Abstract
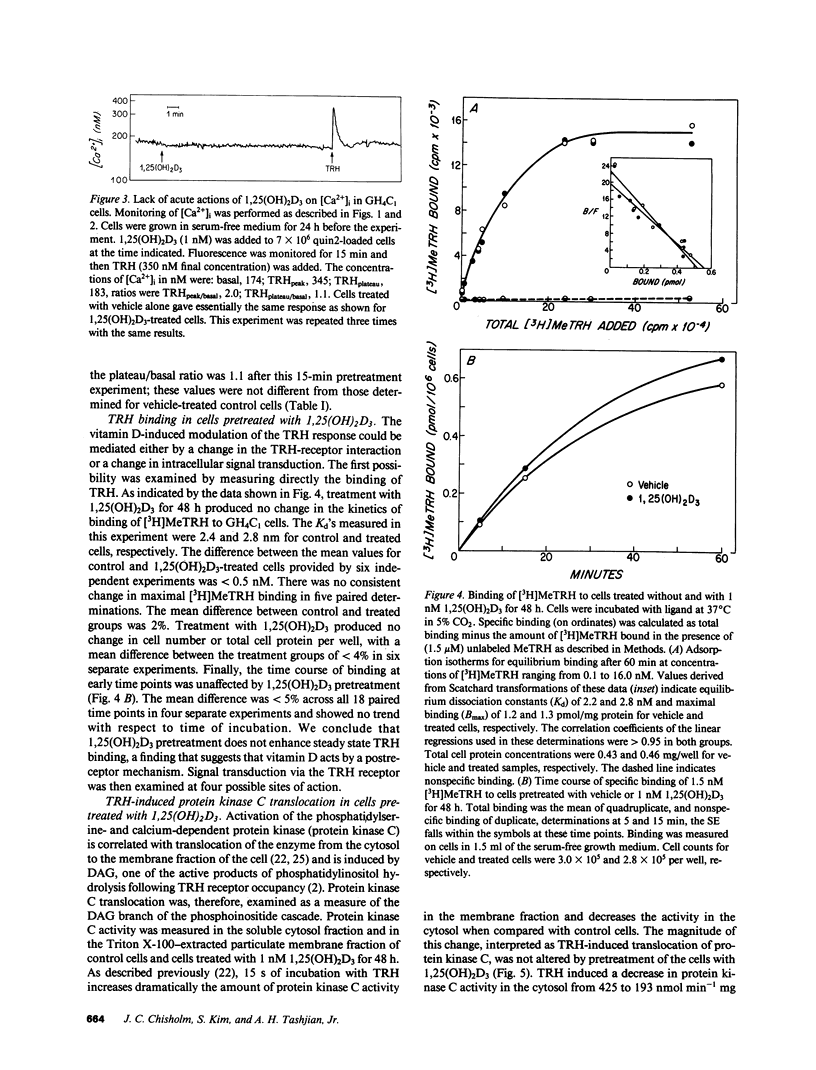
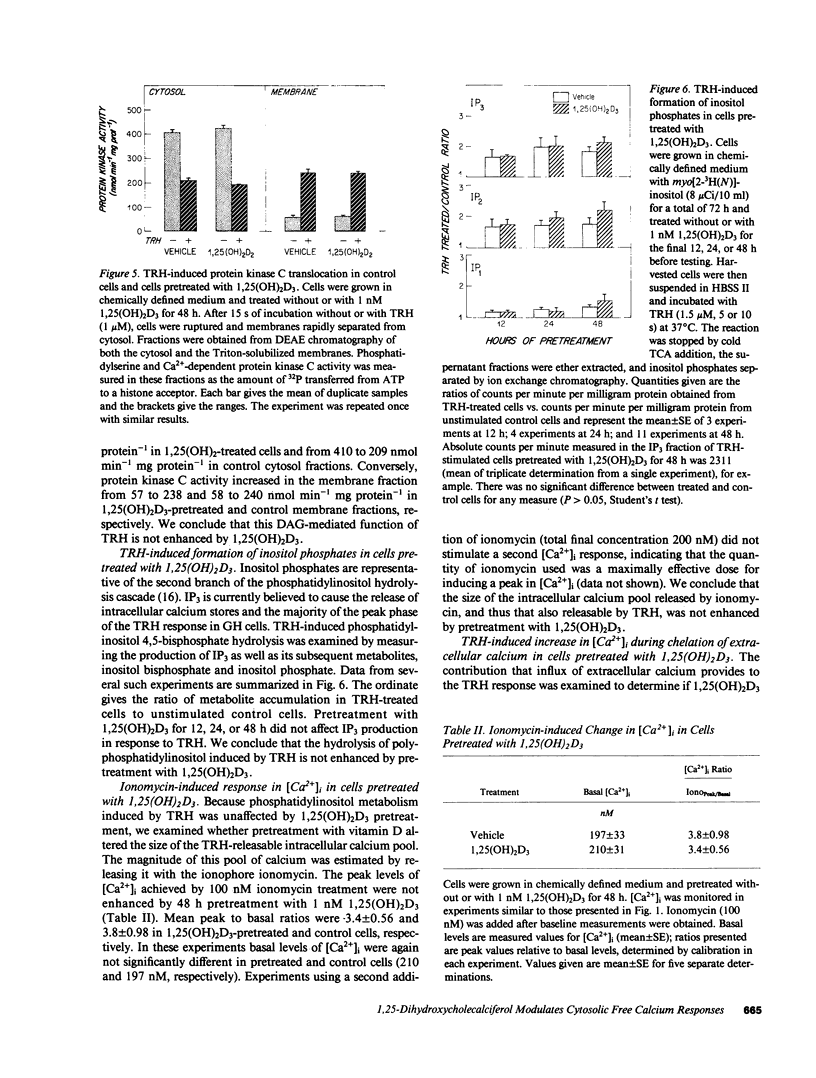
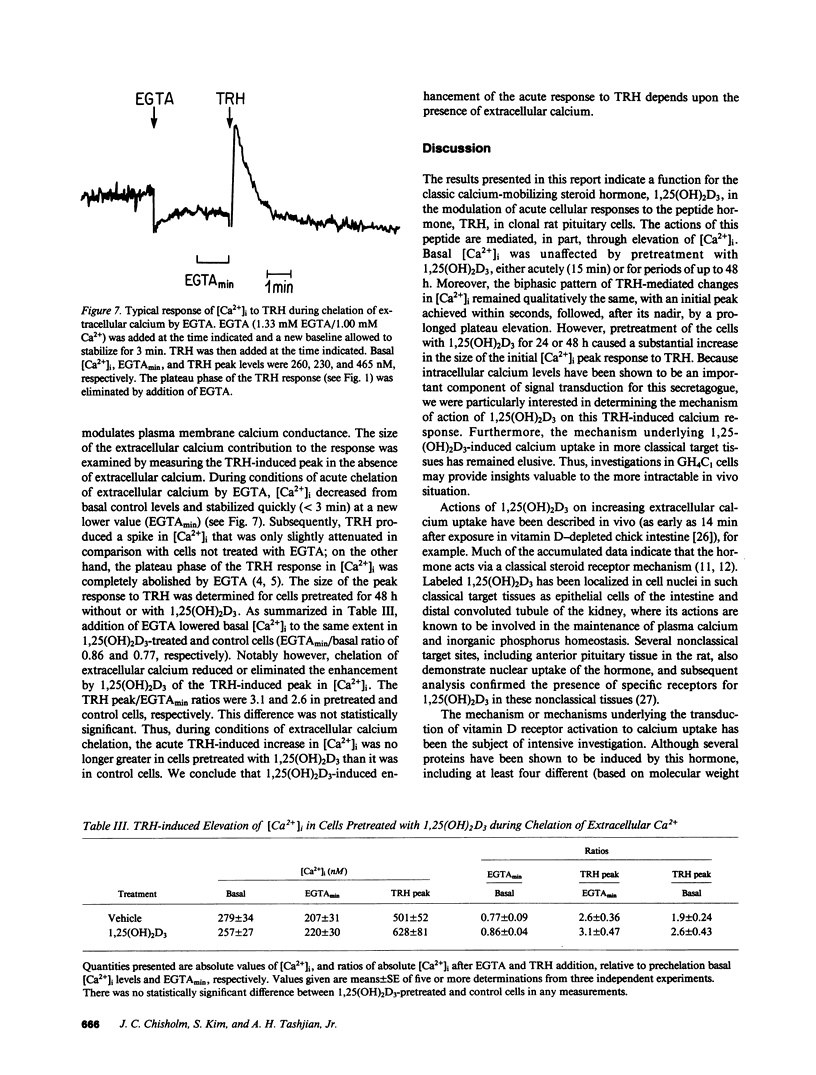
Receptor-mediated regulation of prolactin synthesis by 1,25-dihydroxycholecalciferol (1,25(OH)2D3) in the pituitary cell strain GH4C1 is dependent on the concentration of extracellular calcium. We have now investigated the actions of 1,25(OH)2D3 on cytosolic free calcium concentrations [( Ca2+]i) in these cells using the fluorescent indicator quin2. Basal resting [Ca2+]i was unchanged in cells treated with 1 nM 1,25(OH)2D3 either acutely (from 0 to 15 min) or for periods of up to 48 h. However, the initial peak of the biphasic change in [Ca2+]i induced by thyrotropin-releasing hormone (TRH) was enhanced more than twofold in cells pretreated for 24 or 48 h with 1,25(OH)2D3. This 1,25(OH)2D3-enhanced calcium response was restricted to the initial phase of TRH action; the secondary plateau phase was unaffected. Neither the affinity nor number of TRH receptors nor the early time course of [3H]MeTRH binding to GH4C1 cells were affected by pretreatment with 1,25(OH)2D3. Because TRH binding was not altered, four sites along the intracellular signal transduction pathway of TRH action were examined. Neither protein kinase C activation nor inositol polyphosphate accumulation were enhanced in response to TRH, in 1,25(OH)2D3 pretreated cells, indicating that phosphatidylinositol hydrolysis was unchanged by pretreatment. A low concentration of ionomycin was used to probe the size of the nonmitochondrial intracellular calcium pool that is sensitive to TRH. Ionomycin was not able to mobilize more calcium from 1,25(OH)2D3 pretreated cells, indicating that TRH-responsive intracellular calcium stores were probably not enhanced by pretreatment. Chelation of extracellular calcium, however, did eliminate enhancement of the TRH response in 1,25(OH)2D3-pretreated cells. We conclude that 1,25(OH)2D3 modulates acute dynamic changes in [Ca2+]i induced by TRH without affecting basal [Ca2+]i. The mechanism of the enhanced response of 1,25(OH)2D3-pretreated cells to TRH appears to depend upon a postreceptor event independent of phosphatidylinositol hydrolysis that involves increased calcium conductance at the level of the plasma membrane. A less likely explanation involves enhancement of intracellular calcium stores in an ionomycin-resistant, EGTA-sensitive, TRH-mobilizable reservoir.
Full text
PDF

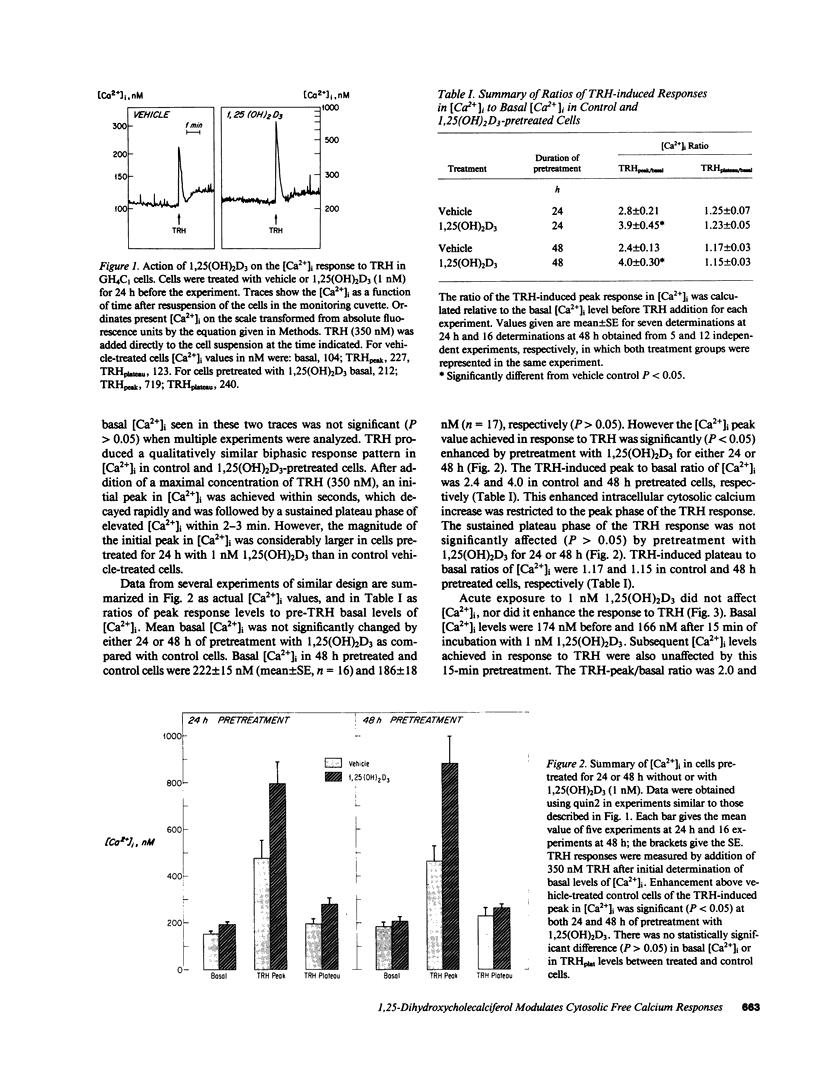


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa T., Hinkle P. M. Thyrotropin-releasing hormone rapidly stimulates a biphasic secretion of prolactin and growth hormone in GH4C1 rat pituitary tumor cells. Endocrinology. 1985 Jan;116(1):73–82. doi: 10.1210/endo-116-1-73. [DOI] [PubMed] [Google Scholar]
- Albert P. R., Tashjian A. H., Jr Relationship of thyrotropin-releasing hormone-induced spike and plateau phases in cytosolic free Ca2+ concentrations to hormone secretion. Selective blockade using ionomycin and nifedipine. J Biol Chem. 1984 Dec 25;259(24):15350–15363. [PubMed] [Google Scholar]
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Bell N. H. Vitamin D-endocrine system. J Clin Invest. 1985 Jul;76(1):1–6. doi: 10.1172/JCI111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Regulation of ion channels by inositol trisphosphate and diacylglycerol. J Exp Biol. 1986 Sep;124:323–335. doi: 10.1242/jeb.124.1.323. [DOI] [PubMed] [Google Scholar]
- D'Emden M. C., Wark J. D. 1,25-Dihydroxyvitamin D3 enhances thyrotropin releasing hormone induced thyrotropin secretion in normal pituitary cells. Endocrinology. 1987 Sep;121(3):1192–1194. doi: 10.1210/endo-121-3-1192. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- Drummond A. H. Inositol lipid metabolism and signal transduction in clonal pituitary cells. J Exp Biol. 1986 Sep;124:337–358. doi: 10.1242/jeb.124.1.337. [DOI] [PubMed] [Google Scholar]
- Drummond A. H., Knox R. J., Macphee C. H. The role of inositol lipids in hormonal mobilization of cell-associated Ca2+. Biochem Soc Trans. 1985 Feb;13(1):58–60. doi: 10.1042/bst0130058. [DOI] [PubMed] [Google Scholar]
- Fearon C. W., Tashjian A. H., Jr Thyrotropin-releasing hormone induces redistribution of protein kinase C in GH4C1 rat pituitary cells. J Biol Chem. 1985 Jul 15;260(14):8366–8371. [PubMed] [Google Scholar]
- Gershengorn M. C., Thaw C. Thyrotropin-releasing hormone (TRH) stimulates biphasic elevation of cytoplasmic free calcium in GH3 cells. Further evidence that TRH mobilizes cellular and extracellular Ca2+. Endocrinology. 1985 Feb;116(2):591–596. doi: 10.1210/endo-116-2-591. [DOI] [PubMed] [Google Scholar]
- Griffin H. D., Hawthorne J. N. Calcium-activated hydrolysis of phosphatidyl-myo-inositol 4-phosphate and phosphatidyl-myo-inositol 4,5-bisphosphate in guinea-pig synaptosomes. Biochem J. 1978 Nov 15;176(2):541–552. doi: 10.1042/bj1760541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., Manolagas S. C., Deftos L. J. Evidence for a 1,25-dihydroxyvitamin D3 receptor-like macromolecule in rat pituitary. J Biol Chem. 1980 Jun 10;255(11):5007–5010. [PubMed] [Google Scholar]
- Hirasawa K., Nishizuka Y. Phosphatidylinositol turnover in receptor mechanism and signal transduction. Annu Rev Pharmacol Toxicol. 1985;25:147–170. doi: 10.1146/annurev.pa.25.040185.001051. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. F., Kowalchyk J. A. Evidence for the role of calcium and diacylglycerol as dual second messengers in thyrotropin-releasing hormone action: involvement of Ca+2. Endocrinology. 1984 Oct;115(4):1527–1536. doi: 10.1210/endo-115-4-1527. [DOI] [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cell. Relationship to its mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3354–3360. [PubMed] [Google Scholar]
- Nemere I., Yoshimoto Y., Norman A. W. Calcium transport in perfused duodena from normal chicks: enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology. 1984 Oct;115(4):1476–1483. doi: 10.1210/endo-115-4-1476. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Ramsdell J. S., Tashjian A. H., Jr Thyrotropin-releasing hormone (TRH) elevation of inositol trisphosphate and cytosolic free calcium is dependent on receptor number. Evidence for multiple rapid interactions between TRH and its receptor. J Biol Chem. 1986 Apr 25;261(12):5301–5306. [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Clonal strains of hormone-producing pituitary cells. Methods Enzymol. 1979;58:527–535. doi: 10.1016/s0076-6879(79)58167-1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Törnquist K., Lamberg-Allardt C. Effect of 1,25-dihydroxyvitamin D3 on TSH secretion from rat pituitary cells in culture. Acta Endocrinol (Copenh) 1987 Mar;114(3):357–361. doi: 10.1530/acta.0.1140357. [DOI] [PubMed] [Google Scholar]
- Wark J. D., Tashjian A. H., Jr Regulation of prolactin mRNA by 1,25-dihydroxyvitamin D3 in GH4C1 cells. J Biol Chem. 1983 Oct 25;258(20):12118–12121. [PubMed] [Google Scholar]
- Wark J. D., Tashjian A. H., Jr Vitamin D stimulates prolactin synthesis by GH4C1 cells incubated in chemically defined medium. Endocrinology. 1982 Nov;111(5):1755–1757. doi: 10.1210/endo-111-5-1755. [DOI] [PubMed] [Google Scholar]