Abstract
We often face the challenge of simultaneously attending to multiple non-contiguous regions of space. There is ongoing debate as to how spatial attention is divided under these situations. While for several years the predominant view was that humans could divide the attentional spotlight, several recent studies argue in favor of a unitary spotlight that rhythmically samples relevant locations. Here, this issue was addressed using high-density electrophysiology in concert with the multifocal m-sequence technique to examine visual evoked responses to multiple simultaneous streams of stimulation. Concurrently, we assayed the topographic distribution of alpha-band oscillatory mechanisms, a measure of attentional suppression. Participants performed a difficult detection task that required simultaneous attention to two stimuli in contiguous (undivided) or non-contiguous parts of space. In the undivided condition, the classical pattern of attentional modulation was observed, with increased amplitude of the early visual evoked response and increased alpha amplitude ipsilateral to the attended hemifield. For the divided condition, early visual responses to attended stimuli were also enhanced and the observed multifocal topographic distribution of alpha suppression was in line with the divided attention hypothesis. These results support the existence of divided attentional spotlights, providing evidence that the corresponding modulation occurs during initial sensory processing timeframes in hierarchically early visual regions and that suppressive mechanisms of visual attention selectively target distracter locations during divided spatial attention.
Keywords: Spatial Attention, Alpha-band oscillations, Event-related potential (ERP), Visual evoked potential (VEP), Spotlight, EEG
Introduction
Attentional processes constantly filter sensory inputs and only a subset of our environment receives fully elaborated perceptual processing. For example each time we make an eye-movement, the eyes bring another part of our environment into the center of gaze for detailed processing. In addition to these overt shifts of attention, humans can deploy spatial attention without moving the eyes or the head, known as covert shifts of attention (von Helmholtz, 1867). One longstanding metaphor for covert spatial attention is the “attentional spotlight”, the notion that attention can only be allocated to one region of space at a time (e.g. Posner, 1980). These models postulate that the attentional spotlight cannot be divided, but that the size of the spotlight can be adapted to task requirements (i.e., the “zoom-lens” model; (Eriksen and St James, 1986). In the attended region of visual space, reaction times are faster and/or detection accuracy is higher compared to unattended regions. This notion of a unitary, indivisible spotlight was supported by earlier visual evoked potential (VEP) studies (e.g. Heinze et al., 1994).
However, a growing number of studies have challenged the idea of a single, non-divisible attentional spotlight. Behavioral experiments provide evidence that humans can divide attention among multiple non-contiguous spatial locations (e.g. Castiello and Umilta, 1992; Awh and Pashler, 2000; Gobell et al., 2004), reporting that reaction time and accuracy are modulated in divided attention designs in the same way as in undivided cued attention paradigms. Another line of evidence for a division of spatial attention has been put forward in steady-state VEP and functional magnetic resonance imaging (fMRI) studies (e.g. Muller et al., 2003a; McMains and Somers, 2004, 2005). These studies reveal brain activation patterns that clearly fit with a divided spotlight account. In recent years, studies providing evidence for a divided spotlight of attention were called into question, on the basis that their results can be explained by a unitary attentional spotlight that simply switches very rapidly between to-be-attended locations (e.g. Jans et al., 2010; VanRullen and Dubois, 2011). Correlates of such a periodic sampling of attention have been observed in electrophysiological experiments in non-human primates (Buschman and Miller, 2009) as well as psychophysical experiments in humans (VanRullen et al., 2007). The dynamics of how attentional resources are redirected in the visual field are strongly debated, with estimates of latencies for attentional shifts between about 70ms (Nakayama and Mackeben, 1989) and 300ms (Duncan et al., 1994). Given these largely deviating estimates for the dynamics of spatial attention, even the use of very short stimulus presentation times (below 200ms) might not be enough to clearly discriminate between the divided attention hypothesis and the “blinking spotlight” model (VanRullen et al., 2007). However, a distinction between the blinking spotlight and divided attention hypothesis might be observed for attentional suppression of distracter locations. The divided spotlight theory predicts that the number of suppressed spatial locations increases from the undivided to the divided attention condition, because the number of distracters increases from one (contiguous) to two or more in the divided case and the attentional system will need to adjust to these changes in order to divide resources appropriately. This should be reflected in topographically specific increases in the amplitude of alpha oscillations, which have been shown to be tightly linked to suppression of visual space (e.g. Worden et al., 2000; Kelly et al., 2006; Thut et al., 2006; Green and McDonald, 2010; Romei et al., 2010; Gould et al., 2011). Given the behavioral findings for the blinking spotlight hypothesis (VanRullen et al., 2007), there are three different possible scenarios for attentional suppression under this model (see Predictions section in the Methods). The current study therefore examined the topographic distribution of suppressive alpha oscillations to examine whether they fit with the predictions of either model.
Another question about the ability to split the attentional spotlight relates to the timing of the attentional modulation. SSVEP and fMRI studies have provided evidence that modulation occurs in early visual cortical areas. However, due to the low temporal resolution of the methods employed, these studies are not suitable for investigating whether or not any cost involved in splitting the spotlight might impact upon the precise temporal locus of attention, i.e. whether the modulation might occur during initial feed-forward processing or if it reflects later feedback from higher cortical areas. The timing of visual cortical activity in humans is generally assessed by VEPs. However, this method is hampered by the need to present suddenly onsetting probe stimuli, which tend to exogenously grab attention and alter evoked responses. This problem can be overcome using the multifocal m-sequence technique (Sutter, 2000; Schmid et al., 2009; Ales et al., 2010a). This method allows for simultaneous recording of independent cortical evoked responses from multiple locations, and for assessment of oscillatory alpha rhythms. In this way, we can examine the timing of attentional modulation and whether these modulations are consistent with a divided spotlight account or one of the single spotlight hypotheses.
Materials and Methods
Subjects
Nineteen healthy subjects (seven female) aged between 20 and 35 participated in the study. In the final dataset 14 participants were included, since five did not have enough usable data after correcting for EEG artefacts and eye movements. All had normal or corrected-to-normal vision. The experimental procedures were approved by the Institutional Review Board at Albert Einstein College of Medicine and conformed to the tenets of the Declaration of Helsinki. All participants provided written informed consent and received a modest fee.
Experimental Stimuli and Paradigm
The stimulus configuration is shown in Figure 1. It consisted of two checkerboard stimuli located 2° above, and on either side of a fixation spot at horizontal eccentricities of 2.5° and 7.9°, respectively. The size of the inner checkerboards was 3.5° × 3.5°, with a spatial frequency of 0.7 cycles per degree, while the outer checkerboards were 4.7° × 4.7°, with a spatial frequency of 0.5 cycles per degree (Figure 1). The larger size of the outer stimuli was chosen to adjust visual stimuli for the reduction in visual cortical area devoted to peripheral space (Adams and Horton, 2003; Frey et al., 2013). Dark checks had a luminance of 0.1 and the white checks were 118.2 cd/m2. The refresh rate of the monitor (model VP2655; ViewSonic, Walnut, CA, USA) was set to 60Hz and on every refresh the checkerboard pattern of each stimulus either remained constant or was inverted as determined by a binary m-sequence of order 7 (e.g. (Sutter, 2000; Schmid et al., 2009). The binary m-sequence technique controls the inversion of the checkerboards displayed in each stimulus location using a pseudo-random sequence, which ensures that inversions in one location were statistically independent from the inversions in all other stimulus locations. Cortical evoked responses are then obtained by cross-correlation of the continuous EEG data around stimulus reversals with the checkerboard reversal sequence. An order of 7 indicates that each sequence was 27 =128 monitor refresh cycles (i.e. 2.1s) long. This duration is sufficient to fit four evoked responses of 500ms duration. In half of the trials we used this sequence and in the other half its inverse. Each trial was 2.95s long, however the m-sequence used for estimating the evoked cortical response was only 2.1s long. In order to minimize stimulus onset artifacts we used another random sequence for the first 850ms of each trial, and this timeframe was excluded from further analysis.
Figure 1.

Stimulus configuration and description of attentional conditions.
For the experimental task, we overlaid each checkerboard with a central red “X” (task-stimulus). At beginning of each block of 20 trials, participants were instructed to simultaneously attend to two of the checkerboards and count how many times their task-stimuli disappeared at the same time. This ensured that participants did not have to switch attention on each trial. Before each experimental trial, the two attended checkerboards were cued again and after a random ISI of 800–1200ms the experimental trial started. Participants were instructed to ignore the uncued checkerboards, since task-stimuli could also disappear in the uncued locations. To ensure that participants attended to both cued checkerboards simultaneously, we also added “fake targets” in which the task-stimulus disappeared in only one of the two cued checkerboards. The task instructions were held constant throughout each block of 20 trials. There were up to 2 targets and “fake targets” in each trial.
In order to analyze all possible aspects of spatial attentional modulation, we asked participants to attend to contiguous and non-contiguous parts of visual space. For the undivided attention conditions, participants were instructed to attend either both stimuli in the contiguous right hemifield (‘attend right’) or the left hemifield (‘attend left’). In order to obtain cortical responses during divided attention, participants had to attend to the inner right and outer left stimulus (‘split left’) or the inner left and outer right stimulus (‘split right’). For each of the divided attention conditions 160 trials were run, while 140 trials were recorded for the conditions in which participants had to attend within a visual hemifield. Target presentation was limited to durations up to 19 frames, i.e., for a maximum duration of approximately 317ms. Given the flickering nature of the checkerboards (see supplemental video for an example of the task with target durations set to 350ms) and the large distance between the stimuli in the divided condition (~10.5°), it would be highly unlikely to achieve a high rate of target-pair detections using a strategy of attending to one of the stimuli and shifting attention to the second stimulus as soon as a possible target is detected.
Data Acquisition
168-channel scalp EEG was recorded, amplified and digitized at 512 Hz using ActiveTwo systems (Biosemi, Amsterdam, Netherlands) with an analogue low-pass filter at 103Hz. The acquisition of the data occurs relative to an active two-electrode reference, which drives the average potential of the participant as close as possible to the reference voltage of the analogue-to-digital converter box (for a description of the Biosemi active electrode system referencing and grounding conventions, visit www.biosemi.com/faq/cms&drl.htm).
Eye movements were recorded using an EyeLink 1000 system (SR Research, Mississauga, Ontario, Canada). Even though participants rested their head on a comfortable chinrest, the eye-tracker was set to head-free mode. In this setting, the eye-tracker corrects for head movements and remains very accurate even with changing head position. Eye-position was recorded at 500 Hz and synchronized with the EEG recording using triggers at the onset of each trial. Every 8 blocks, the eye-tracker was re-calibrated using a 9 point grid.
Eye-tracking analysis
The raw eye-tracking data were filtered using a 4th order Butterworth low-pass filter with 15 Hz cut-off to eliminate rarely occurring high-frequency errors. Due to calibration error, the eye-tracker may represent the participants horizontal gaze position up to 1° to the left or right of the intended position. This “misrepresentation” will be consistent for all blocks during a calibration period. We therefore used the following method to determine the coordinate for fixation: if within one set of eight blocks with the same calibration, the difference between median eye-position in the different blocks was less than 1°, we used the median x- and y-values across all blocks as the fixation point coordinate. Otherwise the eye-tracking data was analyzed without this correction. On this filtered data, we removed all trials in which the subjects’ eyes moved more than 1.75° from fixation point. Two participants were excluded due to excessive eye-movements.
EEG analysis
All EEG data analyses were performed in Matlab using the Fieldtrip Toolbox (Oostenveld et al., 2011) as well as custom scripts. The recorded EEG was high-pass filtered with a low-cut-off of 0.5 Hz using 4th order Chebyshev filters with zero phase-shift. This filter has the advantage of very high attenuation in the stop band with minimal attenuation in the pass-band (<0.1 dB). After filtering, bad channels were determined using statistics of neighboring channels and interpolated using linear, distance-weighted interpolation. The EEG data were then referenced to the average. In addition to the deletion of trials based on eye-movements, there was also an EEG threshold of +/−125μV. If more than six channels or any of the occipital electrodes of interest exceeded this threshold, the trial was discarded. Otherwise, high amplitude channels were interpolated using linear, distance-weighted interpolation. Three participants were excluded due to large numbers of trials with EEG artifacts, bringing the total number of participants used in further analysis to 14. After removing artifact trials, an average 117 trials per condition and participant remained.
Temporal second-order kernels (see e.g. (Sutter, 2000) representing evoked cortical responses were extracted for each electrode and each of the four stimulus locations, by reverse-correlating the EEG response with the known sequence of pattern reversals. The second-order response takes into account the history of visual stimulation, i.e. whether the current pattern is the same as the one presented one monitor refresh before.
Statistical analysis
Given findings of previous studies on spatial attention (e.g. (Lalor et al., 2007; Kelly et al., 2008; Frey et al., 2010), we expect attentional modulation of the evoked responses during early cortical processing as represented by responses in the C1 and P1 time frame. Since evoked response kernels represent activity in early retinotopic cortex, which is very variable across participants (Ales et al., 2010a), the topographical distribution of peak activity was inconsistent across participants. For each stimulus location, we therefore selected two electrodes for each participant by determining mean activity across all four experimental conditions and selecting the two electrodes on the peak of the C1 and P1 topography, respectively. Therefore, for each participant and stimulus location, the two electrodes of interest were constant across experimental conditions. There is considerable variability of early visual cortical geometry between individuals and locations for which reliable C1 components can be elicited are participant-specific (Kelly et al., 2008). However we did have to present stimuli in the same stimulus locations for all participants to be able to examine the topographic distribution of attentional modulation. Therefore not all stimulus locations were optimal for observing C1 modulations.
The amplitude in the time-frame of the early components was extracted for each participant using the mean of a 20ms (C1) and 30ms (P1) window centered on the peak of the grand average. For the C1, the time range was 65 to 85ms, while for the P1 it was 110 to 140ms. These amplitudes were analyzed using repeated measures ANOVA (SPSS v.21.0) using attention (attended/unattended) and spotlight (split/non-split) as factors, as well as location (inner/outer) as a covariate.
Alpha oscillation analysis
An important aspect of providing evidence for a divided spotlight of attention is to examine the “landscape” of attentional modulation during the task (Jans et al., 2010). In the current study we examine the topographic distribution of attentional suppression for the different experimental conditions, because enhancive and suppressive effects of attention are tightly linked (Pinsk et al., 2004; Frey et al., 2010). Brain oscillations in the alpha (8–14Hz) range are known to index attentional suppression of regions of visual space (Foxe et al., 1998; Worden et al., 2000; Romei et al., 2010; Foxe and Snyder, 2011) and the topography of alpha power reflects which part of visual space needs to be ignored (Rihs et al., 2007). Since experimental trials are more than 2s long, we were able to analyze alpha amplitude and its topography concurrently with evoked activity. Alpha oscillations are not expected to be differentially affected by the m-sequence, as the flickering was present in all conditions and only task demands were varied.
For determining alpha amplitude EEG trial data were filtered between 8 and 13 Hz using a 4th order Butterworth filter. This band-pass filtered data was Hilbert-transformed and the absolute value taken. We removed the first and last 100ms of data of each trial, since these contained edge artefacts of the filter. For each time-point, the average of all different conditions was used as baseline. For displaying alpha topographies, the remaining 1.9s was averaged in order to yield one amplitude value per channel and trial. Alpha topographies were normalized (z-score) for every participant and the grand average of z-scores across participants is displayed.
A common finding for undivided spatial attention tasks is a lateralized increase in alpha amplitude when participants attend to one hemifield, with higher amplitude over the right hemisphere for the “attend right” condition and higher alpha amplitude over the left hemisphere for the “attend left” condition. Twelve participants had clean alpha oscillatory data that allowed us to quantify the number of topographic peaks and were included in the analysis of the number of peaks.
In order to determine peaks of alpha amplitude, channels with alpha amplitudes larger than median amplitude plus 1.5 times the Median Absolute Deviation (a robust measure of variability in a sample) across all channels were selected in an occipito-parietal region of interest (ROI), which covered the back of the head. A peak was defined as a group of at least two neighbouring channels. Since the number of peaks was in a very limited range and not normally distributed, we determined the mean number for the divided and undivided conditions for each participant and used the Wilcoxon signed rank test to compare the means between conditions. In addition, we determined the centre location of each alpha peak and determined the great-circle distance (the shortest distance between two points on a sphere) using the haversine formula (Sinnott, 1984). Assuming that the occipito-parietal part of the skull approximates a sphere, we used the width of a template head model as the diameter. If there were more than two alpha peaks (one participant with four detectable peaks in “split right” and two participants with three peaks in the “split left” condition) we chose the peaks with the largest distance.
Predictions
Different attentional theories predict different patterns of excitatory and suppressive modulation of cortical activity when attention is allocated to non-contiguous parts of the visual field (Figure 2A).
Figure 2.
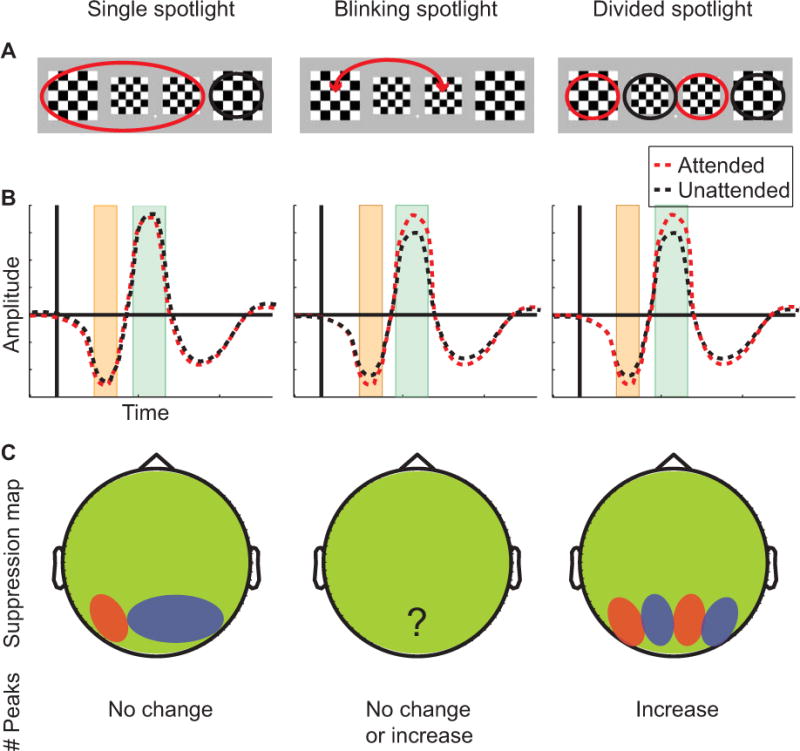
Predictions for different attentional theories.
A: Representation of how attention is thought to be allocated in the “split left” condition.
B: Hypothetical evoked cortical responses for the inner stimuli. The C1 time-frame is highlighted in orange and the P1 time-frame in green. According to the single spotlight theory, the inner stimuli are not expected to exhibit any attentional modulation.
C: Hypothetical topographies of suppressive alpha oscillations. Red areas indicate high alpha amplitude, i.e. suppression. Blue areas indicate regions of high excitability, i.e. low alpha amplitude.
For the evoked responses, we expect excitatory attentional modulation of the evoked responses for the inner stimuli in different conditions during early cortical processing. Examining the inner left stimulus, the single spotlight theory predicts that the evoked cortical response is similar / identical for the “split left” and “split right” conditions (Figure 2B), since the attentional spotlight will encompass this stimulus for both of these conditions. The same holds for the right inner stimulus. In contrast, the blinking and divided spotlight theories predict that for the inner left stimulus, the evoked response in the “split left” and “split right” conditions differ, with the “split right” response being modulated by attention.
For suppression of distracter locations (Figure 2C), single spotlight theory predicts no change in the number of alpha peaks, since there is only one stimulus which receives suppression. However, the topographic map of alpha suppression should change in order to adjust for the increase in attended space. While divided and blinking spotlight hypotheses predict the same pattern of attentional modulation for evoked responses, the two theories do not provide identical predictions for suppression of distracter locations. The divided spotlight theory predicts that the number of suppressed spatial locations increases from the undivided to the divided attention condition, because the number of distracters increases from one in the contiguous to two in the divided conditions and the attentional system will need to adjust to these changes in order to divide resources appropriately. This should be reflected in an increase in the number of peaks in the alpha topography from the undivided to the divided condition. For the blinking spotlight model of attention (VanRullen et al., 2007) we derived three possible predictions for suppression of the to-be-ignored stimuli. In this theory, the attentional spotlight is thought to constantly move between all available stimuli. Therefore the first prediction is that all unattended stimuli are suppressed individually. That is, we assume a similar mechanism exists for both suppression and excitation. For the current experimental paradigm such a mechanism would result in two peaks of suppression for the divided as well as the undivided attention condition. The second prediction is that there is no suppression of to-be-ignored stimuli, since the blinking spotlight of attention might only selectively enhance target locations. This should obviously result in alpha topographies that do not possess distinctive occipito-parietal peaks. The third prediction is that while the attentional focus switches rhythmically between all possible target locations, suppression is allocated to distracter locations in a static fashion. This would result in the same topographic distribution and increase in the number of peaks in the divided attention condition as for the divided spotlight account and indicate a static split of suppression.
Results
Behaviour
Participants were successful at performing the difficult attentional tasks. With chance level at 33.3%, the mean percentage of correct responses was around 50% for the attentional task conditions involving the outer right stimulus and around 45% for the ones involving the left outer stimulus (Figure 3). These performance values are somewhat lower than in other studies of attention, but the experimental task was more difficult due to the randomly flickering stimuli that were necessary to estimate the brain’s impulse response to all four stimuli.
Figure 3.

Percentage of correct responses (with SEM) in the four different experimental conditions.
Electrophysiology
For the C1 time-frame the repeated measures ANOVA revealed no significant main effects (F(1,54) = .2; p = .657). Only for the inner left stimulus there is a significant modulation of activity with attention (F(1,13) = 4.78; p = 0.048). This indicates that there was no influence of attention on cortical processing in this very early time-frame or that the locations of the four different stimuli were not optimal for obtaining C1 responses.
However, for the P1 component repeated measures ANOVA revealed a main effect of attention, with the mean amplitude during the P1 time frame significantly larger for the conditions in which the participant had to attend to a stimulus (F(1,54) = 4.58, p = 0.037). For both conditions (divided and undivided) the amplitude is significantly larger for attended than unattended stimuli (Figure 4). This pattern of evoked responses is in line with the predictions of the divided spotlight hypothesis.
Figure 4.
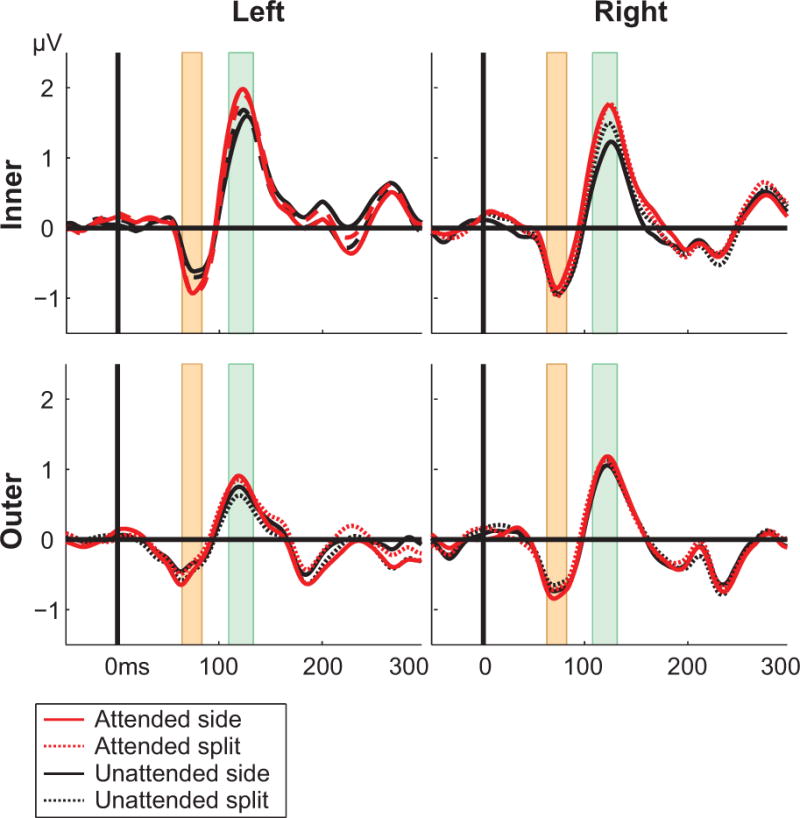
Evoked cortical responses for all stimuli and conditions. The upper panel represents responses obtained for the inner stimulus locations, the lower panel those for the outer stimuli. The C1 time-frame is highlighted in orange and the P1 time-frame in green.
To examine the attentional modulations observed in more detail, we analyzed the topographic distribution of alpha oscillatory amplitude for the different conditions. Since alpha is closely linked to attentional suppression we expected additional foci of alpha synchronization in the divided compared to the attend hemifield conditions, if humans are able to divide the attentional spotlight.
We find additional foci of alpha synchronization in the divided attention condition (Figure 5). The median number of alpha peaks in the attend hemifield condition across participants is 1.25, while it is 2 for the divided attention conditions (p < 0.05 Wilcoxon signed rank test). The median distance of the peak centres on the scalp for the “attend right” condition is 12.3 cm, while it is 10.8 cm for the “attend left” condition (Figure 5C). Only one peak was detected for four participants in the “split right” and for two participants in the “split left” condition. Topographic distribution of suppressive oscillatory activity therefore is in line with the predictions of the divided spotlight theory of attention.
Figure 5.
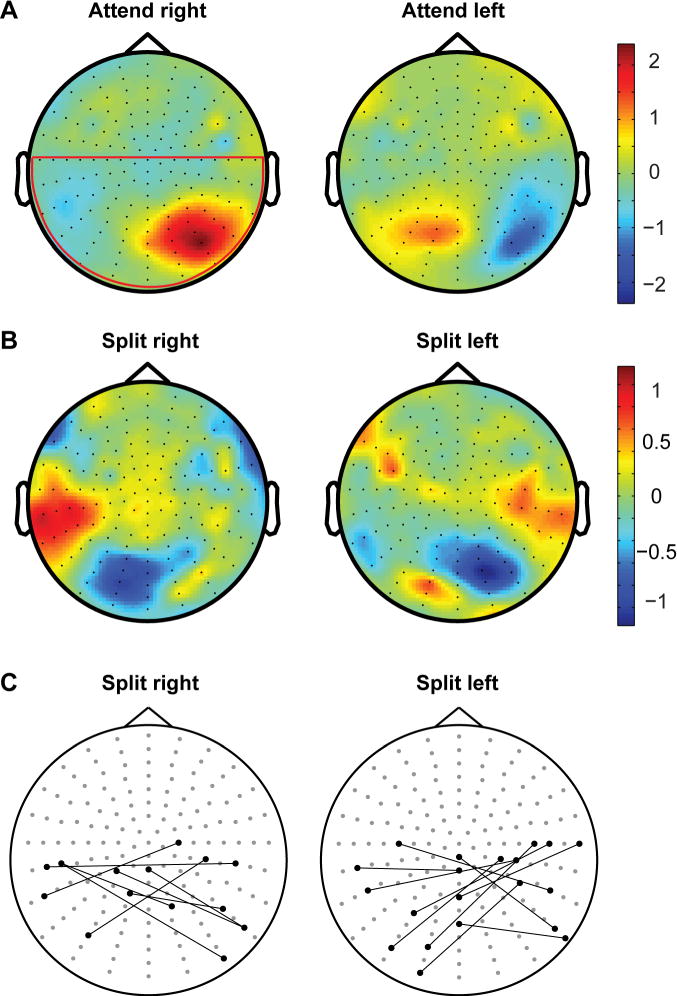
Topographic distribution of grand average alpha amplitude for A) attention to one hemifield and B) divided attention to two non-contiguous stimulus locations. Colour-maps represent z-scores averaged across participants. The red area in the “attend right” topography in panel A) represents the ROI used for analyses. Panel C depicts the centers (nearest electrode) of alpha peaks in the divided attention conditions. For each participant these peak electrodes are connected by a solid line.
Discussion
The present results support previous research providing evidence for the divided spotlight hypothesis. Topographic analyses showed that oscillatory suppressive mechanisms flexibly adjust to task demands and that whenever more than one spatial location has to be ignored there is a corresponding increase in the number of alpha oscillatory foci over occipito-parietal scalp. In addition, we provide evidence that attentional modulation for each attended stimulus, whether in contiguous or non-contiguous parts of space, occurs during early sensory-perceptual processing in extrastriate visual areas (Di Russo et al., 2002; Frey et al., 2010).
Divided versus blinking spotlight
While the results obtained for attentional enhancement and suppression match with the predictions of the divided attention model, it is not clear whether they also fit with a blinking spotlight of attention account. The idea that attention constantly samples the visual environment (VanRullen et al., 2007) is a very elegant solution to the problem of dividing attention. However, this account does not provide a clear prediction for suppression of unattended stimuli, because it assumes that the attentional system constantly samples all target stimuli. There is ample evidence that the brain employs an active mechanism of attentional suppression. Brain oscillations in the alpha range have been shown to be an index of suppression of unattended visual space (e.g. (Worden et al., 2000; Kelly et al., 2006; Thut et al., 2006; Romei et al., 2010; Gould et al., 2011; Belyusar et al., 2013), visual features (Snyder and Foxe, 2010), and even modalities (Fu et al., 2001; Banerjee et al., 2011). The experiments examining attentional allocation to contiguous parts of visual space reveal topographically specific increases in visual cortex ipsilateral to the attended visual hemifield (e.g. (Worden et al., 2000; Kelly et al., 2006; Thut et al., 2006). Under the divided spotlight of attention account it follows that the number of topographic foci of alpha should increase from the undivided to the divided attention condition, since an additional stimulus needs to be ignored. This is exactly what we find in the current study.
Based on the description of the blinking spotlight model of attention (VanRullen et al., 2007) we derived three possible predictions for suppression of the to-be-ignored stimuli. Since the spotlight is thought to constantly move between all possible target stimuli, the first prediction is that all unattended stimuli are suppressed individually. That is, we assume a similar mechanism exists for both suppression and excitation. For the current experimental paradigm such a mechanism would result in two peaks of suppression for the divided as well as the undivided attention condition. The second prediction is that there is no suppression of to-be-ignored stimuli, since the blinking spotlight of attention can be focused selectively on possible targets. Obviously this should result in alpha topographies without peaks over occipito-parietal brain areas. The results of the current study are not in line with either of these possible predictions of the blinking spotlight model. A third prediction refers to the possibility that while the attentional focus switches rhythmically between all possible target locations, suppression is static, as for the divided spotlight account. Such a prediction fits with the current results, but would indicate that at least attentional suppression behaves according to the divided attention hypothesis. Taken together, the current results provide evidence that humans are able to divide spatial attention across two locations for a considerable amount of time, if the task requires them to do so.
Suppressive mechanisms in divided attention
A very interesting observation can be made for the alpha topographies in the divided attention conditions. For the undivided conditions, where participants try to suppress a whole visual hemifield, we find a large increase in alpha amplitude ipsilateral to the ignored hemifield. However, in the divided attention conditions alpha amplitudes exhibit a large peak over contralateral visual cortex. For example, in the “split right” conditions, in which the inner left and outer right stimuli are attended, we find a large alpha peak over left occipito-parietal cortex. This peak has higher amplitude and a larger extent than the alpha peak over right visual cortex. A very similar pattern holds for the “split left” condition.
A likely explanation for this finding is that during divided attention conditions, stimuli appearing in locations between target stimuli are especially distracting, since they are positioned between the targets and are close to the fixation location. It therefore seems especially important to suppress this intervening distracter location. In contrast the unattended outer stimulus should interfere less with task demands and therefore can receive less suppression. These results indicate that the brain can flexibly adjust suppression to changing task demands.
Attentional demand
Why do some studies find evidence for a divided attention model and others not? Reviewing the scientific literature, we find that a common difference between those studies in support of and against the divided spotlight is the number and nature of distracter stimuli. In most electrophysiological and neuroimaging studies providing evidence for a divided attentional spotlight (Muller et al., 2003a; McMains and Somers, 2004; Niebergall et al., 2011) as well as the current study, the experimental task contained a small number of distracting stimuli that were continuously present and placed between to be attended stimuli at known locations. This experimental design allows participants to prepare for suppression of the distracters in order to deal more efficiently with the to-be attended stimuli. Only one electrophysiological study using a comparable experimental design did not find any evidence for divided spotlight (Heinze et al., 1994). However, this study employed a visual evoked potential (VEP) paradigm with suddenly onsetting probe stimuli at distracter locations, which likely captured exogenous attention. Therefore it is not clear whether attentional modulation of the distracter stimuli was due to a failure to divide the attentional spotlight or exogenous grabbing of attention by the probe stimuli.
Most studies providing support for serial attentional deployment did not provide a-priori defined distracters located between attended stimuli. For example, in the electrophysiological studies of Woodman and Luck (Woodman and Luck, 1999, 2003) a visual search paradigm was used, providing evidence that possible target locations are examined in a serial fashion. In this visual search paradigm, participants do not know a priori where distracters or possible targets will occur. Therefore the optimal strategy is to enhance only possible target locations, which were defined by colors. Other studies employ designs with a circular arrangement of stimuli around the fixation spot, asking participants to detect targets in a number of possible locations (Barriopedro and Botella, 1998; Muller et al., 2003b; Thornton and Gilden, 2007; VanRullen et al., 2007; Dubois et al., 2009) Even though two of these studies find some evidence for a divided spotlight of attention (Thornton and Gilden, 2007; Dubois et al., 2009), they are often regarded as supporting a single spotlight model. The circular arrangement of stimuli works in a way that the distracter stimuli are not directly between the attended stimuli. Having distracters either in locations where they are not very distracting or their locations not defined a-priori likely affects the demand of the attentional system to suppress them.
Attentional resources in humans are limited in terms of the number of objects or locations that can be processed simultaneously (e.g. Trick and Pylyshyn, 1993); for a review see (Cavanagh and Alvarez, 2005). In the current study there might be a neurophysiological correlate of this limitation. We find that the peak alpha amplitude in the divided attention condition is about half the amplitude of the undivided condition. The divided spotlight of attention account predicts that the number of to-be ignored locations increases from one in the undivided case to two in the divided attention condition. Our data therefore indicate that there is a relationship between increase in the number of suppressed locations and reduction in the amplitude of the measure of attentional suppression. Such a relationship logically would result in a limit on the number of locations/objects that can be suppressed, since at some point the amplitude of suppressive alpha oscillations might become too small to be effective. Since in many circumstances, enhancing and suppressive effects of attention are closely related (Pinsk et al., 2004; Frey et al., 2010) this decrease in suppressive alpha amplitude might directly affect the number of objects that can be processed simultaneously. Given this reasoning, it seems reasonable that the brain is able to employ a divided spotlight of attention only for a limited number of stimuli/objects. Whenever the threshold is crossed, the attentional system might set into a blinking mode (VanRullen et al., 2007) or settle into a serial search.
We therefore hypothesize that a divided spotlight of attention can only be achieved with a limited number of stimuli and distracters, which forces the attentional system to suppress them based on their location and nature. It may even be that attentional suppression is a necessary pre-requisite to observe a divided spotlight of attention. This idea is somewhat at odds with the hypothesis of Cave and colleagues (Cave et al., 2010), who proposed a model with four different modes of attention, with selection of noncontiguous regions of space and inhibition of distracter locations as separate modes. To examine the limits of divided attention and its relation to suppression are therefore interesting avenues for future research.
Theoretical considerations
In their review on attention to multiple stimulus locations, Jans and colleagues (2010) introduce several lines of evidence for their argument that divided attention is unlikely to be a standard feature of the attentional system. For example, they point out that the saliency map (Koch and Ullman, 1985), an influential model for visual attention, encodes relevance in a single spatial location. However, Jans and colleagues do not clearly mention that the original description of the saliency map model is based on psychophysical experiments in support of a serial model of visual search (Treisman and Gelade, 1980) and therefore implements a winner-take-all process selecting a single location at a time. Put another way, the saliency map model was defined based on the experimental results at the time it was invented and the predominant view of visual attention was the one of a serial process. As such, the saliency map is not a valid model to generate hypotheses regarding whether the attentional spotlight can be divided or not.
Timing of attentional modulation
The current study did not provide evidence that the earliest detectable evoked activity is modulated by attention for all stimuli across the visual field. Only in one of the four locations we find a significant modulation of this C1 component. The evoked activity in this time range is thought to largely represent processing in V1 (Kelly et al., 2013), with possible contributions from extrastriate areas V2 and V3 (Ales et al., 2010b). Our results could therefore be interpreted as evidence for attention not modulating afferent activity in early visual areas. However, they could also indicate that only one stimulus was in a location, for which we could observe attentional modulation. The difficulty to obtain robust C1 responses has been described in detail (Kelly et al., 2008). For a large number of participants in their study a stimulus in the upper left hemifield was optimal. This location is comparable to the one for which we find clear modulations in the C1 time frame. Therefore we interpret our results as indicating that divided spatial attention likely modulates earliest evoked cortical activity. However, a paradigm with stimulus locations mapped to individual participants is necessary to provide evidence that this modulation occurs across the visual field.
Acknowledgments
Funding
This work was primarily supported by a grant from the US National Science Foundation (NSF) to J.J.F (BCS0642584) and grants from the U.S. National Institute of Health (RO1 MH085322 to J.J.F. and S.M.). The work of Author A.M.S. on this project was supported by RO1 EY9314 to Professor Jonathan D. Victor of Weill Cornell Medical College. The Human Clinical Phenotyping Core, where the participants enrolled in this study were recruited and evaluated, is a facility of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (RFK-IDDRC) which is funded by a center grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P30 HD071593). Ongoing support of The Cognitive Neurophysiology Laboratory is provided through a grant from the Sheryl and Daniel R. Tishman Charitable Foundation.
Footnotes
Conflict of Interest Statement
All authors of this paper declare no conflicts-of-interest, financial or otherwise, that could have biased their contributions to this work. The senior author, Foxe, attests that all authors had access to the full dataset and to all stages of the analyses.
References
- Adams DL, Horton JC. A precise retinotopic map of primate striate cortex generated from the representation of angioscotomas. J Neurosci. 2003;23:3771–3789. doi: 10.1523/JNEUROSCI.23-09-03771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ales J, Carney T, Klein SA. The folding fingerprint of visual cortex reveals the timing of human V1 and V2. Neuroimage. 2010a;49:2494–2502. doi: 10.1016/j.neuroimage.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ales JM, Yates JL, Norcia AM. V1 is not uniquely identified by polarity reversals of responses to upper and lower visual field stimuli. Neuroimage. 2010b;52:1401–1409. doi: 10.1016/j.neuroimage.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Pashler H. Evidence for split attentional foci. J Exp Psychol Hum Percept Perform. 2000;26:834–846. doi: 10.1037//0096-1523.26.2.834. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Snyder AC, Molholm S, Foxe JJ. Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9923–9932. doi: 10.1523/JNEUROSCI.4660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriopedro MI, Botella J. New evidence for the zoom lens model using the RSVP technique. Perception & psychophysics. 1998;60:1406–1414. doi: 10.3758/bf03208001. [DOI] [PubMed] [Google Scholar]
- Belyusar D, Snyder AC, Frey HP, Harwood MR, Wallman J, Foxe JJ. Oscillatory alpha-band suppression mechanisms during the rapid attentional shifts required to perform an anti-saccade task. Neuroimage. 2013;65:395–407. doi: 10.1016/j.neuroimage.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations. Neuron. 2009;63:386–396. doi: 10.1016/j.neuron.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello U, Umilta C. Splitting focal attention. J Exp Psychol Hum Percept Perform. 1992;18:837–848. doi: 10.1037//0096-1523.18.3.837. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Alvarez GA. Tracking multiple targets with multifocal attention. Trends in cognitive sciences. 2005;9:349–354. doi: 10.1016/j.tics.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cave KR, Bush WS, Taylor TG. Split attention as part of a flexible attentional system for complex scenes: comment on Jans, Peters, and De Weerd (2010) Psychological review. 2010;117:685–696. doi: 10.1037/a0019083. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Human brain mapping. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hamker FH, VanRullen R. Attentional selection of noncontiguous locations: the spotlight is only transiently “split”. Journal of vision. 2009;9(3):1–11. doi: 10.1167/9.5.3. [DOI] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369:313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, St James JD. Visual attention within and around the field of focal attention: a zoom lens model. Perception & psychophysics. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Frontiers in psychology. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Frey HP, Kelly SP, Lalor EC, Foxe JJ. Early spatial attentional modulation of inputs to the fovea. J Neurosci. 2010;30:4547–4551. doi: 10.1523/JNEUROSCI.5217-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey HP, Molholm S, Lalor EC, Russo N, Foxe JJ. Atypical cortical representation of peripheral visual space in children with an Autism Spectrum Disorder (ASD) Eur J Neurosci. 2013 doi: 10.1111/ejn.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain research Cognitive brain research. 2001;12:145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Gobell JL, Tseng CH, Sperling G. The spatial distribution of visual attention. Vision Res. 2004;44:1273–1296. doi: 10.1016/j.visres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Gould IC, Rushworth MF, Nobre AC. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J Neurophysiol. 2011;105:1318–1326. doi: 10.1152/jn.00653.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JJ, McDonald JJ. The role of temporal predictability in the anticipatory biasing of sensory cortex during visuospatial shifts of attention. Psychophysiology. 2010;47:1057–1065. doi: 10.1111/j.1469-8986.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Luck SJ, Munte TF, Gos A, Mangun GR, Hillyard SA. Attention to adjacent and separate positions in space: an electrophysiological analysis. Perception & psychophysics. 1994;56:42–52. doi: 10.3758/bf03211689. [DOI] [PubMed] [Google Scholar]
- Jans B, Peters JC, De Weerd P. Visual spatial attention to multiple locations at once: the jury is still out. Psychological review. 2010;117:637–684. doi: 10.1037/a0019082. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial attention modulates initial afferent activity in human primary visual cortex. Cerebral cortex. 2008;18:2629–2636. doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Schroeder CE, Lalor EC. What does polarity inversion of extrastriate activity tell us about striate contributions to the early VEP? A comment on Ales et al. (2010) Neuroimage. 2013;76:442–445. doi: 10.1016/j.neuroimage.2012.03.081. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. Journal of neurophysiology. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Human neurobiology. 1985;4:219–227. [PubMed] [Google Scholar]
- Lalor EC, Kelly SP, Pearlmutter BA, Reilly RB, Foxe JJ. Isolating endogenous visuospatial attentional effects using the novel visual-evoked spread spectrum analysis (VESPA) technique. Eur J Neurosci. 2007;26:3536–3542. doi: 10.1111/j.1460-9568.2007.05968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMains SA, Somers DC. Multiple spotlights of attentional selection in human visual cortex. Neuron. 2004;42:677–686. doi: 10.1016/s0896-6273(04)00263-6. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC. Processing efficiency of divided spatial attention mechanisms in human visual cortex. J Neurosci. 2005;25:9444–9448. doi: 10.1523/JNEUROSCI.2647-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of the attentional spotlight. Nature. 2003a;424:309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- Muller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA. A physiological correlate of the “Zoom Lens” of visual attention. J Neurosci. 2003b;23:3561–3565. doi: 10.1523/JNEUROSCI.23-09-03561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Res. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Niebergall R, Khayat PS, Treue S, Martinez-Trujillo JC. Multifocal attention filters targets from distracters within and beyond primate MT neurons’ receptive field boundaries. Neuron. 2011;72:1067–1079. doi: 10.1016/j.neuron.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational intelligence and neuroscience. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, Kastner S. Push-pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol. 2004;92:622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. The Quarterly journal of experimental psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid AM, Purpura KP, Ohiorhenuan IE, Mechler F, Victor JD. Subpopulations of neurons in visual area v2 perform differentiation and integration operations in space and time. Frontiers in systems neuroscience. 2009;3:15. doi: 10.3389/neuro.06.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RW. Virtues of the Haversine. Sky and Telescope. 1984;68:159. [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory attentional suppression of visual features indexed by oscillatory alpha-band power increases: a high-density electrical mapping study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4024–4032. doi: 10.1523/JNEUROSCI.5684-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter E. The interpretation of multifocal binary kernels. Documenta ophthalmologica Advances in ophthalmology. 2000;100:49–75. doi: 10.1023/a:1002702917233. [DOI] [PubMed] [Google Scholar]
- Thornton TL, Gilden DL. Parallel and serial processes in visual search. Psychological review. 2007;114:71–103. doi: 10.1037/0033-295X.114.1.71. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Trick LM, Pylyshyn ZW. What enumeration studies can show us about spatial attention: evidence for limited capacity preattentive processing. J Exp Psychol Hum Percept Perform. 1993;19:331–351. doi: 10.1037//0096-1523.19.2.331. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Dubois J. The psychophysics of brain rhythms. Frontiers in psychology. 2011;2:203. doi: 10.3389/fpsyg.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Carlson T, Cavanagh P. The blinking spotlight of attention. Proc Natl Acad Sci U S A. 2007;104:19204–19209. doi: 10.1073/pnas.0707316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. J Exp Psychol Hum Percept Perform. 2003;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


