Abstract
Although emerging evidence suggests that low levels of vitamin D may contribute to the development of autoimmune disease, the relationship between vitamin D reduction and autoimmune thyroid disease (AITD), which includes Graves’ disease (GD) and Hashimoto thyroiditis (HT), is still controversial. The aim was to evaluate the association between vitamin D levels and AITD through systematic literature review. We identified all studies that assessed the association between vitamin D and AITD from PubMed, Embase, CENTRAL, and China National Knowledge Infrastructure (CNKI) databases. We included studies that compared vitamin D levels between AITD cases and controls as well as those that measured the odds of vitamin D deficiency by AITD status. We combined the standardized mean differences (SMD) or the odds ratios (OR) in a random effects model. Twenty case-control studies provided data for a quantitative meta-analysis. Compared to controls, AITD patients had lower levels of 25(OH)D (SMD: −0.99, 95% CI: −1.31, −0.66) and were more likely to be deficient in 25(OH)D (OR 2.99, 95% CI: 1.88, 4.74). Furthermore, subgroup analyses result showed that GD and HT patients also had lower 25(OH)D levels and were more likely to have a 25(OH)D deficiency, suggesting that low levels of serum 25(OH)D was related to AITD.
Keywords: vitamin D, autoimmune thyroid disease, Graves’ disease, Hashimoto thyroiditis, meta-analysis
1. Introduction
Because an estimated one billion people worldwide have vitamin D deficiency or insufficiency [1], vitamin D has become an important focus of current medical research. Although the biological activities of vitamin D are mainly manifested in the regulation of calcium-phosphorus metabolism, studies in the past 30 years indicate vitamin D may play an important role in the immune system [2,3]. Results show that 1,25-dihydroxyvitamin D3 can either prevent or markedly suppress experimental autoimmune encephalomyelitis, rheumatoid arthritis, systemic lupus erythematosus, type 1 diabetes, and inflammatory bowel disease [4,5,6,7,8]. Clinical trials have also shown that vitamin D supplements may reduce the incidence of rheumatoid arthritis, multiple sclerosis, and type 1 diabetes in children [1]. In the past two decades, vitamin D receptors have been found not only in bone, kidney, and intestine, but also in the immune system (T and B cells, macrophages, and monocytes), reproductive system, endocrine system, muscles, brain, skin, and liver [9], suggesting that the role of vitamin D is not limited to the skeletal system.
Recently, many studies have shown that low levels of vitamin D contribute to Graves’ disease (GD) and Hashimoto thyroiditis (HT) and that combining vitamin D with anti-thyroid drugs or thyroid hormone contributes to the treatment of autoimmune thyroid disease (AITD) by suppressing the autoimmune reaction and reducing serum levels of thyroid autoantibodies [10,11]. However, other authors have proposed that vitamin D deficiency does not increase the risk of AITD and is not associated with early-stage AITD [12,13]. Because the association between vitamin D levels and AITD is still controversial, we conducted a systematic review of the published studies that investigated the relationship between serum 25(OH)D levels and AITD.
2. Methods
2.1. Bibliographic Search
A bibliographic search was performed on PubMed, Embase, CENTRAL, and China National Knowledge Infrastructure (CNKI) (updated to 20 December 2014) by two investigators (Jiying Wang and Shishi Lv) using the key words “vitamin D”, in combination with “autoimmune thyroid disease”, “thyroid autoimmunity”, “Graves’ disease” or “Hashimoto thyroiditis”. Articles were only considered if they were in English or Chinese and were not hand-searched.
2.2. Eligibility Criteria and Excluded Studies
Articles were included in this meta-analysis if (1) they described a population-based case-control study; (2) the case group consisted of AITD patients and the control group included healthy individuals; (3) the outcome measures reported quantitative vitamin D levels (mean ± SD) and qualitative vitamin D levels (odds of vitamin D deficiency); (4) the study was a high-quality study (≥7 points according to the Cochrane’s Newcastle-Ottawa Scale evaluation standard for case-control studies [14]); and (5) was written in English or Chinese. After reading the title and abstract, we excluded a study if it was an animal or in vitro experiment, did not contain original data (e.g., was a medical recapitulate), was not related to AITD, did not contain data on vitamin D, or was not a case-control study, case reports, and studies consisting of duplicate data. After reading the full text, we excluded from the study if the comparator group did not conform to the requirements (e.g., compared female patients and male patients), duplicate publication, conference abstracts, no data about vitamin D level (mean ± SD), inconsistent data, or it did not refer to AITD. Disagreement was resolved by discussion between the authors (Jiying Wang and Shishi Lv). If they could not reach a consensus, another investigator (Yong Xu) was consulted regarding the disagreements.
2.3. Data Extraction
The following information was extracted from each study: the author, publication year, participant characteristics (age, gender, number), season, type of serum vitamin D assay, serum vitamin D (reported in ng/mL; for studies that reported vitamin D in nmol/L, we converted the values to ng/mL by dividing by 2.496 [15]), the number of patients with vitamin D deficiency, the cut-off for defining vitamin D deficiency, p value, and study quality. Information was independently extracted by Jiying Wang and Guo Chen, and all data were confirmed by another author (Chenlin Gao).
2.4. Statistical Method
For studies that reported quantitative vitamin D levels for AITD participants and controls, we combined the standardized mean differences (SMD) in a random effects model. For studies that reported qualitative vitamin D levels, we pooled the odds ratios (OR) in a random effects model. We assessed statistical heterogeneity using Q-tests and the I2 statistic. Publication bias was assessed using Egger’s test (p < 0.1 was considered to be publication bias). All analyses were carried out using the commands metan and metabias in Stata software, version 12.0 (Stata Corp).
3. Results
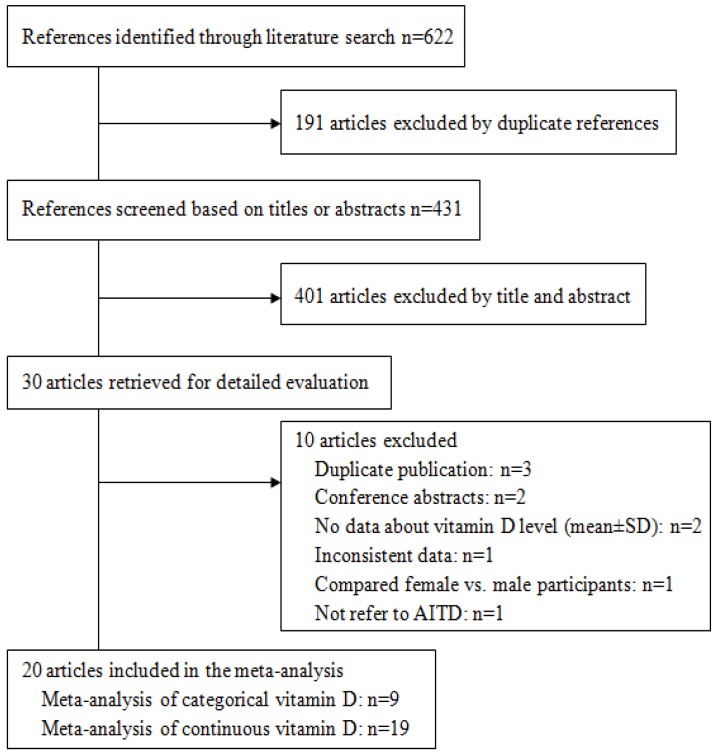
Our search identified 431 unique references, of which 411 did not meet our inclusion criteria. We conducted meta-analyses on the remaining 20 articles [10,11,13,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. (Figure 1). Of the 20 included articles, 19 were used to analyze continuous data on vitamin D levels (Table 1) and nine were used to analyze dichotomous data on vitamin D (deficiency or no deficiency) (Table 2).
Figure 1.
Flow diagram showing study selection.
Table 1.
Studies with continuous data on vitamin D levels in AITD and controls.
| First Author and Year | AITD (N)/Total (N) | AITD, males, % | AITD, Year, mean ± SD | Assay Method | Season of Collected Samples | 25(OH)D in AITD, ng/mL mean ± SD | 25(OH)D in Control, ng/mL mean ± SD | p-Value | Quality of Study (Score) |
|---|---|---|---|---|---|---|---|---|---|
| Yusuda T 2013 | 36/85 | 0 | 37.8 ± 8.1 | CPBA | Sum, A | 14.5 ± 2.9 | 18.6 ± 5.3 | <0.0005 | 8 |
| Tamer G 2011 | 161/323 | 6/161 | 35.4 ± 7.9 | RIA | W | 16.3 ± 10.4 | 29.6 ± 25.5 | <0.0001 | 9 |
| Yusuda T 2012 | 26/72 | 0 | 37.3 ± 13.0 | CPBA | W, S | 14.4 ± 4.9 | 17.1 ± 4.1 | <0.05 | 8 |
| Bozkurt NC 2013 | 360/540 | 114/360 | 42.55 ± 11.35 | ELISA | Sum | 12.2 ± 5.6 | 15.4 ± 6.8 | <0.001 | 8 |
| Effraimidis G 2012 | 67/134 | NG | 38.3 ± 11.5 | RIA | ALL | 21.6 ± 9.2 | 21.2 ± 9.3 | NS | 8 |
| Han Y 2013 | 30/50 | 6/30 | 35.7 ± 7.3 | HPLC | W, S | 17.51 ± 6.14 | 58.84 ± 8.01 | <0.01 | 7 |
| Miao W 2013 | 70/140 | 22/70 | 40 ± 15.2 | ECLIA | W, S | 12.7 ± 5.25 | 16.56 ± 5.8 | <0.01 | 9 |
| Huang ZL 2013 | 40/60 | 6/40 | 44.6 ± 8.5 | ECLIA | S, A | 16.26 ± 4.16 | 49.5 ± 8.68 | <0.01 | 8 |
| Liu XH 2012 | 160/325 | 25/160 | 43.25 ± 8.55 | ECLIA | W, S, Sum | 13.51 ± 5.88 | 19.48 ± 10.12 | <0.05 | 8 |
| Xuan LY 2014 | 89/134 | 32/89 | 33.92 ± 12.70 | ELISA | ALL | 19.04 ± 9.72 | 29.95 ± 13.86 | <0.01 | 7 |
| Shin DY 2014 | 111/304 | 21/111 | 48.7 ± 12.7 | RIA | ALL | 12.6 ± 5.5 | 14.5 ± 7.3 | <0.001 | 8 |
| Li YB 2014 | 40/90 | 0 | 34 ± 14 | ELISA | W, S | 13 ± 5 | 29 ± 5 | <0.05 | 8 |
| Zhang H 2014 | 70/140 | 28/70 | 31.77 ± 10.32 | ELISA | S | 21.15 ± 4.41 | 24.28 ± 4.37 | <0.05 | 8 |
| Jyotsna VP 2012 | 80/160 | 18/80 | 36.33 ± 11.15 | RIA | ALL | 12.67 ± 6.24 | 10.99 ± 7.05 | <0.05 | 7 |
| Mansournia N 2014 | 41/86 | NG | 42.3 ± 15.3 | HPLC | A | 15.9 ± 12.1 | 24.4 ± 17.3 | <0.01 | 8 |
| Zheng Y 2014 | 33/72 | 14/33 | 35.3 ± 9.23 | ELISA | ALL | 15.71 ± 6.79 | 30.84 ± 8.57 | <0.01 | 7 |
| Wang YC 2014 | 60/90 | 22/60 | 35.1 ± 7.95 | ECLIA | W, S, Sum | 12.28 ± 5.83 | 18.1 ± 5.92 | <0.01 | 7 |
| Kang DH 2013 | 280/719 | 100/280 | 42.5 ± 7.9 | ELISA | A | 21.68 ± 9.54 | 24.05 ± 9.58 | <0.01 | 7 |
| Wang ZS 2014 | 28/79 | 0 | NG | ECLIA | ALL | 26.98 ± 9.02 | 19.05 ± 5.47 | <0.01 | 7 |
(Introductions of Table 1: (1) Assay method: ELISA, enzyme-linked immunosorbent assay; HPLC, high performance liquid chromatography; ECLIA, chemiluminescence immunoassay; CPBA, competitive protein binding assay; RIA, radioimmunoassay; (2) Season: S, spring; Sum, summer; A, autumn; W, winter. (3) N, number; NG, not given; NS, not significant.)
Table 2.
Studies with dichotomous data on vitamin D deficiency and no deficiency in AITD and controls.
| First Author and Year | AITD(N)/Total (N) | AITD, Males, % | AITD, year (Mean or Range) | Assay Method | Season of Collected Samples | 25(OH)D Deficiency in AITD (N) | 25(OH)D Deficiency in Control (N) | Criterion of 25(OH)D Deficiency | p-Value | Quality of Study (Score) |
|---|---|---|---|---|---|---|---|---|---|---|
| Yusuda T 2012 | 26/72 | 0 | 37.3 ± 13.0 | CPBA | W, S | 17 | 15 | <15 ng/mL | <0.05 | 8 |
| Bozkurt NC 2013 | 360/540 | 57/180 | 42.55 ± 11.35 | ELISA | Sum | 150 | 37 | <10 ng/mL | <0.001 | 8 |
| Effraimidis G 2012 | 67/134 | NG | 38.3 ± 11.5 | RIA | ALL | 33 | 23 | <20 ng/mL | =0.05 | 8 |
| HanY 2013 | 30/50 | 6/30 | 35.7 ± 7.3 | HPLC | W, S | 16 | 0 | <20 ng/mL | <0.01 | 7 |
| Miao W 2013 | 70/140 | 22/70 | 40 ± 15.2 | ECLIA | W, S | 65 | 54 | <20 ng/mL | <0.05 | 9 |
| Kivity S 2011 | 50/148 | 6/50 | 45 ± 16 | DCCLIA | S | 35 | 37 | <10 ng/mL | <0.001 | 8 |
| Zhang H 2014 | 70/140 | 28/70 | 31.77 ± 10.32 | ELISA | S | 30 | 10 | <20 ng/mL | <0.05 | 8 |
| Kang DH 2013 | 280/719 | 100/280 | 42.5 ± 7.9 | ELISA | A | 133 | 158 | <20 ng/mL | <0.01 | 7 |
| Mansourria N 2014 | 41/86 | NG | 42.3 ± 15.3 | HPLC | A | 34 | 24 | <20 ng/mL | 0.82 | 8 |
(Introductions of Table 2: (1) Assay method: ELISA, enzyme-linked immunosorbent assay; HPLC, high performance liquid chromatography; ECLIA, chemiluminescence immunoassay; CPBA, competitive protein binding assay; DCCLIA, direct competitive chemiluminescence immunoassay; RIA, radioimmunoassay. (2) Season: S, spring; Sum, summer; A, autumn; W, winter. (3) N, number; NG, not given; NS, not significant.)
Overall, most studies showed a higher prevalence of vitamin D deficiency and lower vitamin D levels in AITD patients compared with controls.
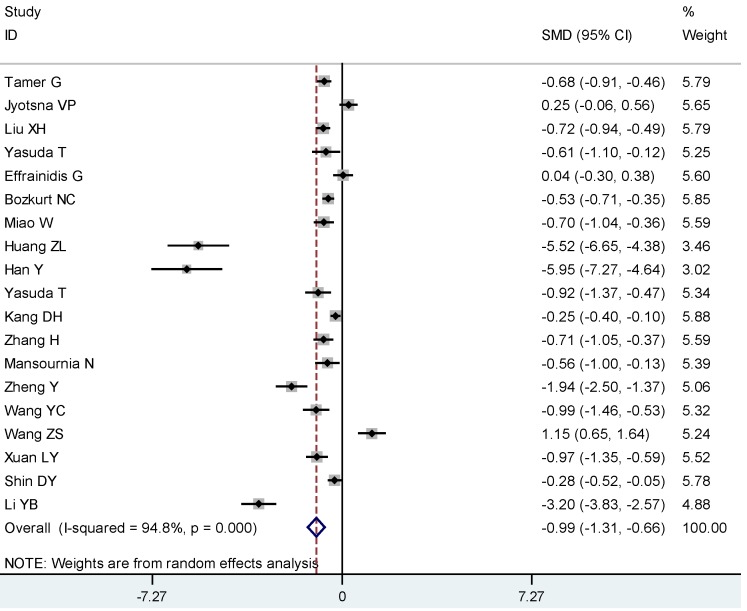
The meta-analysis of the continuous vitamin D by AITD status included 3603 participants (1782 AITD cases and 1821 controls). On average, AITD patients had lower levels of 25(OH)D compared to controls (SMD: −0.99, 95% CI: −1.31, −0.66) (I2 94.8%, p < 0.01) (Figure 2). We found evidence of publication bias as evidenced by Egger’s test (p = 0.009).
Figure 2.
Meta-analysis of studies (chronologically ordered) reporting 25(OH)D levels in autoimmune thyroid disease (AITD) vs. controls, standardized mean difference with 95% confidence interval.
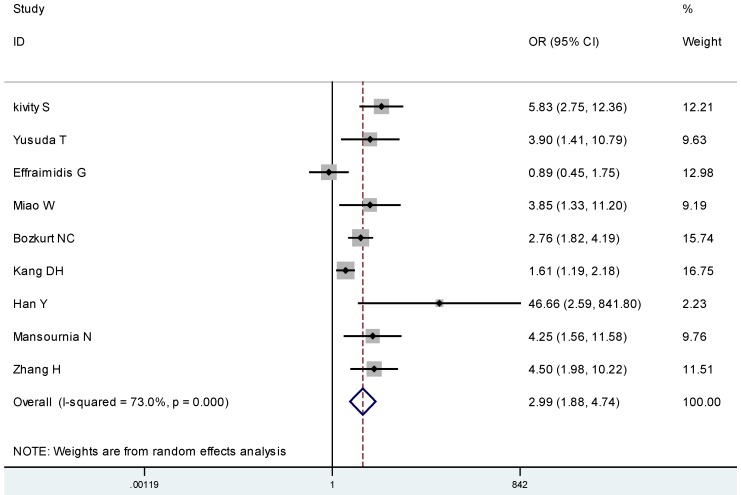
For the presence of vitamin D deficiency, nine studies totaling 994 AITD participants and 1035 controls were included. AITD participants were more likely to be deficient in 25(OH)D (OR 2.99, 95% CI: 1.88, 4.74) (I2 73.0%, p < 0.01) compared to their controls (Figure 3). We found evidence of publication bias as evidenced by Egger’s test (p = 0.056).
Figure 3.
Meta-analysis of studies (chronologically ordered) reporting dichotomous data on 25(OH)D levels in autoimmune thyroid disease (AITD) vs. controls and estimated odds ratios (ORs) with 95% confidence interval.
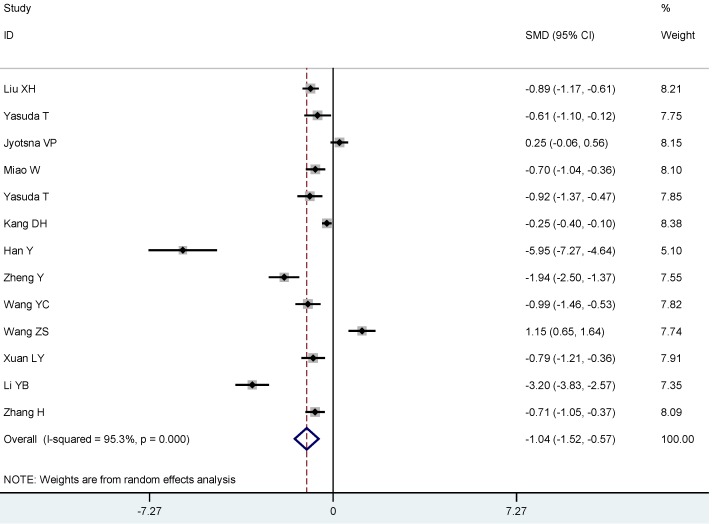
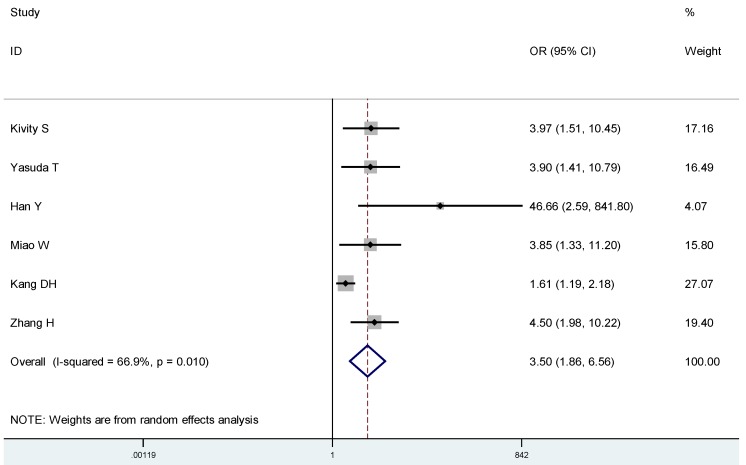
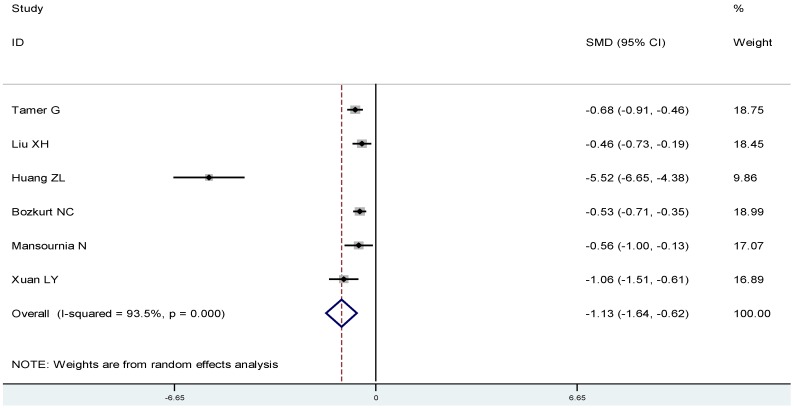
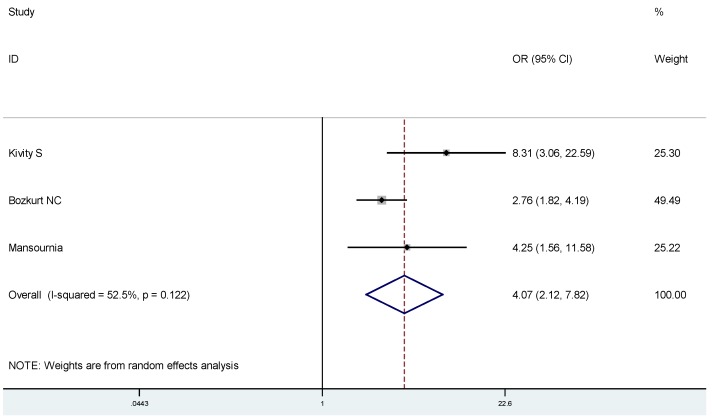
To estimate the association between 25(OH)D and Graves’ disease or Hashimoto thyroiditis, respectively, we conducted subgroup analyses: On average, Graves’ disease patients had lower 25(OH)D compared to controls (SMD: −1.04, 95% CI: −1.52, −0.57) (Figure 4), and were more likely to have a 25(OH)D deficiency(OR 3.50, 95% CI: 1.86, 6.56) (Figure 5). Likewise, Hashimoto thyroiditis patients had lower 25(OH)D compared to controls (SMD: −1.13, 95% CI: −1.64, −0.62) (Figure 6), and were more likely to have a 25(OH)D deficiency (OR 4.07, 95% CI: 2.12, 7.82) (Figure 7).
Figure 4.
Meta-analysis of studies (chronologically ordered) reporting 25(OH)D levels in Graves’s disease vs. controls, standardized mean difference with 95% confidence interval.
Figure 5.
Meta-analysis of studies (chronologically ordered) reporting dichotomous outcomes of 25(OH)D levels in Graves’ disease vs. controls and estimated ORs with 95% confidence interval.
Figure 6.
Meta-analysis of studies (chronologically ordered) reporting 25(OH)D levels in Hashimoto thyroiditis vs. controls, standardized mean difference with 95% confidence interval.
Figure 7.
Meta-analysis of studies (chronologically ordered) reporting dichotomous data of 25(OH)D levels in Hashimoto thyroiditis vs. controls and estimated ORs with 95% confidence interval.
4. Discussion
The association between low serum vitamin D and autoimmune diseases has been generally accepted by researchers. Bellastella G. found that automimmune disease patients showed 25(OH)D levels significantly lower than healthy controls [33]. A meta-analysis of vitamin D receptor gene polymorphisms and AITD showed a significant correlation between certain vitamin D receptor gene polymorphisms (such as BsmI and TaqI) and autoimmune thyroid diseases [34], but no meta-analysis of serum vitamin D levels and AITD has been published to date. In the present study, the serum 25(OH)D was lower in AITD patients compared to healthy control individuals, and AITD was more likely to develop in individuals who showed serum 25(OH)D deficiencies, which suggested that vitamin D deficiency may play a role in the pathological process of AITD.
AITD has been traditionally thought to be related to unbalanced ratio of T helper cell type 1 (Th1) and Th2 cells. Graves’ disease occurs when a high proportion of Th2 cells are present and secrete the cytokine IL-4 [35,36,37], and a complete lack of IL-4 has been shown to eliminate Graves’ disease in animal model [38]. Conversely, Hashimoto thyroiditis patients have a high proportion of Th1 cells, which secrete the cytokine IFN-γ [39]. Recent studies showed the secretion of cytokines from Th17 is involved in the development of AITD [40,41]. IF-γ and IF-17A mRNA expression is significantly higher in Hashimoto thyroiditis patients than in healthy controls [42,43]. Interestingly, vitamin D plays an important role in regulating Th1, Th2, and Th17 cells, as well as the secretion of IFN-γ, IL-4, and IL-17 [44,45,46,47]. These findings may explain why lower levels of vitamin D contribute to thyroid gland immune disorder. On the other hand, Graves’ disease is an autoimmune thyroid disorder in which thyrotrophin receptor antibody (TRAb) causes hyperthyroidism [48]. Low vitamin D status is associated with increased TRAb in this disease [22]. Results also show that levels of 25O (HD) <50 nmol/L are a risk factor for positive thyroid autoantibody (Thyroid peroxidase antibody (TPOAb) and thyroglobulin antibodies (TgAb)) [49]. Thus, this increased thyroid autoantibody in AITD, may be a consequence of the lower levels of vitamin D contributes to AITD.
The levels of vitamin D may dictate the prognosis of Graves’ disease [50], and may create an opportunity for vitamin D supplementation for patients? Research by Kawakami-Tani shows that concomitant administration (such as thyroid hormones or anti-thyroid drugs) of 1α(OH)D3 is useful for treating hyperthyroidism in patients with Graves’ disease [51]. Moreover, preliminary results of a small randomized controlled trial also showed that vitamin D treatment significantly decreased TPOAb and TgAb compared with placebo treatment in AITD patients [52]. Current evidence, however, is not definitive, the cost-effectiveness of vitamin D supplementation in AITD patients, as well as its optimal safe doses require further investigation.
To our knowledge, this was the first meta-analysis to investigate the association between vitamin D levels and AITD. The inclusion of Embase, PubMed, CENTRAL, and the CNKI database added strength to our study. However, our study had some limitations. Firstly, many of the original studies did not adjust for potentially important confounders, such as season or assay method. The prevalence of vitamin D deficiency and mean 25(OH)D levels did not distinctly different between winter and summer weather [53]. Limitations in reaching significant difference may be due to interassay and interlaboratory variability in measurements of vitamin D [54], the cut-off for defining vitamin D deficiency and the method of AITD diagnosis, which varied across studies, along with the language differences among the studies and publication bias may have contributed to the heterogeneity of our findings. Criterion of vitamin D deficiency include <10 ng/mL, <15 ng/mL and <20 ng/mL, but result showed that cut-points of vitamin D deficiency should be assay specific rather than universal and that greater consistency between laboratories is required [53]. In the studies, AITD was diagnosed by thyroid function test, anti-thyroid antibodies, with or without ultrasonography. Thirdly, due to the nature of the abstracted case control studies in our review, further prospective studies are needed to clarify whether reduced vitamin D level is a causal factor in the pathogenesis of autoimmune diseases or a consequence of this. Finally, we found statistical heterogeneity in our analysis. However, we did not find any major clinical heterogeneity and therefore the pooled analysis was appropriate for our study.
5. Conclusions
In conclusion, we have demonstrated that vitamin D deficiency is prevalent in AITD subjects and that these subjects have lower levels of serum 25(OH)D, suggesting that lower serum vitamin D is related to AITD and the deficiency in vitamin D may plays a role in the development of the disease. Large-sample multi-center randomized controlled trials will help to consolidate whether there is an association between vitamin D and AITD, and consequently give directions as to the beneficial effect of vitamin D supplementation in those patients.
Acknowledgments
The authors would like to thank BioMed Proofreading LLC for English expression polishing.
Author Contributions
Jiying Wang and Shishi Lv performed bibliographic search; Jiying Wang, Guo Chen and Chenlin Gao collected data and performed statistical analyses; Jiying Wang and Haihua Zhong drafted the manuscript, Jianhua He and Yong Xu revised the manuscript and contributed to the discussion. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Lemire J.M., Adams J.S., Sakai R., Jordan S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rigby W.F., Stacy T., Fanger M.W. Inhibition of T lymphocyte mitogenesis by 1, 25-dihydroxyvitamin D3 (Calcitriol) J. Clin. Investig. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deluca H.F., Cantorna M.T. Vitamin D: Its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Raya A., Abou-Raya S., Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: A randomized placebo-controlled trial. J. Rheumatol. 2013;40:265–272. doi: 10.3899/jrheum.111594. [DOI] [PubMed] [Google Scholar]
- 6.Grishkan I.V., Fairchild A.N., Calabresi P.A., Gocke A.R. 1,25-Dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proc. Natl. Acad. Sci. USA. 2013;110:21101–21106. doi: 10.1073/pnas.1306072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ananthakrishnan A.N., Cagan A., Gainer V.S., Cheng S.C., Cai T., Szolovits P., Shaw S.Y., Churchill S., Karlson E.W., Murphy S.N., et al. Higher plasma vitamin D is associated with reduced risk of Clostridium difficile infection in patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2014;39:1136–1142. doi: 10.1111/apt.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skaaby T., Husemoen L.L., Thuesen B.H., Linneberg A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine. 2015 doi: 10.1007/s12020-015-0547-4. [DOI] [PubMed] [Google Scholar]
- 9.Verstuyf A., Carmeliet G., Bouillon R., Mathieu C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010;78:140–145. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z.L. Master Dissertation. Jilin University; Jilin, China: 2013. The Study on Relationship between Serum 25-Hydroxyvitamin D3 Concentration and Hashimoto Thyroiditis. [Google Scholar]
- 11.Liu X.H. Master Dissertation. Zhengzhou University; Zhengzhou, China: 2012. The Study on Relation between Vitamin D3 Level and Immune Disorder in Patients with Autoimmune Thyroid Disease. [Google Scholar]
- 12.Sezgin G., Esref O.M. Relationship of vitamin D deficiency and autoimmune thyroid diseases. Eur. J. Internal Med. 2011;22:87. doi: 10.1016/S0953-6205(11)60355-5. [DOI] [Google Scholar]
- 13.Effraimidis G., Badenhoop K., Tijssen J.G., Wiersinga W.M. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur. J. Endocrinol. 2012;167:43–48. doi: 10.1530/EJE-12-0048. [DOI] [PubMed] [Google Scholar]
- 14.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. [(accessed on 1 December 2014)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Eliades M., Spyrou E., Agrawal N., Lazo M., Brancati F.L., Potter J.J., Koteish A.A., Clark J.M., Guallar E., Hernaez R. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013;38:246–254. doi: 10.1111/apt.12377. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda T., Okamoto Y., Hamada N., Miyashita K., Takahara M., Sakamoto F., Miyatsuka T., Kitamura T., Katakami N., Kawamori D., et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine. 2013;43:230–232. doi: 10.1007/s12020-012-9789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamer G., Arik S., Tamer I., Coksert D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid. 2011;21:891–896. doi: 10.1089/thy.2009.0200. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda T., Okamoto Y., Hamada N., Miyashita K., Takahara M., Sakamoto F., Miyatsuka T., Kitamura T., Katakami N., Kawamori D., et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine. 2012;42:739–741. doi: 10.1007/s12020-012-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozkurt N.C., Karbek B., Ucan B., Sahin M., Cakal E., Ozbek M., Delibasi T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 2013;19:479–484. doi: 10.4158/EP12376.OR. [DOI] [PubMed] [Google Scholar]
- 20.Han Y., Cheng Y.K., Chen Y.J., Li Y. M., Jiang Y. Q., Zhang S. F. Abnormality of serum 25(OH)D level and its associations with hormones and auto-antibody in patients with Graves’ disease. Chin. J. Clin. Res. 2013;26:642–646. [Google Scholar]
- 21.Miao W., Ma J., Guo R., Wang Y. J., Wang G., Guan H. X. The correlation between serum 25(OH)D and Graves’ disease. Chin. J. Pract. Med. 2013;33:394–395. [Google Scholar]
- 22.Zhang H., Liang L.Y., Xie Z.J. Low Vitamin D status is associated with increased titers of thyroid stimulating hormone receptor antibodies in Graves’ disease. Endocr. Pract. 2014 doi: 10.4158/EP14191.OR. [DOI] [PubMed] [Google Scholar]
- 23.Shin D.Y., Kim K.J., Kim D., Hwang S., Lee E.J. Low serum vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med. J. 2014;55:476–481. doi: 10.3349/ymj.2014.55.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y.B., Xue X.H., Liu S.W., Xi G.X., Zhao L. X., Zhang X.L. Serum vitamin D of early Graves’ disease patients: A clinical research. Chin. Rem. Clin. 2014;14:242–243. [Google Scholar]
- 25.Kivity S., Agmon L.N., Zisappl M., Shapira Y., Nagy E.V., Dankó K., Szekanecz Z., Langevitz P., Shoenfeld Y. Vitamin D and autoimmune thyroid diseases. Cell Mol. Immunol. 2011;8:243–247. doi: 10.1038/cmi.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jyotsna V.P., Sahoo A., Ksh S.A., Sreenivass V., Gupta N. Bone mineral density in patients of Graves’ disease pre- & post-treatment in a predominantly vitamin D deficient population. Indian J. Med. Res. 2012;135:36–41. doi: 10.4103/0971-5916.93422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansournia N., Mansournia M.A., Saeedi S., Dehghan J. The association between serum 25(OH)D levels and hypothyroid Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2014;37:473–476. doi: 10.1007/s40618-014-0064-y. [DOI] [PubMed] [Google Scholar]
- 28.Xuan L.Y., Yang Y.H., Lai X.Y. The association between the serum 25(OH)D3 of autoimmune thyroid diseases patients and the level of sFas. Shandong Med. J. 2014;38:61–63. [Google Scholar]
- 29.Zheng Y., Zheng F.P., Li H. The relationship between the blood uric acid level of Graves’ disease and bone mineral density of lumbar vertebra. Chin. J. Gerontol. 2014;11:3017–3019. [Google Scholar]
- 30.Wang Y.C. Master Dissertation. Anhui Medical University; Hefei, China: 2014. Analysis of the Relationship between 25(OH)D, IGF-1 and Bone Metabolism in Patients with Graves’ Disease. [Google Scholar]
- 31.Kang D.H., Wang Y., Cao W., Wang P., Zhang H.M. Higher prevalence of vitamin D deficiency in female patients with Graves’ disease. Acta Nutrimenta Sin. 2014;35:299–301. [Google Scholar]
- 32.Wang Z.S., Wu Y.P., Song Q.H. Bone mineral density and bone metabolism of Graves’ disease in premenopausal women. Guangdong Med. J. 2014;35:1743–1746. [Google Scholar]
- 33.Bellastella G., Maiorino M.I., Petrizzo M., de Bellis A., Capuano A., Esposito K., Giugliano D. Vitamin D and autoimmunity: What happens in autoimmune polyendocrine syndromes? J. Endocrinol. Investig. 2015 doi: 10.1007/s40618-014-0233-z. [DOI] [PubMed] [Google Scholar]
- 34.Feng M., Li H., Chen S.F., Li W.F., Zhang F.B. Polymorphism in the vitamin D receptor gene and risk of autoimmune thyroid disease: A meta-analysis. Endocrine. 2013;43:318–326. doi: 10.1007/s12020-012-9812-y. [DOI] [PubMed] [Google Scholar]
- 35.Tsatsoulis A. The role of stress in the clinical expression of thyroid autoimmunity. Ann. N.Y. Acad. Sci. 2006;1088:382–395. doi: 10.1196/annals.1366.015. [DOI] [PubMed] [Google Scholar]
- 36.Mullins R.J., Cohen S.B., Webb L.M., Chernajovsky Y., Dayan C.M., Londei M., Feldmann M. Identification of thyroid stimulating hormone receptor-specific T cells in Graves’ disease thyroid using autoantigen-transfected Epstein-Barrvirus-transformed B cell lines. J. Clin. Investig. 1995;96:30–37. doi: 10.1172/JCI118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallmann B.A., Hüther M., Tubes M., Feldkamp J., Bertrams J., Gries F.A., Lampeter E.F., Kolb H. Systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Graves’ disease. Diabetes. 1997;46:237–243. doi: 10.2337/diab.46.2.237. [DOI] [PubMed] [Google Scholar]
- 38.Dogan R.N.E., Vasy C. Absence of IL-4 and not suppression of the Th2 response, prevents development of experimental autoimmune thyroid Graves’ disease. J. Immunol. 2003;170:2195–2204. doi: 10.4049/jimmunol.170.4.2195. [DOI] [PubMed] [Google Scholar]
- 39.Karanikas G., Schuetz M., Wahl K., Paul M., Kontur S., Pietschmann P., Kletter K., Dudczak R., Willheim M. Relation of anti-TPO autoantibody titre and T-lymphocyte cytokine production patterns in Hashimoto’s thyroiditis. Clin. Endocrinol. (Oxf.) 2005;63:191–196. doi: 10.1111/j.1365-2265.2005.02324.x. [DOI] [PubMed] [Google Scholar]
- 40.Peng D., Xu B., Wang Y., Guo H., Jiang Y. A high frequency of circulating Th22 and Th17 cells in patients with new onset Graves’ disease. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0068446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li D., Cai W., Gu R., Zhang Y., Zhang H., Tang K., Xu P., Katirai F., Shi W., Wang L., et al. Th17 cells plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin. Immunol. 2013;149:411–420. doi: 10.1016/j.clim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Qin Q., Liu P., Liu L., Wang R., Yan N., Yang J., Wang X., Pandey M., Zhang J.A. The increased but non-predominant expression of Th17- and Th1-specific cytokines in Hashimoto’s thyroiditis but not in Graves’ disease. Braz. J. Med. Biol. Res. 2012;45:1202–1208. doi: 10.1590/S0100-879X2012007500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito C., Watanabe M., Okuda N., Watanabe C., Iwatani Y. Association between the severity of Hashimoto’s disease and the functional +874A/T polymorphism in the interferon-gamma gene. Endocr. J. 2006;53:473–478. doi: 10.1507/endocrj.K06-015. [DOI] [PubMed] [Google Scholar]
- 44.Staeva-Vieira T.P., Freedman L.P. 1,25-Dihydroxyvitamin D3 inhabit IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J. Immunol. 2002;168:1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 45.Palmer M.T., Lee Y.K., Maynard C.L., Oliver J.R., Bikle D.D., Jetten A.M., Weaver C.T. Lineage-specific Effects of 1, 25-Dihydroxyvitamin D3 on the development of effect CD4 T cells. J. Biol. Chem. 2011;286:997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pichler J., Gerstmayr M., Szépfalusi Z., Urbanek R., Peterlik M., Willheim M. 1α,25 (OH)2D3 inhabits not only Th1 but also Th2 differentiation in human blood T cells. Pediatr. Res. 2002;52:12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Joshi S., Pantalena L.C., Liu X.K., Gaffen S.L., Liu H., Rohowsky-Kochan C., Ichiyama K., Yoshimura A., Steinman L., Christakos S., et al. 1,25-Dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell Biol. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muscogiuri G., Mitri J., Mathieu C., Badenhoop K., Tamer G., Orio F., Mezza T., Vieth R., Colao A., Pittas A. Mchanisms in endocrinology: Vitamin D as a potential contributor in endocrine health and disease. Eur. J. Endocrinol. 2014;171:101–110. doi: 10.1530/EJE-14-0158. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q.Q., Sun M., Wang Z.X., Fu Q., Shi Y., Yang F., Zheng S., Xu J.J., Huang X.P., Liu X.Y., Cui D., Yang T. Relationship between serum 25-hydroxy vitamin D and thyroid autoimmunity among middle-aged and elderly individuals. Acta Univ. Med. Nanjing (Nat. Sci.) 2014;34:486–489. [Google Scholar]
- 50.Shin D., Hwang S. Baseline vitamin D level could be a short-term prognostic marker in patients with Graves’ disease. Thyroid. 2011;21:A48. [Google Scholar]
- 51.Kawakami-Tani T., Fukawa E., Tanaka H., Abe Y., Makino I. Effect of 1 alpha-hydroxyvitamin D3 on serum levels of thyroid hormones in hyperthyroid patients with untreated Graves’ disease. Metabolism. 1997;46:1184–1188. doi: 10.1016/S0026-0495(97)90214-6. [DOI] [PubMed] [Google Scholar]
- 52.De Remigis P., Vianale L., De Remingis A., Napolitano G. Vitamin D and autoimmune thyroid disease (at): Preliminary results. Thyroid. 2013;23:A81–A82. [Google Scholar]
- 53.Amrein K., Zajic P., Schnedl C., Waltensdorfer A., Fruhwald S., Holl A., Purkart T., Wünsch G., Valentin T., Grisold A., et al. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit. Care. 2014;18 doi: 10.1186/cc13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai J.K., Lucas R.M., Clements M.S., Harrison S.L., Banks E. Assessing vitamin D status: Pitfalls for the unwary. Mol. Nutr. Food Res. 2010;54:1062–1071. doi: 10.1002/mnfr.200900468. [DOI] [PubMed] [Google Scholar]