Abstract
In continuation of our endeavor towards the development of potent and effective antimicrobial agents, three series of halophenyl bis-hydrazones (14a–n, 16a–d, 17a and 17b) were synthesized and evaluated for their potential antibacterial, antifungal and antimycobacterial activities. These efforts led to the identification of five molecules 14c, 14g, 16b, 17a and 17b (MIC range from 0.12 to 7.81 μg/mL) with broad antimicrobial activity against Mycobacterium tuberculosis; Aspergillus fumigates; Gram positive bacteria, Staphylococcus aureus, Streptococcus pneumonia, and Bacillis subtilis; and Gram negative bacteria, Salmonella typhimurium, Klebsiella pneumonia, and Escherichia coli. Three of the most active compounds, 16b, 17a and 17b, were also devoid of apparent cytotoxicity to lung cancer cell line A549. Amphotericin B and ciprofloxacin were used as references for antifungal and antibacterial screening, while isoniazid and pyrazinamide were used as references for antimycobacterial activity. Furthermore, three Quantitative Structure Activity Relationship (QSAR) models were built to explore the structural requirements controlling the different activities of the prepared bis-hydrazones.
Keywords: synthesis, antimicrobial activity, halophenyl bis-hydrazones, antimycobacterial, 2D-QSAR
1. Introduction
Despite the harmful impact of microbial threats to public health, large pharmaceutical companies have left the area of antimicrobial discovery and the number of scientists involved in the search for novel broad antimicrobial leads was reduced dramatically [1]. Unfortunately, the vast discoveries of new potent antimicrobial agents in the 1950s and 1960s led to over-confidence of eradication of the infectious diseases [2]. Moreover, only few classes of antibiotics (oxazolidinone, ketolide and lipoglycopeptide) were produced in the last 50 years, since the introduction of nalidixic acid in 1962. However, all of the antibacterial agents that have entered the market during this period were just modifications of existing molecules [2,3]. In the absence of an effective platform for antibiotic discovery [3] and overuse of antibiotics in humans and animals [4,5], bacteria exploited this opportunity by progressively developing resistance to most of the used antibiotics. Therefore, there is a great need to develop novel and effective antimicrobial and antimycobacterial drugs to combat this resistance.
Tuberculosis (TB) is a serious continuing major global health crisis and is ranked as the second leading cause of worldwide death among infectious diseases [6]. Nearly, 8.6 million people infected with Mycobacterium tuberculosis and 1.3 million died from the disease (including 320,000 deaths among HIV-positive people) in 2012, according to World Health Organization (WHO) global tuberculosis report 2013 [6]. The disease is aggravated by the worldwide continuous emergence of multidrug-resistant strains of M. tuberculosis (MDR-TB), extensively drug-resistant tuberculosis (XDR-TB) and totally drug-resistant tuberculosis (TDR-TB) [7,8]. The concern that tuberculosis may again become an incurable disease has increased nowadays, due to the magnitude and extent of drug-resistant strains [9,10]. Moreover, the longer duration of TB therapy and the increasing incidences of tuberculosis in immunocompromised patients emphasize the urgent need for discovery of new lead compounds to extend the range of effective TB treatment options [11,12,13].
Owing to their significant biological and pharmacological profiles, hydrazides and hydrazones have stood out as a prime scaffold for the discovery of innovative therapies. They were reported to possess antibacterial–antifungal [14,15], anticonvulsant [16], anti-inflammatory [17], antimalarial [18] and antitubercular activities [19,20,21,22]. Nifuroxazide, salinazid and verazide are examples of clinically approved hydrazone-containing antibacterial and antimycobacterial drugs [23,24]. Moreover, isonicotinoyl hydrazide (INH) and its isonicotinoyl hydrazone analogs, related to the hydrazide class, are well-established antitubercular agents [25,26,27]. INH also exhibits bacteriostatic effects on bacillus [28]. Additionally, several research groups have pointed out the importance of halogens incorporation in different scaffolds for the antitubercular activity enhancement [29,30]. In view of the antimicrobial property of the hydrazone pharmacophore, it was envisaged that its combined effect with an active moiety, such as benzothiazole and benzofuran of reported significant antitubercular activity [31,32,33,34], may result in increased antitubercular and antimicrobial activities.
With regard to anti-TB agents, and to antibacterials generally, the limitations of target-based screening approach as a tool of drug discovery paved the way for the resurgence of interest in the use of phenotypic screening in antimicrobial hits discovery [1]. So after extensive literature search and in continuation of our research work on hydrazides and hydrazones as antimicrobial agents [35,36], it was contemplated to synthesize some novel (hydrazono)propane hydrazonoyl chlorides (14a–n), ((hydrazono)-1-(phenylsulfonyl/tosyl)propan-2-ylidene) benzohydrazide/benzo[d]thiazole-2-carbohydrazides (16a–d) and ((hydrazono)-4-phenyl-1-(piperidin-1-yl)but-3-en-2-ylidene)benzohydrazide/benzofuran-2-carbohydrazides (17a,b), and evaluate their antimicrobial and antitubercular activity. In addition, we described three valid 2D-QSAR models in order to explore the structural requirements controlling these observed antibacterial and antitubercular activities.
2. Results and Discussion
2.1. Chemistry
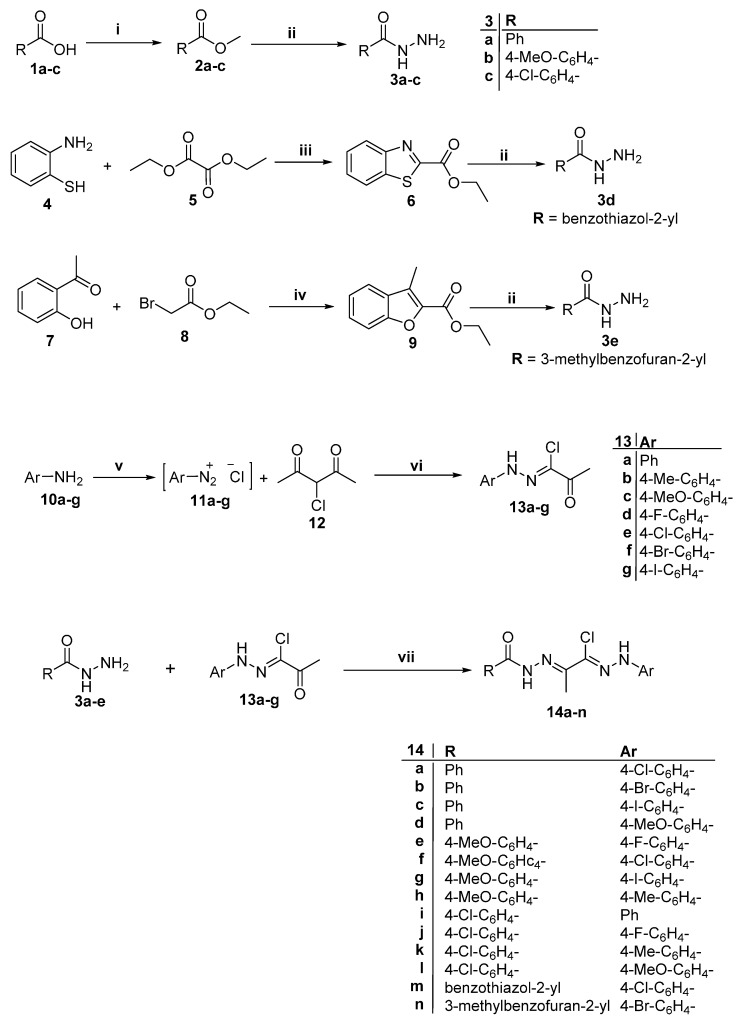
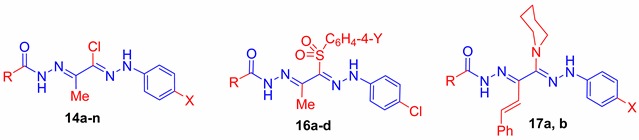
In an extension of our ongoing efforts towards developing potent antimicrobial agents [35,36,37,38,39], we synthesized eighteen novel bis-hydrazone derivatives bearing different aryl and heteroaryl rings. The synthetic route was initiated with the preparation of carbohydrazides 3a–c [40], benzo[d]thiazole-2-carbohydrazide 3d [35] and 3-methylbenzofuran-2-carbohydrazide 3e [36] (Scheme 1). Diazodization of aromatic amines 10a–g with hydrochloric acid and sodium nitrite gave diazonium salts 11a–g, which were subsequently coupled with 3-chloropentane-2,4-dione 12 in ethanolic sodium acetate (Japp-Klingemann reaction) and afforded oxo-N-arylpropanehydrazonoyl chlorides 13a–g, respectively [41] (Scheme 1). The reaction of carbohydrazides 3a–e with 2-oxo-N'-(4substitutedphenyl)propanehydrazonoyl chloride 13a–g in refluxing tetrahydrofuran (THF) afforded bis-hydrazones 14a–n (Scheme 1).
Scheme 1.
Synthesis of compounds 14a–n. Reagents and conditions: i, MeOH/H2SO4/reflux 8 h; ii, NH2NH2.H2O/EtOH/reflux 4 h; iii, Neat, reflux 6 h; iv, MeOH/MeONa/reflux 8 h; v, HCl/NaNO2/H2O/0–5 °C; vi, CH3COONa/EtOH/0–5 °C; and vii, THF/reflux 10 h.
The Fourier transform infrared spectroscopy (FTIR) of bis-hydrazones 14a–l showed the presence of stretching vibrations of carbonyl group in the region 1667–1683 cm−1 in addition to the absorption bands of two NH functions in the region 3167–3404 cm−1. In the 1H NMR spectra two NH groups showed two D2O exchangeable signals in the regions δ: 10.00–10.27 and δ: 10.64–10.90 ppm, in addition to the singlet signal of the methyl group in the region δ: 2.29–2.38 ppm. Recently, we reported the X-ray diffraction structure for an analogue of compounds 14a–n, which confirmed the (1Z,2E)-configuration of these bis-hydrazones in solid state [36].
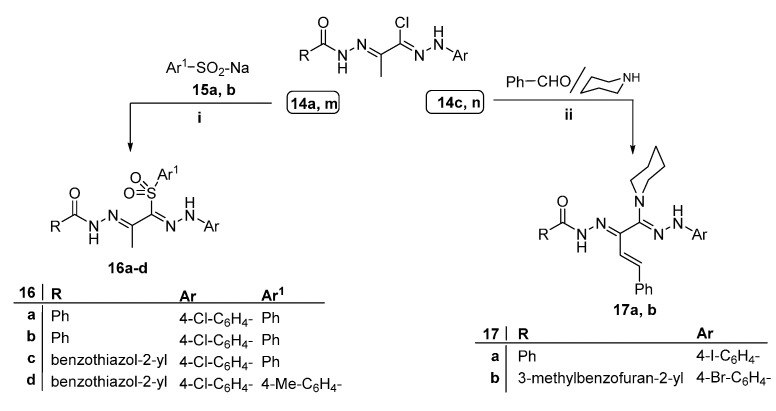
Arylsulfones are a promising class of antimicrobial agents [42,43]. To explore the influence of incorporating the sulfone moiety in the bis-hydrazone scaffold on the antimicrobial activity, some derivatives of the first series 14a and 14m have been selected for the synthesis of new bis-hydrazones bearing a phenylsulfone and tosyl moieties. Thus, reaction of 14a or 14m with sodium benzenesulfinate 15a or sodium p-methylbenzenesulfinate 15b afforded the corresponding sulfones 16a–d, respectively (Scheme 2). The IR spectra of 16a–d exhibited characteristic absorption band at 1639–1684 cm−1 due to acetyl C=O, while that of the sulfonyl functionality was observed in the regions 1134–1137 and 1247–1290 cm−1. Their 1H NMR spectra exhibited the two D2O-exchangeable signal of hydrazone NH at δ 11.67–13.39 ppm for =NNH– and δ 14.17–14.58 ppm for –CONH–. The compounds 16a and 16d showed characteristic 13C NMR signals at 15.06 and 13.00 ppm corresponding to the carbon of methyl groups and signals at 165.28 and 167.35 ppm attributed to the carbons of carbonyl function, respectively.
Scheme 2.
Synthesis of 16a–d and 17a,b. Reagents and conditions: i, EtOH/reflux 10 h; and ii, EtOH/reflux 15 h.
The formation of piperidine derivatives 17a and 17b was accomplished by heating each of bis-hydrazones 14c or 14n with piperidine in ethanol, which resulted in elimination of hydrogen chloride and the formation of a single product, in each case. The 1H NMR of 17a and 17b revealed the protons of piperidine moiety in the regions δ 1.56–1.64 and 3.21–3.28 ppm while the 13C NMR of 17b showed the signals of five sp3 carbons at around 23.95 and 46.60 ppm, respectively.
2.2. Anti-Microbial Activity
Antibacterial and antifungal activities were performed at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. Initially, target compounds 14a–n and reference drugs were evaluated in vitro for their antibacterial and antifungal activity, by inhibition zone technique and minimum inhibitory concentration (MIC), using one fungi: A. fumigatus (RCMB 02568), three Gram positive bacteria: S. aureus (RCMB 010028) S. pneumonia (RCMB 010010) and B. subtilis (RCMB 010069), four Gram-negative bacteria: P. aeruginosa (RCMB 010043), S. typhimurium (RCMB 010315), K. pneumonia (RCMB 0010093) and E. coli (RCMB 010052) with the addition of M. tuberculosis (RCMB 010126), For the optimization purpose, the inactive and moderate active agents, 14a, 14m and 14n were selected for further modification, hoping to increase the antimicrobial as well as the antimycobacterial activities. Compounds 14a and 14m modified to the sulfone derivatives 16a, 16b and 16c, 16d, respectively, while compound 14n optimized to the piperidine derivative 17b. Encouraged by the promising results of these modified analogs, the most active compound 14c was further modified to the piperidine counterpart 17a.
2.2.1. Anti-Fungal Activity
Aspergillus fumigatus is largely responsible for increased the incidence of invasive aspergillosis (IA) in immunocompromised patients [44]. Moreover, the mortality rate due to invasive fungal diseases is still unacceptable high, because of a limited number of antifungal agents [45].
Data in Table 1 and Table 2 revealed that compounds 14c, 14e, 14g, 16b, 17a and 17b showed a remarkable activity against Aspergillus fumigatus.
Table 1.
Antimicrobial activities of the synthesized bis-hydrazones against the pathological organisms expressed as inhibition diameter zones in millimeters (mm) based on well diffusion assay.
| Comp. | R | X or Y | Fungi | Gram Positive Bacteria | Gram Negative Bacteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Af | Sa | Sp | Bs | Pa | St | Kp | Ec | |||
| 14a | Ph- | Cl | NA | NA | NA | NA | NA | NA | NA | NA |
| 14b | Ph- | Br | NA | 13.9 ± 0.44 | 12.4 ± 0.63 | 14.8 ± 0.58 | NA | 15.1 ± 0.58 | 16.4 ± 0.72 | 10.4 ± 0.44 |
| 14c | Ph- | I | 21.2 ± 0.25 | 21.9 ± 0.44 | 23.2 ± 0.37 | 23.9 ± 0.25 | 17.6 ± 0.25 | 20.4 ± 0.63 | 19.2 ± 0.25 | 20.7 ± 0.63 |
| 14d | Ph- | OMe | 11.2 ± 0.32 | 9.4 ± 0.58 | 11.4 ± 0.72 | 13.2 ± 0.72 | NA | 11.6 ± 0.25 | 13.7 ± 0.44 | 10.2 ± 0.58 |
| 14e | 4-MeOC6H4- | F | 19.7 ± 0.32 | 20.1 ± 0.58 | 20.9 ± 0.58 | 20.2 ± 0.32 | NA | 21.3 ± 0.58 | 19.2 ± 0.24 | 20.4 ± 0.58 |
| 14f | 4-MeOC6H4- | Cl | 16.9 ± 0.58 | 17.1 ± 0.58 | 18.8 ± 0.44 | 20.6 ± 0.44 | NA | 17.8 ± 0.63 | 16.4 ± 0.37 | 18.5 ± 0.58 |
| 14g | 4-MeOC6H4- | I | 21.4 ± 0.44 | 20.8 ± 0.58 | 22.3 ± 0.63 | 23.2 ± 0.58 | 15.3 ± 0.25 | 22.4 ± 0.44 | 19.9 ± 0.25 | 21.3 ± 0.44 |
| 14h | 4-MeOC6H4- | Me | 15.6 ± 0.25 | 17.2 ± 0.44 | 17.8 ± 0.37 | 19.9 ± 0.25 | NA | 20.3 ± 0.63 | 18.6 ± 0.25 | 19.7 ± 0.63 |
| 14i | 4-ClC6H4- | H | NA | NA | NA | NA | NA | NA | NA | NA |
| 14j | 4-ClC6H4- | F | NA | NA | NA | NA | NA | NA | NA | NA |
| 14k | 4-ClC6H4- | Me | 15.7 ± 0.58 | 16.9 ± 0.37 | 15.6 ± 0.25 | 17.2 ± 0.58 | NA | 19.1 ± 0.63 | 16.5 ± 0.63 | 18.1 ± 0.72 |
| 14l | 4-ClC6H4- | OMe | 15.3 ± 0.25 | 18.4 ± 0.58 | 16.2 ± 0.63 | 17.3 ± 0.58 | NA | 17.9 ± 0.58 | 18.2 ± 0.72 | 15.8 ± 0.63 |
| 14m | Benzothiazol-2-yl | Cl | NA | 16.2 ± 0.63 | 14.7 ± 0.44 | 15.3 ± 0.44 | NA | 18.6 ± 0.58 | 19.8 ± 0.58 | 18.6 ± 0.44 |
| 14n | 3-Methylbenzofuran-2-yl | Br | NA | NA | NA | NA | NA | NA | NA | NA |
| 16a | Ph- | H | 14.3 ± 0.37 | 17.2 ± 0.63 | 16.8 ± 0.44 | 18.3 ± 0.44 | NA | 16.8 ± 0.25 | 13.4 ± 0.44 | 15.2 ± 0.63 |
| 16b | Ph- | Me | 21.2 ± 0.58 | 22.3 ± 0.63 | 22.8 ± 0.25 | 24.2 ± 0.25 | 18.9 ± 0.25 | 22.9 ± 0.37 | 21.4 ± 0.58 | 20.7 ± 0.63 |
| 16c | Benzothiazol-2-yl | H | 18.1 ± 0.58 | 14.8 ± 0.58 | 13.8 ± 0.58 | 16.3 ± 0.44 | NA | 19.8 ± 0.25 | 18.7 ± 0.44 | 16.1 ± 0.63 |
| 16d | Benzothiazol-2-yl | Me | 19.1 ± 0.63 | 15.7 ± 0.15 | 17.3 ± 0.18 | 19.8 ± 0.22 | NA | 19.7 ± 0.48 | 16.5 ± 0.37 | 17.6 ± 0.25 |
| 17a | Ph- | I | 20.6 ± 0.58 | 21.3 ± 0.58 | 21.9 ± 0.44 | 23.1 ± 0.44 | 17.2 ± 0.58 | 21.3 ± 0.58 | 20.4 ± 0.37 | 20.6 ± 0.58 |
| 17b | 3-Methylbenzofuran-2-yl | Br | 20.4 ± 0.58 | 20.9 ± 0.44 | 21.3 ± 0.58 | 21.9 ± 0.36 | 19.1 ± 0.44 | 22.4 ± 0.58 | 21.4 ± 0.19 | 22.9 ± 0.58 |
| AB | 19.5 ± 0.21 | nt | nt | nt | nt | nt | nt | nt | ||
| CF | nt | 20.0 ± 0.34 | 20.3 ± 0.58 | 20.4 ± 0.14 | 19.2 ± 0.15 | 19.8 ± 0.63 | 19.7 ± 0.12 | 20.3 ± 0.44 | ||
NA: No Activity; The screening organisms, Mould: Aspergillus fumigatus (RCMB 02568, Af); Gram positive bacteria: Staphylococcus aureus (RCMB 010028, Sa), Streptococus pneumoniae (RCMB 010010, Sp), and Bacillus subtilis (RCMB 010069, Bs); Gram negative bacteria: Pseudomonas aeruginosa (RCMB 010043, Pa), Salmonella typhimurium (RCMB 010315, St), Klebsiella penumoniae (RCMB 0010093, Kp) and Escherichia coli (RCMB 010052, Ec); AB: Amphotericin B; CF: Ciprofloxacin; Comp.: Compound; nt: not tested; Results shown in bold letters indicate more antituberculosis activity of compounds compared to others.
Table 2.
Antimicrobial activities of the tested standards and synthesized compounds as MICs (μg/mL).
| Comp. | Fungi | Gram Positive Bacteria | Gram Negative Bacteria | |||||
|---|---|---|---|---|---|---|---|---|
| Af | Sa | Sp | Bs | Pa | St | Kp | Ec | |
| 14a | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 14b | >125 | 125 | 125 | 62.50 | >125 | 62.50 | 31.25 | 125 |
| 14c | 0.98 | 0.49 | 0.24 | 0.12 | 7.81 | 1.95 | 3.90 | 0.98 |
| 14d | >125 | >125 | 125 | 125 | >125 | 125 | 125 | 125 |
| 14e | 1.95 | 1.95 | 0.98 | 1.95 | >125 | 0.98 | 3.90 | 1.95 |
| 14f | 15.63 | 15.63 | 3.90 | 0.98 | >125 | 7.81 | 15.63 | 3.90 |
| 14g | 0.98 | 0.98 | 0.49 | 0.24 | 62.50 | 0.49 | 1.95 | 0.98 |
| 14h | 31.25 | 31.25 | 7.81 | 1.95 | >125 | 1.95 | 3.90 | 1.95 |
| 14i | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 14j | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 14k | 31.25 | 15.63 | 31.25 | 15.63 | >125 | 3.90 | 15.63 | 7.81 |
| 14l | 62.50 | 7.81 | 31.25 | 15.63 | >125 | 7.81 | 7.81 | 31.25 |
| 14m | >125 | 31.25 | 62.50 | 62.50 | >125 | 3.90 | 1.95 | 3.90 |
| 14n | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 16a | 125 | 15.63 | 15.63 | 7.81 | >125 | 15.63 | 125 | 62.50 |
| 16b | 0.49 | 0.49 | 0.24 | 0.12 | 3.90 | 0.24 | 0.98 | 0.98 |
| 16c | 7.81 | 62.50 | 125 | 31.25 | >125 | 3.90 | 3.90 | 31.25 |
| 16d | 3.90 | 31.25 | 15.63 | 1.95 | >125 | 3.90 | 15.63 | 7.81 |
| 17a | 0.98 | 0.98 | 0.49 | 0.24 | 15.63 | 0.98 | 1.95 | 0.98 |
| 17b | 1.95 | 0.98 | 0.98 | 0.98 | 3.90 | 0.49 | 0.98 | 0.24 |
| AB | 1.95 | nt | nt | nt | nt | nt | nt | nt |
| CF | nt | 1.95 | 1.95 | 1.95 | 3.90 | 3.90 | 3.90 | 1.95 |
The screening organisms, Mould: Aspergillus fumigatus (RCMB 02568, An); Gram positive bacteria: Staphylococcus aureus (RCMB 010028, Sa), Streptococus pneumoniae (RCMB 010010, Sp), and Bacillus subtilis (RCMB 010069, Bs); Gram negative bacteria: Pseudomonas aeruginosa (RCMB 010043, Pa), Salmonella typhimurium (RCMB 010315, St), Klebsiella penumoniae (RCMB 0010093, Kp) and Escherichia coli (RCMB 010052, Ec); AB: Amphotericin B; CF: Ciprofloxacin; Comp.: Compound; nt: not tested; Results shown in bold letters indicate more or equal activity of the compounds compared to the standard drugs.
The sulfone derivative 16b (MIC = 0.49 μg/mL) exhibited the highest potency against Aspergillus fumigatus organism where it was four times more active than the reference drug Amphotericin B (MIC = 1.95 μg/mL), a membrane-active polyene macrolide antibiotic and an antifungal compound [46]. Para-iodo substituted members; 14c, 14g and 17a revealed twofold increase in activity than the reference drug with MIC = 0.98 μg/mL. Compounds 14e and 17b were equipotent to the Amphotericin B.
2.2.2. Antibacterial Activity
As indicated in Table 1 and Table 2, compounds 14c, 14e, 14g, 16b and 17a and 17b displayed broad-spectrum antibacterial activity against the tested Gram positive and Gram negative bacteria as compared with ciprofloxacin. Ciprofloxacin is a broad spectrum widely used antibacterial and is considered the most potent marketed fluoroquinolone against gram-negative bacteria [47].
Regarding Gram positive bacteria, compounds 14c and 16b emerged as the most potent analogs. There were found to be fourfold more active against S. aureus, eightold more active than S. pneumonia and 16 times more active than B. subtilis compared to the standard drug (MIC = 1.95 μg/mL), while 14g and 17a showed twofold increased inhibition against S. aureus, fourfold against S. pneumonia and eightfold against B. subtilis. Compound 17b showed double the activity of ciprofloxacin against the selected Gram positive bacteria. On the other hand, the counterparts, 14e and 14f also displayed double the activity of the standard drug against S. pneumonia and B. subtilis, respectively. Moreover, 14e was equipotent to ciprofloxacin against S. aureus, compounds 14e, 14h and 16d were also equipotent to the standard drug against B. subtilis. Rest of the compounds elicited moderate to fair activity towards Gram positive bacteria.
Concerning Gram negative bacteria, 16b and 17b counterparts (MIC = 3.90 μg/mL) were equipotent to the ciprofloxacin in inhibiting the growth of P. aeruginosa. All the compounds were screened against Salmonella typhimurium to evaluate their anti-typhoid activity. Compound 16b was the most active against S. Typhimurium as it exhibited 16-fold increased activity than ciprofloxacin (MIC = 3.90 μg/mL). Moreover, compounds 14g and 17b were eightfold, 14e and 17a were fourfold while 14c and 14h were twofold more potent than the standard drug. Furthermore, 14k, 14m, 16c and 16d counterparts were equipotent to the reference drug in inhibiting S. Typhimurium.
The activity of all compounds against K. pneumonia was investigated. Highest activity observed in 16b and 17b counterparts, followed by 14g, 14m and 17a.
Compound 17b showed the best activity in inhibiting E. coli, whereas, 14c, 14g, 16b and 17a showed good activity. Finally, 14c, 14e, 14h and 16c were equipotent to ciprofloxacin in inhibiting K. pneumonia, while, compounds 14e and 14h were also equipotent to the reference drug against E. coli.
2.2.3. Antimycobacterial Activity
By investigating the antitubercular activity of a limited focused library of twenty bis-hydrazone derivatives 14a–n, 16a–d, 17a and 17b (Table 3), it was observed that, in general, best activity is found in compounds of higher lipophilicity, as observed by Maccari et al. [48].
Table 3.
Anti-tubercular activities and C logP measurements of bis-hydrazones.
| Comp. | I.Z | MIC | C LogP a |
|---|---|---|---|
| 14a | NA | NA | 5.615 |
| 14b | NA | NA | 5.746 |
| 14c | 18.3 ± 0.25 | 7.81 | 6.02 |
| 14d | NA | NA | 4.994 |
| 14e | NA | NA | 5.157 |
| 14f | NA | NA | 5.672 |
| 14g | 18.4 ± 0.25 | 7.81 | 6.077 |
| 14h | NA | NA | 5.442 |
| 14i | NA | NA | 5.615 |
| 14j | NA | NA | 5.779 |
| 14k | NA | NA | 6.064 |
| 14l | NA | NA | 5.672 |
| 14m | NA | NA | 5.780 |
| 14n | NA | NA | 6.687 |
| 16a | NA | NA | 5.504 |
| 16b | 19.4 ± 0.25 | 3.90 | 5.952 |
| 16c | NA | NA | 5.669 |
| 16d | 11.3 ± 0.37 | 125 | 6.118 |
| 17a | 19.3 ± 0.37 | 3.90 | 8.283 |
| 17b | 20.2 ± 0.19 | 1.95 | 8.69 |
| Isoniazide | nt | 0.40 | −0.969 |
| Pyrazinamide | nt | 3.21 | −0.711 |
a Calculated by http://www.molinspiration.com/; NA: No Activity; MIC: Minimum Inhibition Zone; Comp.: Compound; I.Z.: Inhibition Zone; nt: not tested; Results shown in bold letters indicate more antituberculosis activity of compounds compared to others.
Regarding 14a–n series, compounds 14c and 14g showed a significant antimycobacterial activity (MIC = 7.81 μg/mL). This indicated that the improvement in the activity is linked to the para substitution of iodo-atom linked to N(1) in the hydrazonoyl chloride moiety and the presence of unsubstituted or p-methoxy phenyl at the carbonyl group. Concerning the sulfone derivatives 16a–d and the piperidine analogs 17a and 17b, the antimycobacterial activity was observed in 16b, 17a and 17b with MIC ranged from 1.95–3.90 μg/mL relative to the reference drugs Isoniazide (MIC = 0.40 μg/mL) and Pyrazinamide (MIC = 3.21 μg/mL). The preliminary antitubercular results afford an excellent lead for further development of these molecules as novel antimycobacterial agents.
2.2.4. In Vitro Cytotoxicity
In vitro cytotoxicity of most active antimicrobial and antitubercular compounds 16b, 17a and 17b was evaluated against human lung cancer cell line (A-549) using SRB method. Doxorubicin was used as the reference drug. The IC50 values obtained for these compounds are listed in Table 4. None of the tested compounds displayed any significant cytotoxicity against lung cells (A549), thereby providing a high therapeutic index.
Table 4.
Levels of cytotoxicity induced by selected compounds on A549 cells.
| Compound | IC50 (μM) |
|---|---|
| 16b | 75.3 |
| 17a | >100 |
| 17b | >100 |
| Doxorubicin | 2.3 |
2.3. 2D QSAR Study
2.3.1. Development of QSAR Models
QSAR analyses for antibacterial and antimycobacterial activities of the prepared derivatives (14a–n, 16a–d, 17a and 17b) were performed in order to correlate the biochemical data with the synthesized compounds, and to identify positive and negative structural features within the three series. The analysis was run by means of the DS 2.5 software (Discovery Studio 2.5, Accelrys, Co., Ltd., San Diego, CA, USA).
A set of the newly synthesized bis-hydrazones (14a–n, 16a–d, 17a and 17b) was used as a training set with their measured pMIC against Bacillis subtilis, Klebsiella pneumonia and Mycobacterium tuberculosis for QSAR modeling. “Calculate Molecular Properties” module was used for calculating different molecular properties for the training set compounds. 2D Descriptors involved: AlogP, molecular properties, molecular property counts, surface area and volume and topological descriptors, while the 3D descriptors involved: Dipole, jurs descriptors, principle moments of inertia, shadow indices and surface area and volume. Genetic function approximation (GFA) was utilized to search for the best possible QSAR regression equation capable of correlating the variations in the biological activities of the training compounds with variations in the generated descriptors, i.e., multiple linear regression modeling (MLR) [49]. QSAR model was validated employing leave one-out cross-validation by setting the folds to a number much larger than the number of samples, r2 (squared correlation coefficient value) and r2 prediction (predictive squared correlation coefficient value), residuals between the predicted and experimental activity of the test set and training set.
2.3.2. QSAR Study Results
Equation (1). Represents the best performing QSAR model for the activity against Bacillis subtilis;
| −logMIC = −17.1387 − 0.2620 Jurs_WPSA_3 + 1.7027 Shadow_nu + 1.4314 Shadow_Ylength | (1) |
Equation (2). Represents the best performing QSAR model for the activity against Klebsiella pneumonia;
| −logMIC = 2.6641 + 0.0174 Jurs_WNSA_1 + 0.1523 Shadow_YZ − 2.13292 Shadow_Zlength | (2) |
Equation (3). Represents the best performing QSAR model for the antimycobacterial activity;
| −logMIC= −7.224 − 0.0015 Jurs_PPSA_2 + 0.6156 Shadow_Ylength | (3) |
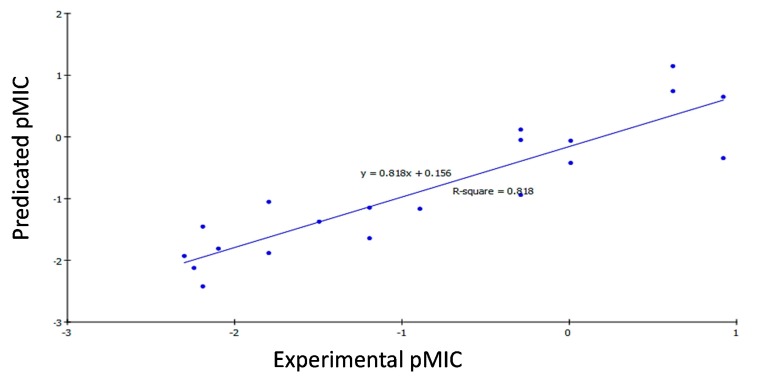
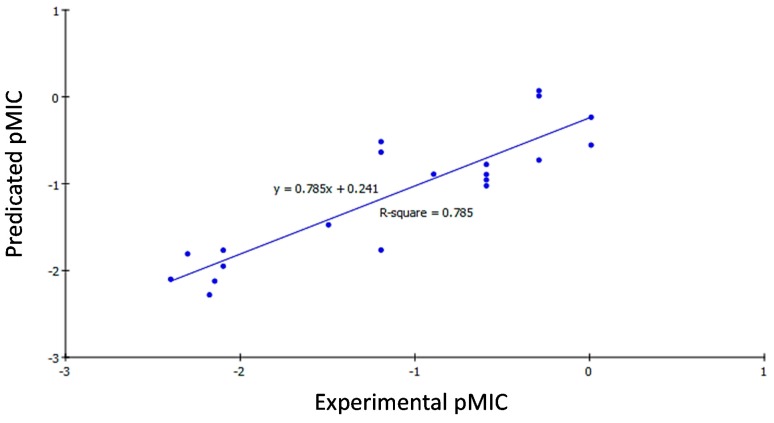
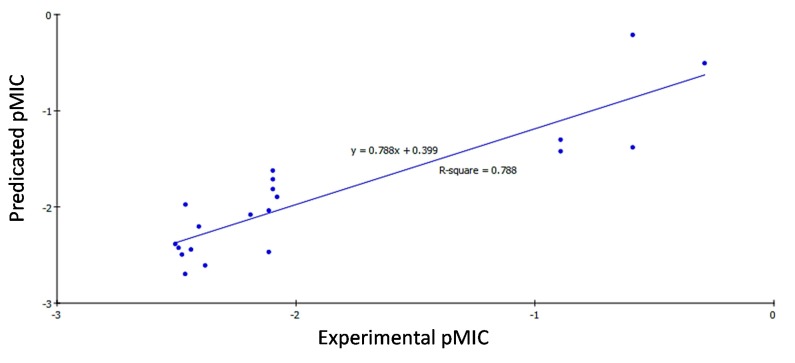
According to Equations (1)–(3), the QSAR models were represented graphically by scattering plots of the experimental versus the predicted bioactivity values −logMIC for the training set compounds as shown in Figure 1, Figure 2 and Figure 3. The method used to build the model was Least-Squares, r2 = 0.818, 0.785 and 0.788, respectively, r2 (adj) = 0.784, 0.745 and 0.763, respectively, r2 (pred) = 0.712, 0.665 and 0.684, respectively, Least-Squared error = 0.2244, 0.1403 and 0.1148, respectively, where r2 (adj) is r2 adjusted for the number of terms in the model; r2 (pred) is the prediction c, equivalent to q2 from a leave-1-out cross validation.
Figure 1.
Predicted versus experimental pMIC of the tested compounds against Bacillis subtilis according to Equation (1) (r2 = 0.818).
Figure 2.
Predicted versus experimental pMIC of the tested compounds against Klebsiella pneumonia according to Equation (2) (r2 = 0.785).
Figure 3.
Predicted versus experimental pMIC of the tested compounds against Mycobacterium tuberculosis according to Equation (3) (r2 = 0.788).
In conclusion, Equations (1)–(3) suggested that the antibacterial activities of the synthesized compounds are mainly affected by jurs descriptors and shadow indices. Jurs descriptors are those ones that combine shape and electronic information to characterize molecules [50].
The descriptors are calculated by mapping atomic partial charges on solvent-accessible surface areas of individual atoms. Jurs_WPSA_3 and Jurs_WNSA_1 are the surface-weighted charged partial surface areas “set of six descriptors (Jurs_WPSA_1, Jurs_WPSA_2, Jurs_WPSA_3, Jurs_WNSA_1, Jurs_WNSA_2 and Jurs_WNSA_3) obtained by multiplying descriptors 1 to 6 by the total molecular solvent-accessible surface area and dividing by 1000”. Jurs_PPSA_2 is atomic charge weighted positive surface area “Sum of the product of solvent-accessible surface area × partial charge for all positively charged atoms”. In addition, shadow indices are set of geometric descriptors to characterize the shape of the molecules [51].
The descriptors are calculated by projecting the model surface on three mutually perpendicular planes: xy, yz, and xz. These descriptors depend not only on conformation, but also on the orientation of the model. To calculate them, the models are first rotated to align the principal moments of inertia with the x-, y-, and z-axes. Shadow_YZ is area of the molecular shadow in the yz plane, Shadow_nu is ratio of largest to smallest dimension, Shadow_Ylength is Length of molecule in the y dimension while, Shadow_Zlength is Length of molecule in the z dimension.
2.3.3. QSAR Validation
Robustness of the established QSAR models (1, 2 and 3) was verified by using; Leave-one-out (LOO) internal validation (r2 = 0.818, 0.785 and 0.788, respectively). Cross-validation was also employed where q2, which is equivalent to r2 (pred), was 0.712, 0.665 and 0.684, respectively. In addition, validation was employed by measuring the residuals between the experimental and the predicted activities of the training set (Table 5). Interestingly, the predicted activities by the QSAR models were very close to those experimentally observed, indicating that these models could be applied for prediction of more effective hits having the same skeletal framework.
Table 5.
Experimental activities of the synthesized derivatives against the predicted activity according to Equations (1)–(3).
| Comp. | Bacillis subtilis | Klebsiella pneumonia | Mycobacterium tuberculosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Activity (pMIC) | Predicted Activity (pMIC) | Residual | Experimental Activity (pMIC) | Predicted Activity (pMIC) | Residual | Experimental Activity (pMIC) | Predicted Activity (pMIC) | Residual | |
| 14a | −2.1903 | −1.4532 | −0.7371 | 2.3010 | −1.8082 | −0.4929 | −2.0792 | −1.9173 | −0.1619 |
| 14b | −1.7959 | −1.0525 | −0.7434 | −1.4949 | −1.4739 | −0.0209 | −2.0969 | −1.7353 | −0.3616 |
| 14c | 0.9208 | −0.3440 | 1.2648 | −0.5911 | −1.0238 | 0.4328 | −0.8927 | −1.4641 | 0.5715 |
| 14d | −2.0969 | −1.8098 | −0.2871 | −2.0969 | −1.9502 | −0.1467 | −2.4771 | −2.5540 | 0.0769 |
| 14e | −0.2900 | −0.9401 | 0.6501 | −0.5911 | −0.9562 | 0.3651 | −2.4065 | −2.2571 | −0.1494 |
| 14f | 0.0088 | −0.4222 | 0.4309 | −1.1940 | −0.5166 | −0.6774 | −2.4624 | −1.9998 | −0.4626 |
| 14g | 0.6198 | 1.1471 | −0.5273 | −0.2900 | 0.0707 | −0.3607 | −0.8927 | −1.3632 | 0.4706 |
| 14h | −0.2900 | 0.1190 | −0.4091 | −0.5911 | −0.7779 | 0.1869 | −2.0969 | −1.9088 | −0.1881 |
| 14i | −2.3010 | −1.9291 | −0.3719 | −2.3979 | −2.1004 | −0.2975 | −2.5052 | −2.3546 | −0.1505 |
| 14j | −2.2430 | −2.1235 | −0.1195 | −2.1461 | −2.1224 | −0.0237 | −2.4393 | −2.3799 | −0.0595 |
| 14k | −1.1940 | −1.6423 | 0.4483 | −1.1940 | −1.7642 | 0.5703 | −2.4914 | −2.3827 | −0.1087 |
| 14l | −1.1940 | −1.1464 | −0.0475 | −0.8927 | −0.8912 | −0.0015 | −2.4639 | −2.6564 | 0.1925 |
| 14m | −1.7959 | −1.8821 | 0.0863 | −0.2900 | −0.7286 | 0.4386 | −2.3802 | −2.5155 | 0.1352 |
| 14n | −2.1903 | −2.4221 | 0.2318 | −2.1761 | −2.2809 | 0.1048 | −2.1139 | −2.4051 | 0.2912 |
| 16a | −0.8927 | −1.1653 | 0.2727 | −2.0969 | −1.7667 | −0.3302 | −2.1139 | −2.1128 | −0.0012 |
| 16b | 0.9208 | 0.6497 | 0.2711 | 0.0088 | −0.5553 | 0.5641 | −0.5911 | −1.4733 | 0.8823 |
| 16c | −1.4949 | −1.3730 | −0.1218 | −0.5911 | −0.8948 | 0.3037 | −2.1903 | −2.0308 | −0.1595 |
| 16d | −0.2900 | −0.0496 | −0.2405 | −1.1940 | −0.6373 | −0.5567 | −2.0969 | −1.5745 | −0.5225 |
| 17a | 0.6198 | 0.7416 | −0.1218 | −0.2900 | 0.0110 | −0.3010 | −0.5911 | −0.2366 | −0.3545 |
| 17b | 0.0088 | −0.0622 | 0.0710 | 0.0088 | −0.2344 | 0.2432 | −0.2900 | −0.3499 | 0.0599 |
3. Experimental Section
3.1. Chemistry
Melting points were measured with a Stuart melting point apparatus and were uncorrected. The NMR spectra were recorded by Varian Gemini-300BB 300 MHz FT-NMR spectrometers (Varian Inc., Palo Alto, CA, USA). 1H and 13C spectra were run at 300 and 75 MHz, respectively, in deuterated dimethylsulfoxide (DMSO-d6). Chemical shifts (δH) are reported relative to TMS as internal standard. All coupling constant (J) values are given in hertz. Chemical shifts (δC) are reported relative to DMSO-d6 as internal standards. The abbreviations used are as follows: s, singlet; d, doublet; m, multiplet. IR spectra were recorded with a Bruker FT-IR spectrophotometer. Electron impact mass spectra were measured on a Varian MAT 311-A (70 Electronvolt) Reaction courses and product mixtures were routinely monitored by thin layer chromatography (TLC) on silica gel precoated F254 Merck plates. Unless otherwise noted, all solvents and reagents were commercially available and used without further purification.
3.1.1. Synthesis of Hydrazones 14a–n
To a stirred solution of the appropriate carbohydrazides 3a–e (5 mmol), 2-oxo-N'-(4-substitutedphenyl)propanehydrazonoyl chloride 13a–g (5 mmol) was added in THF (30 mL). The reaction mixture was heated under reflux for 10 h. The solid product obtained upon cooling was filtered off and recrystallized from dioxane to afford the corresponding hydrazones 14a–n with 65%–80% yield.
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-chlorophenyl)propanehydrazonoyl chloride (14a)
Yellow powder (yield 75%), melting point (m.p.): 199–200 °C; IR (KBr, ν cm−1): 3303, 3353 (2NH), 1643 (C=O) and 1588 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.38 (s, 3H, CH3), 7.31–7.37 (m, 4 H, Ar–H), 7.49–7.59 (m, 3H, Ar–H), 7.87 (d, 2H, J = 6.9 Hz, Ar–H), 10.27 (s, D2O exchangeable (exch.), 1H, =NNH–), 10.84 (s, D2O exch., 1H, –CONH–);13C NMR (DSMO-d6) δ ppm: 16.50, 115.33, 124.50, 124.57, 128.31, 128.95, 131.52, 133.72, 135.50, 137.00, 142.52, 155.00; MS m/z [%]: 350 [(M + 2)+, 4.7], 348 [M+, 7.5], 105 [100].
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-bromophenyl)propanehydrazonoyl chloride (14b)
Yellow powder (yield 78%), m.p.: 197–199 °C; IR (KBr, ν cm−1): 3352, 3302 (2NH), 1641 (C=O) and 1583 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.38 (s, 3H, CH3), 7.28 (d, 2H, J = 9.0 Hz, H-2 and H-6 of 4-BrC6H4), 7.43 (d, 2H, J = 9.0 Hz, H-3 and H-5 of 4-BrC6H4), 7.48–7.59 (m, 3H, H-3, H-4 and H-5 of –C6H5), 7.87 (d, 2H, J = 8.1 Hz, H-2 and H-6 of –C6H5), 10.27 (s, D2O exch., 1H, =NNH–), 10.84 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 396 [(M + 2)+, 1.2], 394 [M+, 4.5], 105 [100].
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-iodophenyl)propanehydrazonoyl chloride (14c)
Brown powder (yield 80%), m.p.: 213–215 °C; IR (KBr, ν cm−1): 3315, 3283 (2NH), 1639 (C=O) and 1560 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.37 (s, 3H, CH3), 7.16 (d, 2H, J = 8.7 Hz, H-2 and H-6 of 4-IC6H4), 7.48–7.56 (m, 3H, H-3, H-4 and H-5 of C6H5), 7.58 (d, J = 8.7 Hz, 2H, H-3 and H-5 of 4-IC6H4), 7.87 (d, 2H, J = 6.9 Hz, H-2 and H-6 of C6H4), 10.25 (s, D2O exch., 1H, =NNH–), 10.84 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 442 [(M + 2)+, 0.8], 440 [M+, 2.5], 404 [100].
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-methoxyphenyl)propanehydrazonoyl chloride (14d)
Yellow powder (yield 65%), m.p.: 189–192 °C; IR (KBr, ν cm−1): 3316, 3291 (2NH), 1645 (C=O) and 1563 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.37 (s, 3H, CH3), 3.72 (s, 3H, OCH3), 6.88 (d, 2H, J = 9 Hz, H-3 and H-5 of 4-OCH3C6H4), 7.26 (d, 2H, J = 9 Hz, H-2 and H-6 of 4-OCH3C6H4), 7.48 (t, 1H, J = 7.4 Hz, H-4 of C6H5), 7.56 (d, 2H, J = 7.8 Hz, H-3 and H-5 of C6H5), 7.87 (d, 2H, J = 7.8 Hz, H-2 and H-6 of C6H4), 10.00 (s, D2O exch., 1H, =NNH–), 10.79 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 14.00, 55.20, 114.43, 114.54, 114.87, 115.02, 122.44, 128.14, 131.50, 133.82, 137.31, 145.00, 154.15; MS m/z [%]: 346 [(M + 2)+, 2.3], 344 [M+, 6.6], 121 [100].
(1Z,2E)-N-(4-Fluorophenyl)-2-(2-(4-methoxybenzoyl)hydrazono)propane hydrazonoyl chloride (14e)
Yellow powder (yield 77%), m.p.: 197–199 °C; IR (KBr, ν cm−1): 3320, 3252 (2NH), 1624 (C=O) and 1556 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.38 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 7.02 (d, 2H, J = 7.2 Hz, H-2 and H-6 of 4-FC6H4), 7.10 (t, 2H, J = 9 Hz, H-3 and H-5 of 4-FC6H4),7.33–7.37 (m, 2H, H-3, H-5 of OCH3C6H5), 7.91 (d, 2H, J = 8.7 Hz, H-2 and H-6 of OCH3C6H5,), 10.10 (s, D2O exch., 1H, =NNH–), 10.64 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 13.61, 55.34, 113.33, 113.50, 115.04 (3JF–C = 7.65 Hz), 115.42 (2JF–C = 22.2 Hz) 115.85, 123.79, 125.68, 130.32, 140.16,155.56 (1JF–C = 235 Hz), 161.93; MS m/z [%]: 364 [(M + 2)+, 0.9], 362 [M+, 2.7], 326 [100].
(1Z,2E)-N-(4-Chlorophenyl)-2-(2-(4-methoxybenzoyl)hydrazono)propane hydrazonoyl chloride (14f)
Yellow powder (yield 71%), m.p.: 200–203 °C; IR (KBr, ν cm−1): 3242–3320 (2NH), 1626 (C=O) and 1557 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.37 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 7.03 (d, 2H, J = 8.7 Hz, H-2 and H-6 of 4-ClC6H4),7.24–7.40 (m, 4H, Ar–H), 7.90 (d, 2H, J = 8.7 Hz, H-2 and H-6 of OCH3C6H5,), 10.24 (s, D2O exch., 1H, =NNH–), 10.68 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 380 [(M + 2)+, 0.8], 378 [M+, 1.2], 135 [100].
(1Z,2E)-N-(4-Iodophenyl)-2-(2-(4-methoxybenzoyl)hydrazono)propanehydrazonoyl chloride (14g)
Yellow powder (yield 74%), m.p.: 195–196 °C; IR (KBr, ν cm−1): 3312, 3268 (2NH), 1659 (C=O) and 1582 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.36 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 7.03 (d, 2H, J = 9 Hz, H-2 and H-6 of 4-IC6H4), 7.16 (d, 2H, J = 9 Hz, H-3 and H-5 of 4-IC6H4), 7.58 (d, 2H, J = 8.7 Hz, H-3 and H-5 of 4-OCH3C6H4), 7.90 (d, 2H, J = 8.7 Hz, H-2 and H-6 of OCH3C6H5), 10.20 (s, D2O exch., 1H, =NNH–), 10.66 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 472 [(M + 2)+, 0.3], 470 [M+, 1.1], 135 [100].
(1Z,2E)-2-(2-(4-Methoxybenzoyl)hydrazono)-N-(p-tolyl)propanehydrazonoyl chloride (14h)
Yellow powder (yield 70%), m.p.: 187–189 °C; IR (KBr, ν cm−1): 3257–3404 (2NH), 1624 (C=O) and 1555 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.24 (s, 3H, CH3), 2.37 (s, 3H, CH3), 3.83 (s, 3H, OCH3), 7.03 (d, 2H, J = 8.7 Hz, H-2 and H-6 of 4-CH3C6H4), 7.08 (d, 2H, J = 8.7 Hz, H-3 and H-5 of 4-CH3C6H4), 7.22 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-OCH3C6H4), 7.89 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-OCH3C6H4), 10.06 (s, D2O exch., 1H, =NNH–), 10.68 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 13.66, 20.24, 55.36, 113.35, 113.51, 113.85, 123.14, 125.72, 129.48, 129.60, 129.80, 130.33, 141.27, 161.91; MS m/z [%]: 360 [(M + 2)+, 1.1], 358 [M+, 3.2], 135 [100].
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-phenylpropanehydrazonoyl chloride (14i)
White powder (yield 75%), m.p.: 222–224 °C; IR (KBr, ν cm−1): 3304, 3167 (2NH), 1653 (C=O) and 1543 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.38 (s, 3H, CH3), 6.89 (t, 1H, J = 6.9 Hz, H-4 of –C6H5), 7.26–7.36 (m, 4H, H-2,H-3, H-5 and H-6 of –C6H5), 7.57 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-ClC6H4), 7.90 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-ClC6H4), 10.16 (s, D2O exch., 1H, =NNH–), 10.90 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 13.72, 113.89, 118.50, 121.16, 126.47, 128.28, 129.16, 130.04, 132.47, 136.32, 143.45, 151.50; MS m/z [%]: 350 [(M + 2)+, 2.1], 348 [M+, 3.0], 312 [100].
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-(4-fluorophenyl)propanehydrazonoyl chloride (14j)
Yellow powder (yield 79%), m.p.: 187–188 °C; IR (KBr, ν cm−1): 3325, 3187 (2NH), 1633 (C=O) and 1564 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.37 (s, 3H, CH3), 7.10 (t, 2H, J = 8.7 Hz, H-3, H-5 of –FC6H5), 7.32–7.37 (q, 2H, J = 4.2, 4.8 Hz, H-2 and H-6 of –FC6H5), 7.57 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-ClC6H4), 7.90 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-ClC6H4), 10.18 (s, D2O exch., 1H, =NNH–), 10.89 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 368 [(M + 2)+, 0.9], 366 [M+, 1.2], 139 [100].
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-(p-tolyl)propanehydrazonoyl chloride (14k)
Yellow powder (yield 73%), m.p.: 205–207 °C; IR (KBr, ν cm−1): 3302, 3247 (2NH), 1667 (C=O) and 1584 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.24 (s, 3H, CH3), 2.37 (s, 3H, CH3), 7.08 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-CH3C6H4), 7.22 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-CH3C6H4), 7.57 (d, 2H, J = 8.7 Hz, H-3 and H-5 of 4-ClC6H4), 7.90 (d, 2H, J = 8.7 Hz, H-2 and H-6 of 4-ClC6H5), 10.06 (s, D2O exch., 1H, =NNH–), 10.87 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 14.00, 20.29, 113.88 (2C), 122.88 (2C), 128.22, 129.46, 129.90, 132.52(2C), 141.21, 150.00; MS m/z [%]: 364 [(M + 2)+, 5.0], 362 [M+, 7.6], 106 [100].
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-(4-methoxyphenyl)propanehydrazonoyl chloride (14l)
Yellow powder (yield 76%), m.p.: 190–192 °C; IR (KBr, ν cm−1): 3326, 3184 (2NH), 1660 (C=O) and 1557 (C=N); 1H NMR (DMSO-d6) δ ppm: 2.36 (s, 3H, CH3), 3.71 (s, 3H, OCH3), 6.87 (d, 2H, J = 8.7 Hz, H-3 and H-5 of 4-OCH3C6H4), 7.26 (d, J = 8.7 Hz, 2H, H-2 and H-6 of 4-OCH3C6H4), 7.56 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-ClC6H4), 7.89 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-ClC6H5), 10.03 (s, D2O exch., 1H, =NNH–), 10.85 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 380 [(M + 2)+, 5.8], 378 [M+, 8.6], 121 [100].
(1Z,2E)-2-(2-(Benzo[d]thiazole-2-carbonyl)hydrazono)-N-(4-chlorophenyl)propane hydrazonoyl chloride (14m)
Yellow powder (yield 77%), m.p.: 23–239 °C [35].
(1Z,2E)-N-(4-Bromophenyl)-2-(2-(3-methylbenzofuran-2-carbonyl)hydrazono)propane hydrazonoyl chloride (14n)
Yellow powder (yield 79%), m.p.: 235–237 °C [36].
3.1.2. Synthesis of Compounds 16a–d
A mixture of hydrazone 14a or 14m (1 mmol) and sodium benzene/toluenesulfinate 15a,b (2 mmol) were refluxed in absolute ethyl alcohol for 10 h. The reaction mixture was left to cool then poured into ice-water. The solid product was filtered off, washed with water, dried and crystallized from EtOH/DMF to furnish the sulfones 16a–d.
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-(phenylsulfonyl)propan-2-ylidene) benzohydrazide (16a)
Yellow powder (yield 70%), m.p.: 245–247 °C; IR (KBr, ν cm−1): 3228–3300 (2NH), 1639 (C=O), 1583 (C=N) and 1284, 1134 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.49 (s, 3H, CH3), 6.91 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-ClC6H4), 7.34 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-ClC6H4), 7.54–7.84 (m, 6H, Ar–H), 7.95–7.99 (m, 4H, Ar–H), 11.72 (s, D2O exch., 1H, =NNH–), 14.58 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 15.06, 115.87, 116.01, 125.00, 127.22, 128.48, 129.36, 129.52, 130.50, 132.27, 133.82, 135.19, 139.20, 141.38, 147.33, 165.28; MS m/z [%]: 456 [(M + 2)+, 4.6], 454 [M+, 11.8], 105 [100].
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-tosylpropan-2-ylidene)benzohydrazide (16b)
Yellow powder (yield 73%), m.p.: 237–239 °C; IR (KBr, ν cm−1): 3267–3310 (2NH), 1648 (C=O), 1577 (C=N) and 1290, 1134 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.48 (s, 3H, CH3), 2.52 (s, 3H, CH3), 6.95 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-ClC6H4), 7.35 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-ClC6H4), 7.50–7.65 (m, 5H, Ar–H), 7.82 (d, 2H, J = 8.1 Hz, H-2 and H-6 of 4-CH3C6H4), 7.97 (d, 2H, J = 6.6 Hz, H-2 and H-6 of C6H5), 11.67 (s, D2O exch., 1H, =NNH–), 14.57 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 470 [(M + 2)+, 2.6], 468 [M+, 6.4], 105 [100].
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-(phenylsulfonyl)propan-2-ylidene) benzo [d]thiazole-2-carbohydrazide (16c)
Yellow powder (yield 68%), m.p.: 240–242 °C; IR (KBr, ν cm−1): 3270–3311 (2NH), 1684 (C=O), 1590 (C=N) and 1289, 1137 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.28 (s, 3H, CH3), 6.91 (d, 2H, J = 9 Hz, H-2 and H-6 of 4-ClC6H4), 7.28 (m, 1H, Ar–H), 7.37 (d, 2H, J = 9 Hz, H-3 and H-5 of 4-ClC6H4), 7.63–7.85 (m, 4H, Ar–H), 7.97 (d, 2H, J = 7.5 Hz, Ar–H), 8.26 (d, 2H, J = 7.5 Hz, Ar–H), 12.39 (s, D2O exch., 1H, =NNH–), 14.20 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 513 [(M + 2)+, 2.0], 511 [M+, 4.4], 370 [100].
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-tosylpropan-2-ylidene)benzo[d] thiazole-2-carbohydrazide (16d)
Yellow powder (yield 70%), m.p.: 239–241 °C; IR (KBr, ν cm−1): 3274–3312 (2NH), 1682 (C=O), 1592 (C=N) and 1247, 1134 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.22 (s, 3H, CH3), 2.37 (s, 3H, CH3), 6.91 (d, 2H, J = 8.4 Hz, H-2 and H-6 of 4-ClC6H4), 7.17–7.35 (m, 3H, Ar–H), 7.37 (d, 2H, J = 8.4 Hz, H-3 and H-5 of 4-ClC6H4), 7.51 (d, 1H, J = 8.1 Hz, Ar–H), 7.59–7.72 (m, 1H, Ar–H), 7.83 (d, 1H, J = 8.1 Hz, Ar–H), 8.25 (m, 2H, Ar–H), 12.38 (s, D2O exch., 1H, =NNH–), 14.17 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 13.00, 21.50, 115.24, 116.13, 123.15, 124.01, 124.49, 125.15, 127.46, 128.62, 128.97, 129.73, 135.33, 136.18, 141.15, 142.47, 144.16, 144.60, 150.97, 152.64, 157.84, 167.35; MS m/z [%]: 527 [(M + 2)+, 2.0], 525 [M+, 4.5], 126 [100].
3.1.3. Synthesis of Compounds 17a,b
To a solution of the appropriate hydrazone 14c or 14n (1 mmol) in absolute ethyl alcohol (30 mL), piperidine (0.34 g, 4 mmol) and benzaldehyde (0.1 g, 1 mmol) were added. The reaction mixture was refluxed for 15 h. The precipitated product was filtered off while hot, washed with ethanol and dried. Recrystallization from EtOH/DMF gave compounds 17a and 17b, respectively.
N'-((1Z,2E,3E)-1-(2-(4-Iodophenyl)hydrazono)-4-phenyl-1-(piperidin-1-yl)but-3-en-2-ylidene)benzohydrazide (17a)
Yellow powder (yield 60%), m.p.: 210–212 °C; IR (KBr, ν cm−1): 3275–3320 (2NH), 1680 (C=O) and 1586 (C=N); 1H NMR (DMSO-d6) δ ppm: 1.56 (m, 6H, 3CH2 of piperidine), 3.21 (m, 4H, 2CH2 of piperidine), 6.81 (d, 2H, J = 8.7 Hz, Ar–H), 6.85 (s, IH, CH=CH), 6.91 (s, 1H, –CH=Ph), 7.07–7.40 (m, 8H, Ar–H), 7.44 (t, 1H, J = 7.5 Hz, Ar–H), 7.58–7.60 (m, 1H, Ar–H), 7.65 (d, 2H, J = 7.8 Hz), 8.39 (s, D2O exch., 1H, =NNH–), 10.39 (s, D2O exch., 1H, –CONH–); MS m/z [%]: 577 [M+, 6.7], 458 [100].
N'-((1Z,2E,3E)-1-(2-(4-Bromophenyl)hydrazono)-4-phenyl-1-(piperidin-1-yl)but-3-en-2-ylidene)-3-methylbenzofuran-2-carbohydrazide (17b)
Orange powder (yield 55%), m.p.: 224–226 °C; IR (KBr, ν cm−1): 3262–3318 (NH), 1679 (C=O) and 1593 (C=N); 1H NMR (DMSO-d6) δ ppm: 1.64 (m, 6H, 3CH2 of piperidine), 2.50 (s, 3H, CH3), 3.28 (m, 4H, 2CH2 of piperidine), 6.87 (s, 1H, –CH=CH–), 6.93 (d, 2H, J = 9 Hz, Ar–H), 7.13 (s, 1H, =CH–Ph), 7.18–7.56 (m, 7H, Ar–H), 7.59 (d, 2H, J = 7.5 Hz, Ar–H), 7.77 (d, 2H, J = 7.5 Hz, Ar–H), 8.54 (s, D2O exch., 1H, =NNH–), 10.58 (s, D2O exch., 1H, –CONH–); 13C NMR (DSMO-d6) δ ppm: 8.64, 23.95, 46.60, 71.37, 108.40, 111.44, 114.21, 114.37, 121.49, 126.57, 123.94, 127.41, 128.09, 128.76, 130.98, 131.15, 131.61, 135.61, 136.67, 141.32, 144.20, 146.67, 148.81, 152.73, 154.89; MS m/z [%]: 585 [(M + 2)+, 2.8], 585 [M+, 2.4], 175 [100].
3.2. Biological Evaluation
3.2.1. Antimicrobial Activity
All strains were provided from culture collection of the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. Antibacterial and antifungal activities were expressed as the diameter of inhibition zones; Agar well diffusion method was used. Holes (1 cm diameter) were digger in the agar using sterile cork borer in sterile malt agar plates for fungi and sterile nutrient agar plates for bacteria, which had previously been uniformly seeded with tested microorganisms. The holes were filled by fungal filtrates (100 μL). Plates were left in a cooled incubator at 4 °C for one hour for diffusion and then incubated at 37 °C for tested bacteria and 28 °C for tested fungi. Inhibition zones developed due to active antimicrobial metabolites were measured after 24 h of incubation for bacteria and 48 h of incubation for fungi. Amphotericin B and ciprofloxacin were used as antifungal and antibacterial positive control, respectively. The experiment was performed in triplicate and the average zone of inhibition was calculated.
3.2.2. Minimum Inhibitory Concentration
MIC was performed by a serial dilution technique described by Irobi et al. [52], starting with 100-mmol concentration of all compounds dissolved in 1 mL DMSO and then reduced by successive twofold dilutions of stock solution using a calibrated micropipette. Amphotericin B and ciprofloxacin were used as the reference compounds for fungi and bacteria; respectively. The final solutions concentrations were 125, 62.50, 31.25, 15.63, 7.81, 3.90, 1.95, 0.98, 0.49, 0.24 and 0.12 μmol/mL. The microtiter plates were incubated at 37 °C for tested bacteria and 28 °C for tested fungi and were readied using microplate reader after 24 h for bacteria and after 48 h for fungi. In each case, triplicate tests were performed and the average was taken as final reading. MIC was expressed as the lowest concentration inhibiting test organism’s growth [53].
3.2.3. Antimycobacterial Activity
M. tuberculosis (RCMB 010126) strain was provided from culture collection of the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. The isolated M. tuberculosis (RCMB 010126) clone was cultivated under agitation on LB medium at 37 °C for 24 h. The antitubercular activity was expressed as the diameter of inhibition zones using agar well diffusion method and determination of MIC using serial dilution technique. Isoniazide and pyrazinamide were used as the reference drugs. The final solutions concentrations were 125, 62.50, 31.25, 15.63, 7.81, 3.90, 1.95, 0.98, 0.49, 0.24 and 0.12 μmol/mL. The zones of inhibition were analyzed after 72 h of incubation at 37 °C. Each test was repeated 3 times. MIC was expressed as the lowest concentration inhibiting test organism’s growth.
3.2.4. In Vitro Cytotoxicity
A549 human lung cancer cells were grown in DMEM, supplemented with 10% heat inactivated FBS, 50 units/mL of penicillin and 50 g/mL of streptomycin and maintained at 37 °C in a humidified atmosphere containing 5% CO2. The cells were maintained as “monolayer culture” by serial subculturing. Cytotoxicity was determined using SRB method as previously described by Skehan et al. [54]. Exponentially growing cells were collected using 0.25% Trypsin-EDTA and seeded in 96-well plates at 1000–2000 cells/well in DMEM supplemented medium. After 24 h, cells were incubated for 72 h with various concentrations of the tested compounds. Following 72 h treatment, the cells will be fixed with 10% trichloroacetic acid for 1 h at 4 °C. Wells were stained for 10 min at room temperature with 0.4% SRB dissolved in 1% acetic acid. The plates were air dried for 24 h and the dye was solubilized with Tris-HCl for 5 min on a shaker at 1600 rpm. The optical density (OD) of each well was measured spectrophotometrically at 564 nm with an ELISA microplate reader (ChroMate-4300, Palm City, FL, USA).The IC50 values were calculated according to the equation for Boltzman sigmoidal concentration–response curve using the nonlinear regression fitting models (Graph Pad, Prism Version 5, GraphPad Software, Inc., San Diego, CA, USA).
4. Conclusions
In summary, the antimicrobial and antimycobacterial activities of halophenyl bis-hydrazones were evaluated. The results evidenced that compounds 14c, 14g, 16b, 17a and 17b can be considered as broad spectrum antimicrobials against aspergillosis, Gram positive bacteria and Gram negative bacteria. Within the screened compounds, the latter five hydrazide/hydrazones with high lipophilicity showed the most promising antitubercular activity with MIC ranging from 1.95–7.81 μg/mL. The attractive results achieved with these analogs suggest that may be worth developing further in order to identify potential broad-spectrum antimicrobial and anti-TB chemotherapeutics. In addition, the generated QSAR models, performed to explore the structural requirements controlling the observed antibacterial properties, hinted that the biological activities were affected by jurs descriptors and shadow indices of the synthesized hydrazones.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-VPP-321. Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Egyptian Russian University, Cairo, Egypt, is highly appreciated for supporting this research.
Author Contributions
Hatem A. Abdel-Aziz and Khalid A. Al-Rashood formulated the research idea and participated in the preparation of manuscript; Wagdy M. Eldehna, Mohamed Fares, Sara T. A. Al-Rashood and Dalia H. Soliman carried out the experimental, interpreted the data and prepared the manuscript; Marwa M. Abdel-Aziz performed the biological screening. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Payne D.J., Gwynn M.N., Holmes D.J., Pompliano D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 2.Coates A., Hu Y., Bax R., Page C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002;11:895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- 3.Lewis K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 4.Ritter T.K., Wong C. Carbohydrate-based antibiotics: A new approach to tackling the problem of resistance. Angew. Chem. Int. Ed. 2001;40:3508–3533. doi: 10.1002/1521-3773(20011001)40:19<3508::AID-ANIE3508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Alekshun M.N., Levy S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Global Tuberculosis Report 2013. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- 7.Zumla A., Nahid P., Cole S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013;12:388–404. doi: 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 8.Goldman R.C., Plumley K.V., Laughon B.E. The evolution of extensively drug resistant tuberculosis (XDR-TB): History, status and issues for global control. Infect. Disord. Drug Targets. 2007;7:73–91. doi: 10.2174/187152607781001844. [DOI] [PubMed] [Google Scholar]
- 9.Benatar S.R. Extensively drug resistant tuberculosis—Problem will get worse in South Africa unless poverty is alleviated. Br. Med. J. 2006;333:705. doi: 10.1136/bmj.333.7570.705-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn S.D., Wilkinson R. Extensively drug resistant tuberculosis-a serious wake-up call for global health. Br. Med. J. 2006;333:559–560. doi: 10.1136/bmj.38971.587222.AB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahle U.R. Extensively drug resistant tuberculosis-beware patients lost to follow-up. Br. Med. J. 2006;333:705. doi: 10.1136/bmj.333.7570.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi N.R., Moll A., Sturm A.W., Pawinski R., Govender T., Lalloo U., Zeller K., Andrews J., Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 13.Manissero D., Fernandez K. Extensive drug-resistant TB: A threat for Europe? Eurosurveillance. 2006;11:E060928. doi: 10.2807/esw.11.39.03056-en. [DOI] [PubMed] [Google Scholar]
- 14.Küçükgüzel S.G., Oruç E.E., Rollas S., Sahin F., Ozbek A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002;37:197–206. doi: 10.1016/S0223-5234(01)01326-5. [DOI] [PubMed] [Google Scholar]
- 15.Loncle C., Brunel J.M., Vidal N., Dherbomez M., Letourneux Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004;39:1067–1071. doi: 10.1016/j.ejmech.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Ragavendran J.V., Sriram D., Patel S.K., Reddy I.V., Bharathwajan N., Stables J., Yogeeswari P. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007;42:146–151. doi: 10.1016/j.ejmech.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Kajal A., Bala S., Kamboj S., Saini V. Synthesis, characterization, and computational studies on phthalic anhydride-based benzylidene-hydrazide derivatives as novel, potential anti-inflammatory agents. Med. Chem. Res. 2014;23:2676–2689. doi: 10.1007/s00044-013-0848-1. [DOI] [Google Scholar]
- 18.Gemma S., Kukreja G., Fattorusso C., Persico M., Romano M.P., Altarelli M., Savini L., Campiani G., Fattorusso E., Basilico N., et al. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Lett. 2006;16:5384–5388. doi: 10.1016/j.bmcl.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Pavan F.R., da S. Maia P.I., Leite S.R., Deflon V.M., Batista A.A., Sato D.N., Franzblau S.G., Leite C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010;45:1898–1905. doi: 10.1016/j.ejmech.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Vavøíková E., Polanc S., Horváti K., Bosze S., Stolaríková J., Vávrová K., Vinsová J. New fluorine-containing hydrazones active against MDR-tuberculosis. Bioorg. Med. Chem. Lett. 2005;15:2509–2513. doi: 10.1016/j.bmcl.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 21.Pelttari E., Karhumaki E., Langshaw J. Carbohydrazones of substituted salicylaldehydes as potential lead compounds for the development of narrow-spectrum antimicrobials. J. Biosci. 2007;62:483–486. doi: 10.1515/znc-2007-7-805. [DOI] [PubMed] [Google Scholar]
- 22.Torres E., Moreno E., Ancizu S., Barea C., Galiano S., Aldana I., Monge A., Pérez-Silanes S. New 1,4-di-N-oxide-quinoxaline-2-ylmethylene isonicotinic acid hydrazide derivatives as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. Lett. 2011;21:3699–3703. doi: 10.1016/j.bmcl.2011.04.072. [DOI] [PubMed] [Google Scholar]
- 23.Tavares L.C., Chiste J.J., Santos M.B., Penna T. Synthesis and biological activity of nifuroxazide and analogs. II. Boll. Chim. Farm. 1999;183:432–436. [PubMed] [Google Scholar]
- 24.More U.A., Joshi S.D., Aminabhavi T.M., Gadad A.K., Nadagouda M.N., Kulkarni V.H. Design, synthesis, molecular docking and 3D-QSAR studies of potent inhibitors of enoyl-acyl carrier protein reductase as potential antimycobacterial agents. Eur. J. Med. Chem. 2014;71:199–218. doi: 10.1016/j.ejmech.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 25.De Logu A., Onnis V., Saddi B., Congiu C., Schivo M.L., Cocco M.T. Activity of a new class of isonicotinoylhydrazones used alone and in combinatin with isoniazid, rifampicin, ethambutol, para-aminosalicylic acid and clofazimine against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2002;49:275–282. doi: 10.1093/jac/49.2.275. [DOI] [PubMed] [Google Scholar]
- 26.Hearn M.J., Cynamon M.H., Chen M.F., Coppins R., Davis J., Kang H.J.-O., Noble A., Tu-Sekine B., Terrot M.S., Trombino D., et al. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur. J. Med. Chem. 2009;54:4169–4178. doi: 10.1016/j.ejmech.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aboul-Fadl T., Mohammed F.A., Hassan E.A. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH) Arch. Pharm. Res. 2003;26:778–784. doi: 10.1007/BF02980020. [DOI] [PubMed] [Google Scholar]
- 28.Hardman J.G., Limbird L.E. The Pharmacological Basis of Therapeutics. 10th ed. McGraw-Hill Hardman; New York, NY, USA: 2001. Goodman & Gilman’s. [Google Scholar]
- 29.Shaharyar M., Siddiqui A.A., Ali M.A., Sriram D., Yogeeswari P. Synthesis and in vitro antimycobacterial activity of N1-nicotinoyl-3-(4'-hydroxy-3'-methyl phenyl)-5-[(sub)phenyl]-2-pyrazolines. Bioorg. Med. Chem. Lett. 2006;16:3947–3949. doi: 10.1016/j.bmcl.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Moussa Z., El-Sharief M.A., El-Sharief A.M. Synthesis and characterization of new types of halogenated and alkylated imidazolidineiminothiones and a comparative study of their antitumor, antibacterial, and antifungal activities. Eur. J. Med. Chem. 2011;46:2280–2289. doi: 10.1016/j.ejmech.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Telvekar V.N., Bairwa V.K., Satardekar K., Bellubi A. Novel 2-(2-(4-aryloxy benzylidene)hydrazinyl)benzothiazole derivatives as anti-tubercular agents. Bioorg. Med. Chem. Lett. 2011;22:649–652. doi: 10.1016/j.bmcl.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 32.Cho Y., Ioerger T.R., Sacchettini J.C. Discovery of novel nitrobenzothiazole inhibitors for Mycobacterium tuberculosis ATP phosphoribosyl transferase (HisG) through virtual screening. J. Med. Chem. 2008;51:5984–5992. doi: 10.1021/jm800328v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira G.A., Massabni A.C., Castellano E.E., Costa L.A.S., Leite C.Q.F., Pavan F.R., Cuin A. A broad study of two new promising antimycobacterial drugs: Ag(I) and Au(I) complexes with 2-(2-thienyl)benzothiazole. Polyhedron. 2012;38:291–296. doi: 10.1016/j.poly.2012.03.016. [DOI] [Google Scholar]
- 34.Liu J., Jiang F., Jiang X., Zhang W., Liu J., Liu W., Fu L. Synthesis and antimicrobial evaluation of 3-methanone-6-substituted benzofuran derivatives. Eur. J. Med. Chem. 2012;54:879–886. doi: 10.1016/j.ejmech.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Aziz H.A., Abdel-Wahab B.F., Badria F.A. Stereoselective synthesis and antiviral activity of (1E,2Z,3E)-1-(Piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol-2-oyl)hydrazono]-4-(ary1)but-3-enes. Arch. Pharm. 2010;343:152–159. doi: 10.1002/ardp.200900195. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Aziz H.A., Mekawey A.A.I. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur. J. Med. Chem. 2009;44:4985–4997. doi: 10.1016/j.ejmech.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Wahab B.F., Abdel-Aziz H.A., Ahmed E.M. Convenient synthesis and antimicrobial activity of new 3-substituted 5-(Benzofuran-2-yl)-pyrazole derivatives. Arch. Pharm. 2008;341:734–739. doi: 10.1002/ardp.200800119. [DOI] [PubMed] [Google Scholar]
- 38.Ghabbour H.A., Qabeel M.M., Eldehna W.M., Al-Dhfyan A., Abdel-Aziz H.A. Design, synthesis, and molecular docking of 1-(1-(4-chlorophenyl)-2-(phenylsulfonyl)ethylidene)-2-phenylhydrazine as potent nonazole anticandidal agent. J. Chem. 2014;2014:154357. doi: 10.1155/2014/154357. [DOI] [Google Scholar]
- 39.Ibrahim H.S., Eldehna W.M., Abdel-Aziz H.A., Elaasser M.M., Abdel-Aziz M.M. Improvement of antibacterial activity of some sulfa drugs through linkage to certain phthalazin-1(2H)-one scaffolds. Eur. J. Med. Chem. 2014;85:480–486. doi: 10.1016/j.ejmech.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Sriram D., Yogeeswari P., Devakaram V.R. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg. Med. Chem. 2006;14:3113–3118. doi: 10.1016/j.bmc.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 41.Shawali A.S., Farghaly T.A. Reactions of hydrazonoyl halides with heterocyclic thiones. Convenient methodology for heteroannulation, synthesis of spiroheterocycles and heterocyclic ring transformation. Arkivoc. 2008;i:18–64. [Google Scholar]
- 42.Padmavathi V., Reddy S.N., Mahesh K. Synthesis, antimicrobial and antioxidant activities of sulfone linked bis heterocycles-pyrazolyl oxadiazoles and pyrazolyl thiadiazole. Chem. Pharm. Bull. 2009;57:1376–1380. doi: 10.1248/cpb.57.1376. [DOI] [PubMed] [Google Scholar]
- 43.Muralikrishna A., Venkatesh B.C., Padmavathi V., Padmaja A., Kondaiah P., Krishna N.S. Synthesis, antimicrobial and cytotoxic activities of sulfone linked bis-heterocycles. Eur. J. Med. Chem. 2012;54:605–614. doi: 10.1016/j.ejmech.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Dagenais T., Keller N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009;3:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morikawa H., Tomishima M., Kayakiri N., Araki T., Barrett D., Akamatsu S., Matsumoto S., Uchida S., Nakai T., Takeda S., et al. Synthesis and antifungal activity of ASP9726, a novel echinocandin with potent Aspergillus hyphal growth inhibition. Bioorg. Med. Chem. Lett. 2014;24:1172–1175. doi: 10.1016/j.bmcl.2013.12.116. [DOI] [PubMed] [Google Scholar]
- 46.Yamasaki M., Harada E., Tamura Y., Lim S., Ohsuga T., Yokoyama N., Morishita K., Nakamura K., Ohta H., Takiguchi M. In vitro and in vivo safety and efficacy studies of amphotericin B on Babesia gibson. Vet. Parasitol. 2014;205:424–433. doi: 10.1016/j.vetpar.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Jacoby G.A., Hooper D.C. Review of the Quinolone Family. Springer US; New York, NY, USA: 2012. [Google Scholar]
- 48.Maccari R., Ottana R., Vigorita M.G. In vitro advanced antimycobacterial screening of isoniazid-related hydrazones, hydrazides and cyanoboranes: Part 14. Bioorg. Med. Chem. Lett. 2005;15:2509–2513. doi: 10.1016/j.bmcl.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 49.Taha M.O., Qandil A.M., Zaki D.D., Al Damen M.A. Ligand-based assessment of factor Xa binding site flexibility via elaborate pharmacophore exploration and genetic algorithm-based QSAR modeling. Eur. J. Med. Chem. 2005;40:701–727. doi: 10.1016/j.ejmech.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Stanton D.T., Jurs P.C. Development and use of charge partial surface area structural descriptors in computer-assisted quantitative structure-property relationship studies. Anal. Chem. 1990;62:2323–2329. doi: 10.1021/ac00220a013. [DOI] [PubMed] [Google Scholar]
- 51.Rohrbaugh R.H., Jurs P.C. Descriptions of molecular shape applied in studies of structure/activity and structure/property relationships. Anal. Chim. Acta. 1987;199:99–109. doi: 10.1016/S0003-2670(00)82801-9. [DOI] [Google Scholar]
- 52.Irobi O.N., Moo-Young M., Anderson W.A. Antimicrobial activity of annatto (Bixa orellana) extract. Pharm. Biol. 1996;34:87–90. doi: 10.1076/phbi.34.2.87.13201. [DOI] [Google Scholar]
- 53.Urzua A., Caroli M., Vasquez L., Mendoza L., Wilkens M., Tojo E. Antimicrobial study of the resinous exudate and of diterpenoids isolated from Eupatorium salvia (Asteraceae) J. Ethnopharmacol. 1998;62:251–254. doi: 10.1016/S0378-8741(98)00068-3. [DOI] [PubMed] [Google Scholar]
- 54.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]