; they were then precipitated with an 8 N NaOH solution, followed by washing and neutral and freeze drying to get the regenerated products. Yields of regenerated products and their physicochemical properties, such as ash content, bulk density, Mw, polydispersity index (PDI), DD, and crystallinity were measured. A higher concentration of acetic acid used resulted in a higher yield. The purity of the regenerated product increased significantly, whereas the bulk density and crystallinity decreased significantly after regeneration. The regeneration process showed its merits of narrowing down the PDI of regenerated products. The DD and structure of chitosan was changed insignificantly after the regeneration process.
Keywords: chitosan, regeneration, molecular weight, polydispersity index, degree of deacetylation, crystallinity, ash content
1. Introduction
Chitosan is a high molecular weight (Mr) polysaccharide and is composed of N-acetyl-d-glucosamine and d-glucosamine, linked by β (1→4) glucoside. It is widely distributed and readily available, with the special features of being a cationic polyelectrolyte in an acid solution, non-toxic, and biodegradable. Chitosan is considered to be both a versatile and environmentally friendly raw material, and can be applied in food processing, agriculture, biomedicine, biochemistry, cosmetics, textiles, and wastewater treatment [1,2,3,4]. This is due to their versatile forms of fibers, hydrogels, membranes, microspheres, microcapsules, nanoparticles, liquid crystalline, etc. [5,6,7,8].
Medical and health care products, such as wound dressings, gauzes, and in vivo absorbable sutures, have the highest market value among the products sold in the above-mentioned areas [9]. Hirano [10] proposed parameters, such as source, appearance, viscosity or Mr, particle size, degree of deacetylation (DD), solubility in aqueous 0.5% acetic acid, moisture content, and ash for the specification of chitosan used in medical, cosmetic, standard, and industrial applications. In addition to the three most commonly used parameters of Mr, DD, and solubility, parameters, such as heavy metal, microbial, protein, and pyrogen contents, are considered to be important, and need to be specified for medical usage.
The physicochemical properties and bioactivity of chitosan, such as aggregation of whole blood, washed erythrocytes and platelets in platelet-rich plasma [11], vaccine adjuvant activity [12], antioxidant activity, antimicrobial activity, enzyme and DNA binding ability and protection, cholesterol, lipid and metal binding capacity, drug delivery property, ionic conductivity and thermal stability of biosensors, rheological property, chain flexibility, mechanical property and pore size of membranes and microcapsules, and water-holding capacity [2,3,13,14,15], depend on intrinsic factors such as the DD, Mr, and polydispersity index (PDI).
The regeneration process is a frequently employed and practical measure to remove unwanted contaminants [16,17,18,19,20,21], to improve the solubility [9,22,23,24,25], or change to physicochemical properties [9,21,24]. These functions have been revealed to be dependent, not only upon the chemical structure, but also on the regeneration method.
The principle of the regeneration process is dissolving the chitosan in an acid solution in order to remove unwanted materials. Acids used include acetic acid [10,16,17,18,19,20,21], formic, propionic, lactic, citric, and sulfuric acid [21]. Next is precipitation by alkali neutralization, such as by using 5% aqueous sodium hydroxide solution at room temperature [19,23,26] or alkali/alcohol/water mixtures [18], or by dialysis [9].
The regeneration procedure is not only employed to remove the unwanted contaminants but also is demonstrated to increase bioactivity, such as immune adjuvant activity, and among different DD regenerated chitosans obtained, the best performance was shown at around 70% of deacetylation [19]. The regenerated chitosan can elevate the absorption capacity of anionic dye [27], or fat or the orange II dye [28]. The absorption of fat and the orange II dye was 1.5–2.0 times higher with regenerated chitosan, with 87% and 96% DD, than that of the 75% DD counterpart, but water absorption capacity was just the opposite. Dutkiewicz et al. [26] reported that regenerated krill chitosan with weight-average molecular weights (Mw) of 800 kDa shows whole blood clotting time two times longer than that of silica gel. The above-mentioned literature indicated that those enhanced functional properties may be attributed to difference in solubility, Mr, and DD, which in turn resulted in different content of positively charged amino groups in an acid solution between original and regenerated chitosan. Protonated amino groups may result in better immune adjuvant activity and fat and anionic dye absorption. However, up to now, the regenerated conditions used and the effect of characters of chitosan, such as the Mr, DD on the yields, and physicochemical of regenerated products have not been systematically explored.
The objective of this study was to explore the effects of the characters of chitosan used and regeneration conditions on the yield and physicochemical characteristics of regenerated products. The different DDs and Mw of chitosans were dissolved in 0.1 and 1.0 M acetic acid, respectively, then chitosan was precipitated with 8 N NaOH, followed by washing, neutral and freeze drying to get the regenerated products. The physicochemical properties, such as Mw, PDI, DD and crystallinity index of regenerated products, were measured.
2. Results and Discussion
2.1. The Yield
Results in Table 1 show that the yield of R-2 was higher than R-1 and the yield of R-4 was higher than R-3. This may be attributed to using a higher concentration of acetic acid (1.0 M) will dissolve more chitosan than using a lower concentration (0.1 M) [29], and the insoluble materials were filtered and discarded prior to the solution proceeding to the regeneration procedure; therefore, the yield of R-2 was higher than R-1, thus R-4 was higher than R-3. The yield of R-4 was higher than R-2, whereas the yield of R-3 was higher than R-1. It indicated that the higher the DDs of chitosan used, the higher the yield of regenerated chitosan was obtained. The result was similar to that of Chen and Liu [9]. This may be due to the fact that the solubility of lower-DD or higher-Mr chitosan was lower than that of the higher-DD or lower-Mr one, respectively [14,23]. The reasons for using higher DD chitosan that ends up with a higher yield than when using lower DD chitosan might be because the insoluble materials were filtered and discarded prior to the solution that proceeded to the regeneration procedure.
Table 1.
Effects of the characters of chitosans used and regeneration conditions on the yield and physicochemical characteristics of regenerated products.
| Sample | Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| DD (%) | Acetic Acid (M) | Product * | Yield (%) | Ash (%) | Bulk Density (g/cm3) | Mw (kDa) | PDI |
| 61.7 | - | O-1 | - | 1.94 ± 0.15 a | 0.55 ± 0.01 a | 176 ± 3.3 a | 2.07 ± 0.03 a |
| 0.1 | R-1 | 53.54 ± 0.31 a | 0.35 ± 0.03 b | 0.49 ± 0.01 b | 155 ± 3.5 b | 1.72 ± 0.02 b | |
| 1.0 | R-2 | 54.73 ± 0.42 b | 0.33 ± 0.03 b | 0.48 ± 0.01 b | 150 ± 1.4 c | 1.73 ± 0.03 b | |
| 94.9 | - | O-2 | - | 1.75 ± 0.13 x | 0.55 ± 0.01 x | 97 ± 1.7 x | 2.09 ± 0.03 x |
| 0.1 | R-3 | 54.63 ± 0.33 x | 0.29 ± 0.04 y | 0.49 ± 0.01 y | 89 ± 1.0 y | 1.73 ± 0.02 y | |
| 1.0 | R-4 | 58.69 ± 0.25 y | 0.27 ± 0.03 y | 0.49 ± 0.01 y | 88 ± 1.1 y | 1.78 ± 0.03 y | |
a–c Reflect mean values (n = 3) and followed by the difference superscripts within the 61.7% DD chitosan are significantly different (p < 0.05); x–y Reflect mean values (n = 3) and followed by the difference superscripts within the 94.9% DD chitosan are significantly different (p < 0.05); * O-1 and O-2 represent original 61.7% and 94.9% DD chitosan, respectively; R-1 and R-2 represent O-1 dissolving in 0.1 and 1.0 M acetic acid to obtain regenerated chitosans, restectively; R-3 and R-4 represent O-2 dissolving in 0.1 and 1.0 M acetic acid to obtain regenerated chitosans, respectively.
2.2. The Ash Content and Bulk Density
Changes of ash content and bulk density of regenerated chitosans are listed in Table 1. Ash content decreased significantly after regeneration. It decreased from 1.94% of O-1 to 0.35% and 0.33% for R-1 and R-2, respectively, and from 1.75% of O-2 to 0.29% and 0.27% for R-3 and R-4, respectively. Results indicated that the different acid concentration treatments and/or different DDs did not differ significantly in ash. Trung [21] reported there was an insignificant difference in ash content removal among different acid treatments. It is conceivable that some minerals were not removed during the extraction of the original chitosan from raw material because they were enclosed in the interior of the solid material and protected against hydrochloric acid used for deminerization. In the regeneration procedure, the chitosan is completely dissolved, the minerals will be freed and will react with acid solvent. Thus, ash measurement is an indicator of the effectiveness of the regeneration step for removal of impurities.
The change in bulk density content decreased significantly after regeneration. It decreased from 0.55 g/cm3 of O-1 to 0.49 and 0.48 g/cm3 for R-1 and R-2 respectively, and from 0.55 g/cm3 of O-2 to 0.49 and 0.49 g/cm3 for R-3 and R-4 respectively. Results indicated that the different acid concentration treatments and different DDs differed insignificantly in bulk density. The results were similar to Trung [21]. The reduction in ash contained might result in lower bulk density and elevate the purity of the resulted products; therefore, the purities of regeneration chitosans were better than the original ones. This may be one of the reasons why regenerated chitosan shows better performance in medical, cosmetics and biochemical studies than the original chitosan [16,17,19,22,23,26,27,28].
2.3. The Mw and PDI
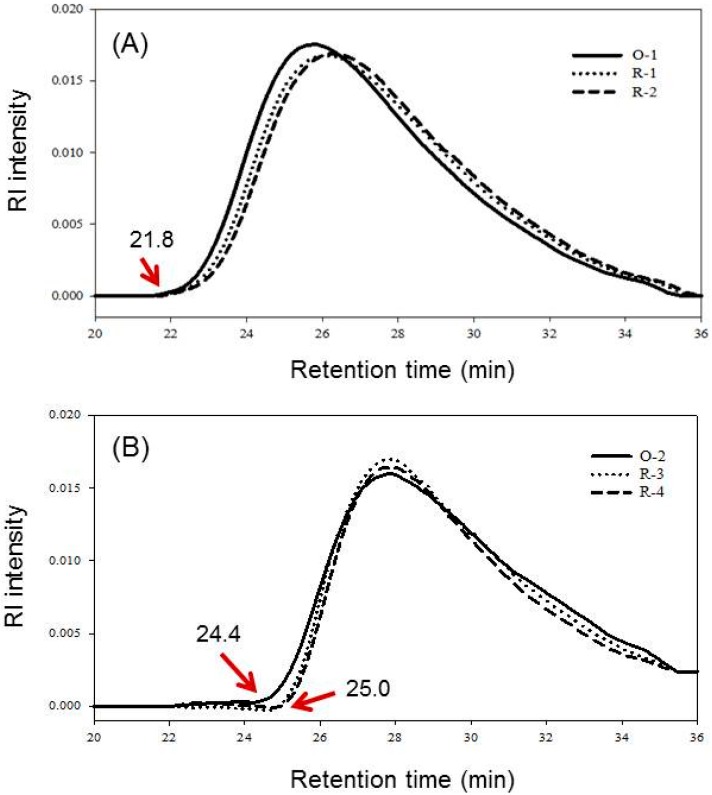
Figure 1 shows the elution patterns of SE-HPLC of original chitosans (O-1 and O-2) and their regenerated chitosans (R-1, R-2, R-3 and R-4). The Mw and PDI of these samples were calculated and listed in Table 1. Mw decreased after regeneration of both high and low DD chitosans. It decreased from 176 kDa of O-1 to 155 and 150 kDa for R-1 and R-2, respectively, and from 97 kDa of O-2 to 89 and 88 kDa for R-3 and R-4, respectively. After regeneration, Mw of regenerated chitosan decreased, possibly due to material loss during the filtering insoluble materials, precipitation process and sieving, as well as acid hydrolysis. Decreases in Mw after being regenerated was more significant of Low DD chitosan (R-1: 11.9%, R-2: 14.8%) than high DD ones (R-3: 8.2%, R-4: 9.3%). The reasons were similar to the previous one just mentioned, i.e., due to different solubilities of O-1 and O-2 chitosans in acetic acid solution, precipitation, and collection process. However, from the size exclusion high performance liquid chromatography (SE-HPLC) elution curve, the decrease in Mw of high DD chitosan might be more significant than the low DD one, i.e., decrease in higher Mw portion was faster in the beginning of elution curve (Figure 1). This is illustrated from the difference of elution curve at starting increase points (arrow) between R-3 and R-4 (both at 25.0 min) to O-2 (at 24.4 min) were more significant than that of the peak in R-1 and R-2 to O-1 (all at 21.8 min). It has been assumed that a high DD chitosan molecule has an extended contour due to more electrostatic force between –NH3+ groups, and the glycosidic linkage is easier to access by H+ hydrolysis reaction [30].
Figure 1.
The SE-HPLC elution patterns of chitosans and regenerated chitosans. (A) 61.7% DD chitosan; (B) 94.9% DD chitosan. Samples O-1, R-1, R-2, O-2, R-3, and R-4, respectively, are described in Table 1.
Results in Figure 1 show the molecular weights distribution of low and high DD chitosans (O-1 and O-2, respectively) and their regenerated chitosans (R-1, R-2, R-3, and R-4). The PDIs of both original chitosans were similar to each other, and their regenerated ones were also similar to each other. The distribution of 61.7% DD chitosan and its regenerated products showed that the high-Mr side (the left-hand side of peak) is steeper, whereas the low-Mr side (the right-hand side of peak) is tailing. After regeneration, the elution curves of R-1 and R-2 were shift to right-hand side of O-1 (Figure 1A). This indicated that the high-Mr fractions (21.8–26.0 min) of R-1 and R-2 decreased, so the retention time were longer than that of O-1, consequently, low-Mr fractions (26.0–35.5 min) increased. The distribution of 94.9% DD chitosan and its regenerated products showed that the high-Mr side is steeper (24.0–28.0 min), whereas the low-Mr side (28.0–35.5 min) is tailing (Figure 1B), similar to Figure 1A. However, the proportion of medium-Mr fractions (27.0–29.0 min) increased significantly after regeneration (Figure 1B). Furthermore, the PDI decreased both after regeneration for high or low DD chitosans used. It decreased from 2.07 of O-1 to 1.72 and 1.73 for R-1 and R-2, respectively, and from 2.09 of O-2 to 1.73 and 1.78 for R-3 and R-4, respectively. Results indicated that the PDI of regenerated products narrows down after the regeneration process.
The merits of the regeneration process can narrow down the PDI have not been reported. The result is unprecedented. The reasons that PDI of chitosan narrows down after regeneration may be due to different solubilities of 61.7% and 94.9% DD chitosans in 0.1 or 1.0 M acetic acid, and different susceptibilities to acid hydrolysis of low and high DD chitosans [31]; it also may be due to the precipitation process and sieving, washing, and collection to get the regenerated products. Therefore, the elution patterns of regenerated products changes; the ratio of medium-Mr fractions increased and PDI decreased. The regeneration procedure of precipitation process and sieving, washing, and collection and it affects the Mw and PDI has not been studied. However, those steps should affect the Mw and PDI of the regenerated product. Therefore, they should be explored in the future studies.
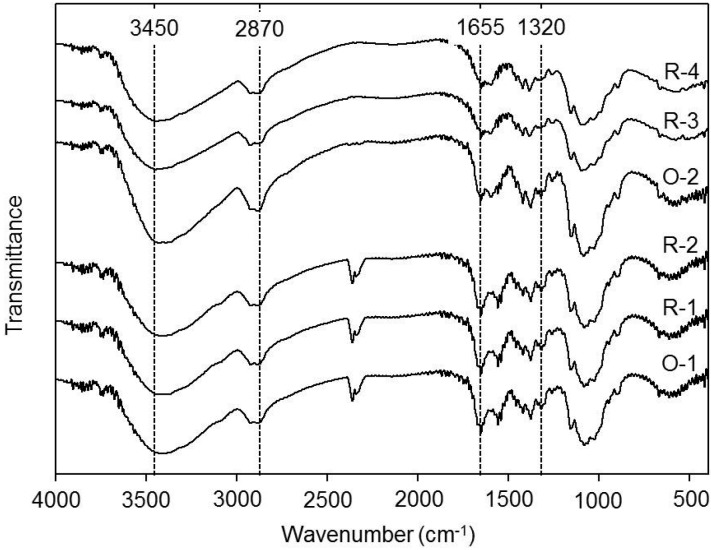
2.4. FTIR Spectrum
In the literature, the OH stretching band at 3450 cm−1 [32]; the CH stretching bands within 2870–2880 cm−1 [33]; the amide I band at 1650–1655 cm−1; the amide II band at 1550–1555 cm−1; the amide III band at 1315–1320 cm−1 [34] have been reported. Results in Figure 2 show that the FTIR spectra of the above-mentioned functional absorption bands have not changed significantly after regeneration. The functional groups on the backbone of different regenerated chitosans either from lower DD chitosans (O-1 to R-1 and R-2) or higher DD chitosans (O-2 to R-3 and R-4) were the same as the original chitosans. Results imply that the regenerated process used in this report did not result in changes of functional groups and DD of polymer chains, suggesting that the acetamido groups were stable during the regenerated treatment. Therefore, the regeneration method is a very good practice to produce final products with higher purity at the same time the functional groups and DD of the chitosan can be preserved.
Figure 2.
Fourier transform infrared spectra (FTIR) of chitosans and regenerated chitosans. Samples O-1, R-1, R-2, O-2, R-3, and R-4, respectively, are described in Table 1.
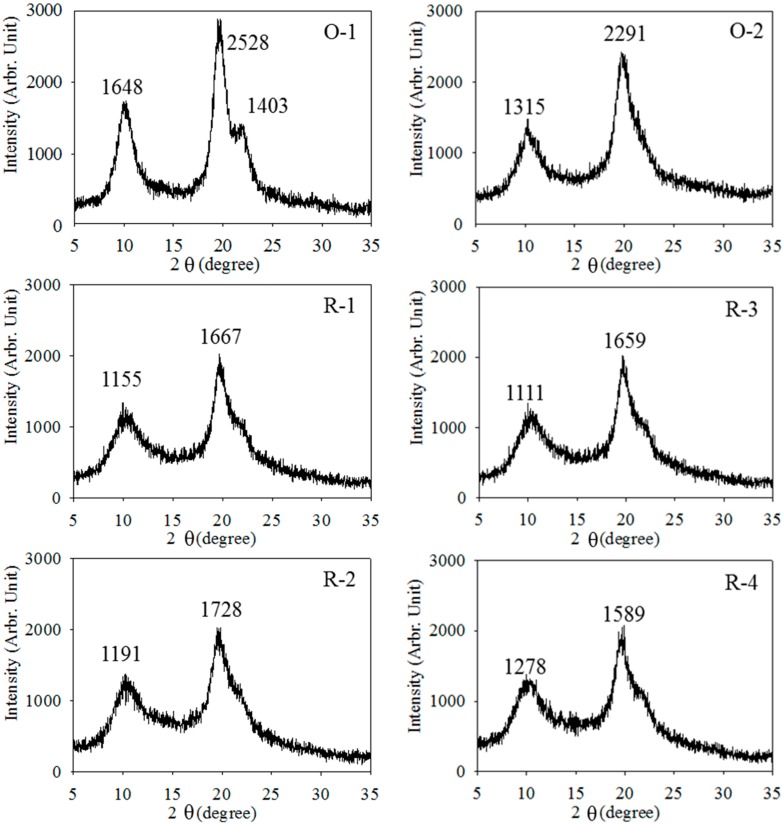
2.5. The Crystallinity
The degree of crystallinity for chitosans (O-1 and O-2) and regenerated chitosans (R-1, R-2, R3, and R4) were evaluated through X-ray powder diffraction method. Webster et al. [35] reported that the diffraction pattern of chitin which exhibits four diffraction peaks at 9°, 12°, 19°, and 26°. The peaks at 10° and 20° are the two prominent peaks in the diffraction pattern, which confirms the partial crystallinity of the polymer. Ogawa [36] reported four crystalline polymorphs, they are tendon, annealed, L-2 and I-2. The tendon and L-2 crystals are hydrated, i.e., water molecules are incorporated. However, the annealed polymorph is anhydrous. The hydrated polymorph (tendon or L-2) showed a strong equatorial reflection spot at 2θ of around 10°, whereas, the anhydrous crystal (annealed) exhibited a strong spot at around 15°, L-2 showed a strong reflection at 2θ at 10.6° and no diffraction spot at around 15°. But I-2 showed diffraction spot at both 2θ of 10.7° and 15.4°. Chitosan after deacetylated to 61.7% (O-1), only three broad peaks appeared at 10°, 20°, and 22°, this may be due to the deacetylaction reaction occurred under high temperature and the strong alkaline condition, displacing the acetamido group to amine group [35]. The resulted showed a similar diffraction pattern that of the hydrated L-2 polymorph of chitosan. After deacetylated to 94.9% (O-2) and regenerated to R-1, R-2, R-3, and R-4, resulted showed that they are hydrated L-2 polymorph, however, only two broad peaks appeared at 10° and 20°, as shown in Figure 3. The results were similar to those of Ogawa [36]. However, Trung [21] reported the diffraction of most regeneration chitosan showed only one major peak at approximately 2θ = 20°. The crystallinity intensity decreased significantly after regeneration. Results in Figure 3 show that I020 (at 2θ = 10°) decreased from 1648 of O-1 to 1155 and 1191 for R-1 and R-2, respectively, and from 1315 of O-2 to 1111 and 1278 for R-3 and R-4, respectively, whereas I110 (at 2θ = 20°) of X-ray intensity decreased from 2528 of O-1 to 1667 and 1728 for R-1 and R-2, respectively, and from 2291 of O-2 to 1659 and 1589 for R-3 and R-4, respectively of X-ray intensity. Results indicated that the structure of regenerated chitosans (R-1, R-2, R-3, and R-4) and their counterparts (O-1 and O-2) differed significantly in crystalline intensity. This is may be due to the fact that original chitosan at 2θ = 10° or 20° decreased with the increase of DD [37] or structure change. However, the DD between original and regenerated chitosans did not change significantly (Figure 2 and Table 1). Thus, the decreases of I020 and I110 of regenerated chitosans should be structure change. On the other hand, the X-ray spectra among regenerated chitosans were similar, which suggest that the same regeneration process including precipitation, washing, neutral and freeze drying might cause the similar structure of regenerated chitosans.
Figure 3.
X-ray diffraction spectra of chitosans and regenerated chitosans. Samples O-1, R-1, R-2, O-2, R-3, and R-4, respectively, are described in Table 1.
Results in Table 2 shows that CrI110 decreased from 80.99% of O-1 to 66.39% and 62.33% for R-1 and R-2, respectively, and decreased from 72.53% of O-2 to 65.71% and 59.11% for R3 and R4, respectively. Results indicated that the regenerated chitosans had a lower crystallinity index than the original chitosans.
Table 2.
Crystalline characteristics of various chitosan and regenerated chitosan products.
| Product | 2θ (degree) | CrI110 (%) | ||
|---|---|---|---|---|
| O-1 | 10.00 | 19.94 | 22.02 | 81.0 ± 0.6 a |
| R-1 | 10.06 | 19.96 | - | 66.4 ± 0.7 b |
| R-2 | 10.02 | 19.94 | - | 62.3 ± 1.2 c |
| O-2 | 10.08 | 20.24 | - | 72.5 ± 0.6 x |
| R-3 | 10.12 | 20.20 | - | 65.7 ± 1.1 y |
| R-4 | 10.10 | 20.20 | - | 59.1 ± 0.2 z |
a–c Reflect mean values (n = 3) and followed by the difference superscripts within the 61.7% DD chitosan are significantly different (p < 0.05); x–z Reflect mean values (n = 3) and followed by the difference superscripts within the 94.9% DD chitosan are significantly different (p < 0.05). Samples O-1, R-1, R-2, O-2, R-3, R-4, respectively, are described in Table 1.
At I110, the crystallinity intensity of R-1 and R-2 those regenerated from O-1 also R-3 and R-4 that regenerated from O-2 were close. However, the crystallinity intensity of O-1 and O-2 are different from each other; furthermore, they are different from the regenerated product derived from them. The difference in I110 between O-1 and O-2 should be due to the deacetylation process. The similarity in I110 among R-1, R-2, R-3, and R-4 may be due to similar processes of dissolving by acetic acid and alkali precipitation, then washing, neutral, freeze drying, which therefore, end up with a similar molecular arrangement and, thus, similar crystallinity intensity.
3. Experimental Section
3.1. Materials
α-Chitin was purchased from the OHKA enterprises Co., Ltd. (Kaohsiung, Taiwan). Acetic acid, sodium acetate, sodium azide, sodium chloride, and potassium bromide were purchased from the Sigma-Aldrich Co. (St. Louis, MO, USA). Hydrochloric acid and sodium hydroxide were purchased from Merck and Co., Inc. (Darmstadt, Germany). Pullulan standards (for SE-HPLC calibration) were purchased from Showa Denko (Tokyo, Japan).
3.2. Preparation of Chitosan
Chitin powder was passed though sieves of 40–60 mesh, and then was alkali-treated (50% NaOH) at 140 °C for 1 or 3 h to get different DD chitosan. Chitosan was washed until neutral and dried at 50 °C to get the final product [5].
3.3. Preparation of Regenerated Chitosan
The modified procedure of Chen and Liu [9] was used to prepare the regenerated chitosans. Chitosans with different DD were dissolved in 0.1 or 1.0 M acetic acid to make 1% solutions, stirred for 10 h then filtered through filter paper (Toyo No. 1, 90 mm, Toyo Roshi Kaisha, Tokyo, Japan) to remove insoluble materials. One liter of 1% chitosan-acetic acid solution was added to two liters of 8 N NaOH solution to precipitate the chitosan. The precipitates were collected with a 325-mesh sieve and washed with distilled water until neutral. The precipitates were freeze dried to obtain the product that was ground and sieved through a 40–60 mesh size to get regenerated chitosans.
3.4. Determination of DD
Infrared spectrometry was used to determine the DD of the chitosan or regenerated chitosan [38]. Chitosan or regenerated chitosan powder was sieved through a 200 mesh and then mixed with KBr (1:100), dried at 60 °C for 3 days to prevent interference of the effect of water molecules on the peak of hydroxyl band in FTIR measurements, and pressed into a pellet. The absorbance of amide I (1655 cm−1) and the hydroxyl band (3450 cm−1) were measured using a Bio-Rad FTS-155 infrared spectrophotometer (Hercules, CA, USA). The band of the hydroxyl group at 3450 cm−1 was used as an internal standard to correct for disc thickness and for differences in chitosan concentration when making the KBr disc. Triplicate measurements were averaged and used to calculate the DD using the following equation:
| DD = 100 − (A1655/A3450) × 115 | (1) |
here, A1655 and A3450 were the absorbance at 1655 and 3450 cm−1, respectively.
3.5. Determination of Molecular Weight and PDI
The weight-average molecular weight (Mw), number-average molecular weight (Mn) and polydispersity index (PDI = Mw/Mn) of samples were measured by size exclusion high performance liquid chromatography (SE-HPLC) [14]. A column (7.8 mm × 30 cm) packed with TSK gel G4000 PWXL and G5000 PWXL (Tosoh Co., Ltd., Tokyo, Japan) was used. The mobile phase consisted of 0.2 M acetic acid/0.1 M sodium acetate and 0.008 M of sodium azide. A sample concentration of 0.1% (w/v) was loaded and eluted with a flow rate of 0.6 mL/min by an LDC Analytical ConstaMetric 3500 pump (Thermo Scientific, Waltham, MA, USA). The elute peak was detected by an RI detector (Gilson model M132, Gilson, Middleton, WI, USA). The data were analyzed by Chem-Lab software (SISC 3.0, Scientific Information Service, Taipei, Taiwan). Pullulan standards with different Mr values were used as markers. The Mr values of the samples were calculated from the pullulan calibration curve with Chem-Lab software.
3.6. Determination of Bulk Density
Chitosan or regenerated chitosan powder was assayed for its bulk density as described by Cho et al. [39]. One gram of chitosan or regenerated chitosan (40–60 mesh particle size) was placed in a 15-mL tapered graduated centrifuge tube, vibrated on a vortex mixer for 1 min, and packed by gently tapping the tube on the bench top 10 times. The volume of the sample was recorded. The procedure was repeated three times for each sample, and the bulk density was computed as grams per milliliter of the sample.
3.7. Determination of Crystallinity
The crystallinity of chitosan or regenerated chitosan was measured by a Miniflex Rigaku X-ray diffractrometer, using Ni filtered Cu Ka radiation generated at 30 kV and 10 mA at a scanning speed of 2° 2θ/min within a range from 5° to 30°. The crystallinity index was also used, based on the method proposed by Zhang et al. [37]. It consisted of measuring the maximum peak intensity, I110, at 2θ = 20° of the (110) lattice diffraction and that of the amorphous diffraction, Iam, at 2θ = 16°. The crystallinity index (CrI110) was calculated using the following formula:
| CrI110 = [(I110 − Iam)/I110] × 100 | (2) |
3.8. Determination of Ash
The ash content was deduced from the difference in weight before and after a thermal treatment of the product in an electric furnace. The crucible containing the dry sample was placed in an electric furnace at 600 °C for 6 h. Ash content was estimated by ignition of a chitosan or regenerated chitosan sample in an electric furnace and quantization of the ash by gravimetric analysis [40].
3.9. Statistical Analysis
All experiments were carried out in triplicate and average values or means (standard deviations) reported. Mean separation and significance for correlation were analyzed using the SPSS (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA) software package.
4. Conclusions
Higher DD of original chitosan and/or higher concentration of acetic acid used resulted in higher purity, however, the bulk density, Mw, PDI, and crystallinity intensity of regenerated chitosan were not affected.
The regeneration process could increase the purity, manipulate the Mw and narrow down the PDI of regenerated chitosan and lower the crystallinity intensity, and bulk density, whereas the DD, structure and functional groups on chitosan molecules were preserved. These changes would be beneficial to chitosan for use in the biomedical field. Therefore, the regeneration process has a bright future in the developing areas of medical applications.
Acknowledgments
The authors wish to express their appreciation for the financial support from the Ministry of Science and Technology, R.O.C. (MOST 103-2313-B-019-003-MY3). Thanks to Sinn Wei Chen (Department of Chemical Engineering, National Tsing Hua University, Taiwan) and Ming-Tsung Yen (Department of Applied Life Science and Health, Chia Nan University of Pharmacy and Science, Taiwan) discussed the crystalline characteristics of original and regenerated chitosans.
Author Contributions
Chu Hsi Hsu performed experiments, analyzed datum and wrote manuscript. Szu Kai Chen and Wei Yu Chen performed partial experiments. Min Lang Tsai and Rong Huei Chen designed and supervised the study as well as guided the manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alishahi A., Aïder M. Applications of chitosan in the seafood industry and aquaculture: A review. Food Bioprocess Technol. 2012;5:817–830. doi: 10.1007/s11947-011-0664-x. [DOI] [Google Scholar]
- 2.Harish Prashanth K.V., Tharanathan R.N. Chitin/chitosan: modifications and their unlimited application potential: An overview. Trends Food Sci. 2007;18:117–131. [Google Scholar]
- 3.Honarkar H., Barikani M. Applications of biopolymers I: Chitosan. Monatsh. Chem. 2009;140:1403–1420. doi: 10.1007/s00706-009-0197-4. [DOI] [Google Scholar]
- 4.Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 5.Chang J.S., Chang K.L.B., Tsai M.L. Liquid-crystalline behavior of chitosan in malic acid. J. Appl. Polym. Sci. 2007;105:2670–2675. doi: 10.1002/app.26475. [DOI] [Google Scholar]
- 6.Chang H.W., Lin Y.S., Tsai Y.D., Tsai M.L. Effects of chitosan characteristics on the physicochemical properties, antibacterial activity, and cytotoxicity of chitosan/2-glycerophosphate/nanosilver hydrogels. J. Appl. Polym. Sci. 2013;127:169–176. doi: 10.1002/app.37855. [DOI] [Google Scholar]
- 7.Chang Y.L., Liu T.C., Tsai M.L. Selective isolation of trypsin inhibitor and lectin from soybean whey by chitosan/tripolyphosphate/genipin co-crosslinked beads. Int. J. Mol. Sci. 2014;15:9979–9990. doi: 10.3390/ijms15069979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai M.L., Bai S.W., Chen R.H. Cavitation effects versus stretch effects resulted in different size and polydispersity of ionotropic gelation chitosan-sodium tripolyphosohate nanoparticle. Carbohydr. Polym. 2008;71:448–457. doi: 10.1016/j.carbpol.2007.06.015. [DOI] [Google Scholar]
- 9.Chen R.H., Liu C.S. Effect of recovery methods and conditions on the yield, solubility, molecular weight, and creep compliance of regenerated chitosan. J. Appl. Polym. Sci. 2002;84:193–202. doi: 10.1002/app.10296. [DOI] [Google Scholar]
- 10.Hirano S. Production and application of chitin and chitosan in Japan. In: Skjåk-Bræk G., Anthonsen T., Sandford P., editors. Chitin and Chitosan Sources, Chemistry, Biochemistry, Physical Properties and Application. Elsevier Applied Science; London, UK: 1989. pp. 34–43. [Google Scholar]
- 11.Hattori H., Ishihara M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015;10 doi: 10.1088/1748-6041/10/1/015014. [DOI] [PubMed] [Google Scholar]
- 12.Scherliess R., Buske S., Young K., Weber B., Rades T., Hook S. In vivo evaluation of chitosan as an adjuvant in subcutaneous vaccine formulations. Vaccine. 2013;31:4812–4819. doi: 10.1016/j.vaccine.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 13.De Moura C.M., de Moura J.M., Soares N.M., de Almeida Pinto L.A. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. Chem. Eng. Process. 2011;50:351–355. doi: 10.1016/j.cep.2011.03.003. [DOI] [Google Scholar]
- 14.Lee W.K., Tsai M.L., Shieh Y.T. Fractionation of chitosan by supercritical carbon dioxide/acetic acid aqueous solution. J. Supercrit. Fluids. 2012;71:86–91. doi: 10.1016/j.supflu.2012.07.012. [DOI] [Google Scholar]
- 15.Tsaih M.L., Tseng L.Z., Chen R.H. Effects of removing small fragment with ultrafiltration treatment and ultrasonic conditions on degradation kinetics of chitosan. Polym. Degrad. Stab. 2004;86:25–32. doi: 10.1016/j.polymdegradstab.2003.10.015. [DOI] [Google Scholar]
- 16.Balassa L.L., Prudden J.F. Application of chitin and chitosan in wound-healing acceleration. In: Muzzarelli R.A.A., Pariser E.R., editors. Proceedings of The First International Conference on Chitin/Chitosan; Cambridge, MA, USA: MIT Sea Grant Information Center; 1978. pp. 296–304. [Google Scholar]
- 17.Kifune K. Biocompatibility of regenerated chitin, porous material. In: Tokura S., Azuma I., editors. Chitin Derivatives in Life Science. Japanese Society for Chitin/Chitosan; Sapporo, Japan: 1992. pp. 40–44. [Google Scholar]
- 18.Seo H., Kinemura Y. Preparation and some properties of chitosan porous beads. In: Skjåk-Bræk G., Anthonsen T., Sandford P., editors. Chitin and Chitosan Sources, Chemistry, Biochemistry, Physical Properties and Application. Elsevier Applied Science; London, UK: 1989. pp. 585–588. [Google Scholar]
- 19.Seo H., Itoyama K., Fukasawa M., Tokura S. Application of regenerated chitosan as biomedical materials. In: Tokura S., Azuma I., editors. Chitin Derivatives in Life Science. Japanese Society for Chitin/Chitosan; Sapporo, Japan: 1992. pp. 32–39. [Google Scholar]
- 20.Seo H., Mitsuhshi K., Tanibe H. Antibacterial and antifungal fiber blended by chitosan. In: Brine C.J., Sandford P.A., Zikakis J.P., editors. Advances in Chitin and Chitosan. Elsevier Applied Science; London, UK: 1992. pp. 34–40. [Google Scholar]
- 21.Trung T.S. Ph.D. Thesis. Asian Institute of Technology; Pathumthani, Thailand: 2003. Value Adding Physico-chemical Modifications of Shrimp Chitosan. [Google Scholar]
- 22.Sannan T., Kurita K., Iwakura Y. Studies on chitin.1. Solubility change by alkaline treatment and film casting. Makromol. Chem. 1975;176:1191–1195. [Google Scholar]
- 23.Sannan T., Kurita K., Iwakura Y. Studies on chitin.2. Effect of deacetylation on solubility. Makromol. Chem. 1976;177:3589–3600. doi: 10.1002/macp.1976.021771210. [DOI] [Google Scholar]
- 24.Shirai A., Takahashi K., Rujiravanit R., Nishi N., Tokura S. Regeneration of chitin using new solvent system. In: Zakaria M.B., Muda W.M.W., Abdullah M.P., editors. Chitin and Chitosan: The Versatile Environmentally Friendly Modern Materials. Universiti Kebangsaan Malaysia; Bangi, Malaysia: 1995. pp. 53–60. [Google Scholar]
- 25.Tokura S., Nishi N. Specification and characterization of chitin and chitosan. In: Zakaria M.B., Muda W.M.W., Abdullah M.P., editors. Chitin and Chitosan: The Versatile Environmentally Friendly Modern Materials. Universiti Kebangsaan Malaysia; Bangi, Malaysia: 1995. pp. 68–86. [Google Scholar]
- 26.Dutkiewicz J., Jukiewicz L., Papiewski A., Kucharska M., Ciszewski R. Some used of kill chitosan as biomaterial. In: Skjåk-Bræk G., Anthonsen T., Sandford P., editors. Chitin and Chitosan Sources, Chemistry, Biochemistry, Physical Properties and Application. Elsevier Applied Science; London, UK: 1989. pp. 719–730. [Google Scholar]
- 27.Trung T.S., Ng C.H., Stevens W.F. Characterization of decrystallized chitosan and its application in biosorption of textile dyes. Biotechnol. Lett. 2003;25:1185–1190. doi: 10.1023/A:1024562900548. [DOI] [PubMed] [Google Scholar]
- 28.Trung T.S., Thein-Han W.W., Qui N.T., Ng C.H., Stevens W.F. Functional characteristics of shrimp chitosan and its membranes as affected by the degree of deacetylation. Bioresour. Technol. 2006;97:659–663. doi: 10.1016/j.biortech.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Rinaudo M., Pavlo G., Desbrieres J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer. 1999;40:7029–32. doi: 10.1016/S0032-3861(99)00056-7. [DOI] [Google Scholar]
- 30.Chen R.H., Chen W.Y., Wang S.T., Hsu C.H., Tsai M.L. Changes in the Mark-Houwink hydrodynamic volume of chitosan molecules in solutions of different organic acids, at different temperatures and ionic strengths. Carbohydr. Polym. 2009;78:902–907. doi: 10.1016/j.carbpol.2009.07.027. [DOI] [Google Scholar]
- 31.Chen R.H., Chang J.R., Shyur J.S. Effects of ultrasonic conditions and storage in acidic solutions on changes in molecular weight and polydispersity of treated chitosan. Carbohydr. Res. 1997;299:287–294. doi: 10.1016/S0008-6215(97)00019-0. [DOI] [Google Scholar]
- 32.Domszy J.G., Roberts G.A.F. Evaluation of infrared spectroscopic techniques for analysing chitosan. Makromol. Chem. 1985;186:1671–1677. doi: 10.1002/macp.1985.021860815. [DOI] [Google Scholar]
- 33.Dong Y., Xu C., Wang J., Wu Y., Wang M., Ruan Y. Influence of degree of deacetylation on critical concentration of chitosan/dichloroacetic acid liquid crystalline solution. J. Appl. Polym. Sci. 2002;83:1204–1208. doi: 10.1002/app.2286. [DOI] [Google Scholar]
- 34.Qin C., Li H., Xiao Q., Liu Y., Zhu J., Du Y. Water solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006;63:367–374. doi: 10.1016/j.carbpol.2005.09.023. [DOI] [Google Scholar]
- 35.Webster A., Osifo P.O., Neomagus H.W.J.P., Grant D.M. A comparison of glycans and polyglycans using solid-state NMR and X-ray powder diffraction. Solid State Nucl. Magn. Reson. 2006;30:150–161. doi: 10.1016/j.ssnmr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa K. Effect of heating an aqueous suspension of chitosan on crystalline, and polymorphism. Agric. Biol. Chem. 1991;55:2375–2379. doi: 10.1271/bbb1961.55.2375. [DOI] [Google Scholar]
- 37.Zhang Y., Xue C., Xue Y., Gao R., Zhang X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005;340:1914–1917. doi: 10.1016/j.carres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Baxter A., Dillon M., Taylor K.D.A., Roberts G.A.F. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992;14:166–169. doi: 10.1016/s0141-8130(05)80007-8. [DOI] [PubMed] [Google Scholar]
- 39.Cho Y.I., No H.K., Meyers S.P. Physico-chemical characteristics and functional properties of various commercial chitin and chitosan products. J. Agric. Food Chem. 1998;46:3839–3843. doi: 10.1021/jf971047f. [DOI] [Google Scholar]
- 40.AOAC . Official Methods of Analysis. 15th ed. Association of Official Analytical Chemists; Arlington, VA, USA: 1990. p. 777. [Google Scholar]