Abstract
During feeding, the gut microbiota contributes to the host energy acquisition and metabolic regulation thereby influencing the development of metabolic disorders such as obesity and diabetes. Short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate, which are produced by gut microbial fermentation of dietary fiber, are recognized as essential host energy sources and act as signal transduction molecules via G-protein coupled receptors (FFAR2, FFAR3, OLFR78, GPR109A) and as epigenetic regulators of gene expression by the inhibition of histone deacetylase (HDAC). Recent evidence suggests that dietary fiber and the gut microbial-derived SCFAs exert multiple beneficial effects on the host energy metabolism not only by improving the intestinal environment, but also by directly affecting various host peripheral tissues. In this review, we summarize the roles of gut microbial SCFAs in the host energy regulation and present an overview of the current understanding of its physiological functions.
Keywords: SCFA, gut microbiota, energy metabolism, GPR41, GPR43
1. Introduction
Diet is a most important factor for daily nutrient acquisition. However, the dysregulation of energy homeostasis by excessive intake leads to obesity. Obesity is currently one of the most serious public health challenges worldwide, especially in the Western world, because of its increasing prevalence and its contribution to a complex of symptoms collectively called the “metabolic syndrome” [1,2]. The progress of obesity is caused by a long-term imbalance between energy intake and expenditure, which in turn influences multiple effector pathways involving metabolites and hormones [3]. Excessive food intake, especially of high-fat and sugar products, together with insufficient exercise and genetic susceptibility, are considered risk factors for developing obesity. Recent research has demonstrated that changes in the gut microbiota are closely linked with metabolic disorders such as obesity and type 2 diabetes [4,5,6]. One of the most important roles of the gut microbiota is to catabolize dietary fibers that are not completely hydrolyzed by the host enzymes during digestion [7]. The main products of intestinal bacterial fermentation of dietary fiber are short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate [8]. SCFAs can be used for de novo synthesis of lipids and glucose, which are the main energy sources for the host [9].
2. Gut Microbial Composition and Metabolic Disease
The gut microbiota is a complex of microorganisms including more than 100 trillion cells of 400 species, which is equivalent to ten times the total number of cells in the human body [10]. Because the majority of the gut microbes are strictly anaerobic their identification and functional analysis has been difficult. However, since the advent of metagenomics, it has been revealed that gut microbes play an important role in host metabolic and immune homeostasis. Recent studies indicate that the gut microbiota can be considered an environmental factor that affects host adiposity and can contribute to obesity [11,12]. The human microbiome contains 150 times more genes than the human genome [13]. Metagenomic analysis of the gut microbiome in obese mice and humans indicated that expression of genes involved in carbohydrate metabolism predominated. Transplantation of the microbiota from obese mice into the gut of germ-free mice significantly increased adiposity in the recipient mice in comparison with transplantation of a lean microbiota. Moreover, cohort studies in Europe and China revealed that, despite the ethnic and dietary differences patients with type 2 diabetes had a lower proportion of butyrate-producing and a larger proportion of non-butyrate-producing Clostridiales [14,15]. In addition, although the functions encoded by the metagenomes of butyrate-producing bacteria were comparable between European and Chinese, the prevalent bacterial taxa were markedly different in the two cohorts. This indicates that the gut microbiota is notably affected by diet and ethnicity. Roux-en-Y gastric bypass (RYGB) induces dramatic and sustained weight loss by restricting the amount of food that can be ingested, thereby improving insulin sensitivity and type 2 diabetes. RYGB is currently the most effective treatment for obesity. The resulting metabolic improvement cannot be explained by the reduced calorie intake alone; the altered gut physiology following RYGB contributes to an altered intestinal microbial ecology in mice, rats, and humans, which may contribute to the improved host metabolism. Liou et al. reported that the change in the gut microbiota upon transplantation of RYGB-related fecal microbiota directly contributed to reduced weight and adiposity [16]. Therefore, the gut microbiota is considered an environmental factor that modulates the host metabolism and may contribute to metabolic disorder.
3. Metabolic Beneficial Effects by SCFAs
Recently, dietary fibers have gained increasing interest because they exert beneficial effects on metabolic functions such as body weight, food intake, glucose homeostasis, and insulin sensitivity [17,18]. Hence, dietary fiber intake reduces risk of inflammatory bowel disease, cardiovascular disease, colon cancer, obesity and diabetes [19,20]. In the last few decades, it has been hypothesized that SCFAs might play a key role in the prevention and treatment of metabolic syndrome, bowel disorders, and cancer [21,22,23]. With regard to the energy metabolism, butyrate improved insulin sensitivity and increased the energy expenditure in dietary-obese mice [24]. Butyrate and propionate were shown to protect against diet-induced obesity and regulated the gut hormones [25]. The oral administration of acetate improved glucose tolerance and suppresses obesity [26]. Clinical studies showed that the administration of SCFAs has a positive effect on the treatment of ulcerative colitis, Crohn's disease, and antibiotic-associated diarrhea and obesity [22,27,28,29]. In obese subjects, propionate significantly increased the release of postprandial plasma peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) from colonic cells, and reduced the energy intake. Inulin-propionate ester administrated at 10 g per day over a period of 24 weeks significantly reduced weight gain, intra-abdominal adipose tissue distribution and intrahepatocellular lipid content, and improved insulin resistance in the inulin control group [29]. On the other hand, SCFAs also utilize as host energy source, therefore SCFAs are regarded as cause of increasing energy harvest from diet, linked to the obese phenotype by changes of gut microbiota composition. To clarify these controversial, the molecular mechanisms of SCFAs was next investigated.
4. Molecular Mechanisms Involved in Host Metabolic Regulation by SCFAs
As gut microbial SCFAs have been reported to exert beneficial effects on the host metabolism, in this paragraph we focus on the elucidation of the molecular mechanisms involved. Total SCFAs in gut lumen is ~100 mM, in blood is, portal ~400 μM and peripheral ~100 μM [30]. Hence, SCFAs exhibit the tissue specific physiological function by this concentration gradient. Butyrate enhances fatty acid oxidation and thermogenesis by increasing the expression of peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) and the phosphorylation of adenosine-monophosphate-activated kinase (AMPK) in muscle and liver tissues, and the expression of PGC-1α and mitochondrial uncoupling protein-1 (UCP-1) in brown adipose tissues [21]. Propionate and butyrate activate intestinal gluconeogenesis via a gut-brain neural circuit, thereby promoting metabolic benefits on body weight and glucose control [31]. Acetate reduces the appetite by changing the expression profiles of appetite regulatory neuropeptides in the hypothalamus through activation of TCA cycle [32]. Various studies that had been focused on the elucidation of the SCFA target molecules in the host demonstrated that G-protein-coupled receptors (GPCR) act as SCFA receptors [33,34].
5. SCFA Receptors
5.1. FFAR2/GPR43
GPR43, also known as FFAR2 has been identified as an SCFA receptor and is mainly activated by acetate and propionate followed by butyrate [35,36]. FFAR2 is expressed in intestinal endocrine L-cells, where it stimulates the release of PYY and GLP-1. SCFA-induced FFAR2 activation was shown to promote the secretion of GLP-1 in mouse colonic primary cultures and in enteroendocrine STC-1 cells [37]. Ffar2-deficient mice exhibited decreased SCFA-induced secretion of GLP-1 both in vitro and in vivo and improved insulin resistance. FFAR2 is abundantly expressed in adipose tissues as well. FFAR2 expression was significantly increased in white adipose tissues of high-fat-diet-induced obese mice in comparison with normal chow-fed mice [38]. In addition, SCFAs inhibited isoproterenol-induced lipolysis in a concentration-dependent manner in mouse 3T3-L1-derived adipocytes [38]. Ge et al. later demonstrated that this effect was dependent on FFAR2 using Ffar2-deficient mice [39]. Moreover, in white adipose tissues, FFAR2 activation by SCFAs suppressed adipose-specific insulin signaling, leading to the inhibition of fat accumulation [40]. Ffar2-deficient mice were shown to exhibit obesity, whereas mice overexpressing adipose-specific Ffar2 overexpressed mice exhibited leanness under normal conditions. However, these mouse strains did not exhibit theses respective phenotypes when grown under germ-free conditions or when treated with antibiotics. This indicated that the source of FFAR2 ligands was dependent on gut microbes and that FFAR2 regulates adipose-insulin signaling by sensing microbial SCFAs, thereby regulating fat accumulation and maintaining body energy homeostasis. Hence, FFAR2 activation by SCFAs promotes GLP-1 secretion in the gut and suppression of fat accumulation in adipose tissue, leading to increased insulin sensitivity. FFAR2 is also expressed in immune tissues. Gut microbiota and FFAR2 regulate inflammatory responses in colitis [41]. In addition, SCFAs regulate the size and function of the colonic pool of regulatory T cells and protect against colitis in a FFAR2-dependent manner in mice [42]. Since the inflammatory response is also related to the development of obesity and type 2 diabetes, the regulation of immune function via FFAR2 may be also related to the metabolic beneficial effects by SCFAs.
5.2. FFAR3/GPR41
FFAR3 has also been identified as an SCFA receptor. However, the ligand affinity to SCFAs differs between FFAR2 and FFAR3; FFAR3 is activated mainly by propionate and butyrate [35,36]. Like FFAR2, FFAR3 is also expressed in PYY- and GLP-1-secreting endocrine L-cells, indicating its involvement in energy homeostasis [43]. The secretion of PYY and GLP-1 was shown to be reduced in primary cultured endocrine cells derived from Ffar3-deficient mice [37,44]. After transplantation of specific microbes to gnotobiotic mice, wild-type but not Ffar3-deficient mice showed an increase in PYY levels. Therefore, the secretion of PYY from intestinal L-cells is due to activation of FFAR3 by SCFAs produced by the gut microbes. FFAR3 is abundantly expressed in sympathetic ganglia as well [45]. FFAR3 activation by propionate increases the heart rate and energy expenditure through sympathetic activation. In addition, sympathetic activation by FFAR3 directly leads to noradrenalin release from the sympathetic neurons [46]. This indicates that FFAR3 regulates sympathetic activity by sensing the nutritional state, thereby maintaining body energy homeostasis. It has been reported that FFAR3 contributes to the improvement of insulin resistance by dietary fibers through activation of FFAR3 expressed in the peripheral nerves by SCFAs produced by gut microbes [30]. This implies that FFAR3 stimulation by SCFAs exhibits beneficial effects on the host metabolism via the peripheral nervous system and hormone secretion in the gut. Similar to FFAR2, FFAR3 affects the inflammatory response. Propionate was shown to affect bone marrow hematopoiesis in an FFAR3-dependent manner in mice, by inducing an enhanced generation of macrophage and dendritic cell precursors thereby influencing the allergic inflammatory response in airway disease via FFAR3 [47]. Therefore, FFAR3 may be involved in the beneficial effects of SCFAs on host metabolism through the regulation of immune responses.
5.3. Other SCFA Receptors (GPR109A and OLFR78)
GPR109A was first identified as a receptor for niacin and is also activated by β-hydroxybutryate and butyrate, but not by acetate and propionate [47]. The EC50 value for GPR109A activation by butyrate is approximately 1mM [47]. GPR109A is expressed in the epithelial cells of the colon and expression decreases in the absence of the gut microbiota in germ-free mice [48]. GPR109A activation by butyrate suppresses colonic inflammation and carcinogenesis by promoting anti-inflammatory properties in colonic macrophages and dendritic cells, which induce the differentiation of regulatory and IL10-producing T cells [49]. In addition, GPR109A is expressed in adipose tissues and activated adipose tissue macrophages where it regulates lipid homeostasis [47,50,51,52]. Olfactory receptor 78 (OLFR78) was identified as SCFAs in a ligand screen for orphan GPCRs [53]. OLFR78 is activated by acetate and propionate but not by butyrate; the EC50 value are 2.35mM and 920 μM, respectively. Olfr78 is expressed in blood vessels, especially localize in renal vessel where it is involved in renin secretion. The binding of gut microbiota-derived SCFAs to OLFR78 induces the release of renin, which is involved in the modulation of the blood pressure [53,54,55]. The metabolic effects of this SCFAs receptor are unclear to date. It is expected that further analysis will elucidate its function in energy metabolism.
6. Epigenetic Regulation by SCFAs
Gene expression is regulated by the modulation of histone acetylation by histone acetyltransferases and histone deacetylases (HDAC). SCFAs produced by gut microbes inhibit histone deacetylase activity and consequently modulate gene expression. Butyrate is the most potent inhibitor of HDAC, with a maximum efficiency of approximately 80% inhibition of HDAC1/2, and its in vitro Ki value is approximately 58 μM [56]. Propionate has a maximum inhibitory efficiently of approximately 60%. SCFAs function as non-competitive inhibitors of HDACs. It has been shown that butyrate and propionate selectively inhibit HDAC1 and HDAC3. With regard to the influence of the inhibition of HDAC by SCFAs on the host physiological functions, it has been reported that propionate and butyrate produced by gut microbes promote the generation of peripheral regulatory T-cells [57,58]. Gut microbe-derived butyrate induces the differentiation of colonic regulatory T cells by enhancing histone H3 acetylation in the promoter and conserved non-coding sequence regions of the Foxp3 locus, and reduces the development of colitis [58]. In addition, the putative link between the microbiota and epigenetic regulation has been examined in obese and type 2 diabetes patients as compared to lean control subjects [59]. The diversity of the gut microbiota and the degree of methylation of the FFAR3 promoter region were significantly lower in the obese and type 2 diabetic patients compared to lean individuals, demonstrating a correlation between a higher body mass index and lower methylation of FFAR3 [59]. Therefore, epigenetic regulation may be also related to the beneficial effects by SCFAs on host metabolism.
7. Conclusions
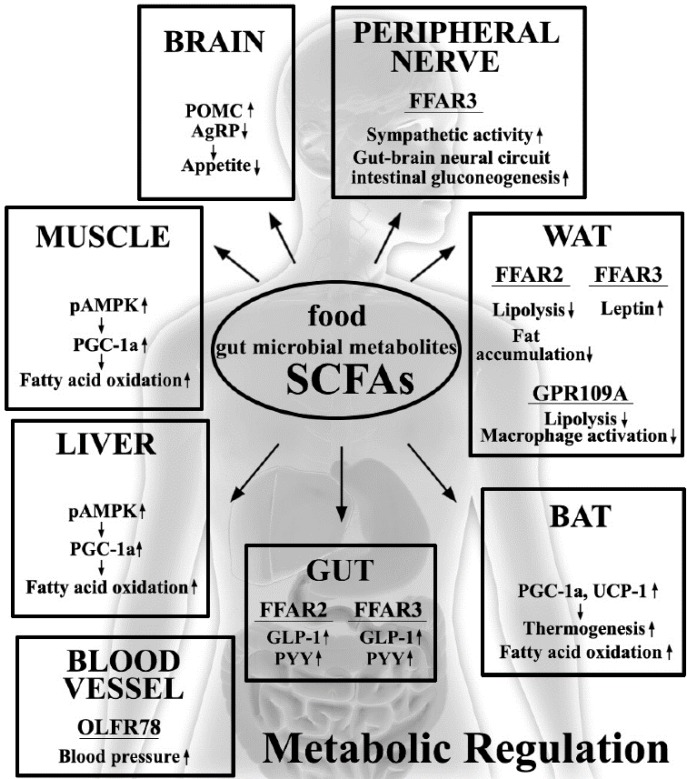
In recent years, various host metabolic and immune response pathways have been reported to be related to diet and gut microbiota. Several studies have provided evidence that SCFAs, which are the end products of the fermentation of dietary fibers by the anaerobic intestinal microbiota, have beneficial effects on the host energy metabolism and inflammatory responses, because the host SCFA receptors and target molecules are expressed in both metabolic and immune tissues (Figure 1). As the inflammatory response is related to the development of obesity and type 2 diabetes, the SCFAs and their target molecules constitute potential therapeutic targets for the treatment of these diseases. Although the exact signaling mechanisms and physiological functions of SCFAs in the host peripheral tissues are unclear, a deeper understanding of the regulation of the energy metabolism and the inflammatory responses by the dietary SCFAs represents an important avenue for research in medicine and drug development for the prevention and treatment of obesity and type 2 diabetes.
Figure 1.
Effects of dietary gut microbial short-chain fatty acids (SCFAs) on the regulation of host metabolic functions. SCFAs affect host homeostasis through the stimulation of SCFA receptors, FFAR2, FFAR3, GPR109A and OLFR78.
Author Contributions
Mayu Kasubuchi, Sae Hasegawa, Takero Hiramatsu, Atsuhiko Ichimura and Ikuo Kimura conceived, designed and drafted the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 2.Sanz Y., Santacruz A., Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc. Nutr. Soc. 2010;69:434–441. doi: 10.1017/S0029665110001813. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood H.C., Bloom S.R., Murphy K.G. Peptides and their potential role in the treatment of diabetes and obesity. Rev. Diabet. Stud. 2011;8:355–368. doi: 10.1900/RDS.2011.8.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greiner T., Backhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011;22:117–123. doi: 10.1016/j.tem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan M.T., Nieuwdrop M., Backhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20:753–760. doi: 10.1016/j.cmet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 8.Flint H.J., Bayer E.A., Rincon M.T., Lamed R., White B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 9.Wolever T.M., Brighenti F., Royall D., Jenkins A.L., Jenkins D.J. Effect of rectal infusion of short chain fatty acids in human subjects. Am. J. Gastroenterol. 1989;84:1027–1033. [PubMed] [Google Scholar]
- 10.Bourlioux P., Koletzko B., Guarner F., Braesco V. The intestine and its microflora are partners for the protection of the host: Report on the Danone Symposium “The Intelligent Intestine”, held in Paris, June 14, 2002. Am. J. Clin. Nutr. 2003;78:675–683. doi: 10.1093/ajcn/78.4.675. [DOI] [PubMed] [Google Scholar]
- 11.Ley R.E., Turnbauqh P.J., Klein S., Gordon J.I. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 12.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 13.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomics sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson F.H., Tremaroli V., Nookaew I., Bergstrom G., Behre C.J., Fagerberg B., Nielsen J., Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 16.Liou A.P., Paziuk M., Lievano J.M., Jr., Machineni S., Turnbaugh P.J., Kaplan L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delzenne N.M., Cani P.D. A place for dietary fiber in the management of the metabolic syndrome. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8:636–640. doi: 10.1097/01.mco.0000171124.06408.71. [DOI] [PubMed] [Google Scholar]
- 18.Delzenne N.M., Neyrinck A.M., Backhed F., Cani P.D. Targeting gut microbiota in obesity: Effect of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 19.Galisteo M., Duarte J., Zarzuelo A. Effect of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008;19:71–84. doi: 10.1016/j.jnutbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.den Besten G., van Eunen K., Groen A.K., Venema K., Reijingoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harig J.M., Soergel K.H., Komorowski R.A., Wood C.M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N. Engl. J. Med. 1989;320:23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 24.Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., Cefalu W.T., Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanism. PLoS ONE, 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita H., Fujisawa K., Ito E., Idei S., Kawaguchi N., Kimoto M., Hiemori M., Tsuji H. Improvement of obesity and glucose tolerance by acetate in type 2 diabetic Otsuka Long-Evans Tokushima fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2007;71:1236–1243. doi: 10.1271/bbb.60668. [DOI] [PubMed] [Google Scholar]
- 27.Binder H.J. Role of colonic short-chain fatty acid transport in diarrhea. Annu. Rev. Physiol. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 28.Di Sabatino A., Moreta R., Ciccocioppo R., Cazzola P., Gotti S., Tinozzi F.P., Tinozzi S., Corazza G.R. Oral butyrate for mildly to moderately active Crohn’s disease. Aliment. Pharmacol. Ther. 2005;22:789–794. doi: 10.1111/j.1365-2036.2005.02639.x. [DOI] [PubMed] [Google Scholar]
- 29.Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E., MacDougall K., Preston T., Tedford D., Finlayson G.S., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2014 doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., MacFarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L., Anastasovska J., Ghourab S., Hankir M., Zhang S., et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blad C.C., Tang C., Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 34.Offermanns S. Free fatty acid (FFA) and hydroxyl carboxylic acid (HCA) receptors. Annu. Rev. Pharmacol. Toxicol. 2014;54:407–434. doi: 10.1146/annurev-pharmtox-011613-135945. [DOI] [PubMed] [Google Scholar]
- 35.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.L., Wigglesworth M.J., Kinghorn I., Fraser N.J., et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 36.Le Poul E., Loison C., Struyf S., Springael J.Y., Lannoy V., Decobecq M.E., Brezillon S., Dupriez V., Vassart G., Van Damme J., et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 37.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong Y.H., Nishimura Y., Hishikawa D., Tsuzuki H., Miyahara H., Gotoh C., Choi K.C., Feng D.D., Chen C., Lee H.G., et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 39.Ge H., Li X., Weiszmann J., Wang P., Baribault H., Chen J.L., Tian H., Li Y. Activation of GPR43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 40.Kimura I., Ozawa K., Inoue D., Imamura T., Kimura K., Maeda T., Terasawa K., Kashihara D., Hirano K., Tani T., et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y. M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tazoe H., Otomo Y., Karaki S., Kato I., Fukami Y., Terasaki M., Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- 44.Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A., Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc. Natl. Acad. Sci. USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue D., Kimura I., Wakabayashi M., Tsumoto H., Ozawa K., Hara T., Takei Y., Hirasawa A., Ishihama Y., Tsujimoto G. Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett. 2012;586:1547–1554. doi: 10.1016/j.febslet.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed K., Tunaru S., Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxyl-carboxylic acid receptors. Trends Pharmacol. Sci. 2009;30:557–562. doi: 10.1016/j.tips.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Cresci G.A., Thangaraju M., Mellinger J.D., Liu K., Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 2010;14:449–461. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- 49.Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., et al. Activiation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh V., Jamwal S., Jain R., Verma P., Gokhale R., Rao K.V. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe. 2012;12:669–681. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Feingold K.R., Moser A., Shigenaga J.K., Grunfeld C. Inflammation stimulates niacin receptor (GPR109A/HCA2) expression in adipose tissue and macrophages. J. Lipid Res. 2014;55:2501–2508. doi: 10.1194/jlr.M050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukasova M., Malaval C., Gille A., Kero J., Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Invest. 2011;121:1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pliznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L.X., Rey F., Wang T., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pluznick J.L. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Natarajan N., Plunznick J.L. From microbe to man: The role of microbial short chain fatty acid metabolites in host cell biology. Am. J. Physiol. Cell Physiol. 2014;307:C979–C985. doi: 10.1152/ajpcell.00228.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cousens L.S., Gallwitz D., Alberts B.M. Different accessibilities in chromatin to histone acetylase. J. Biol. Chem. 1979;254:1716–1723. [PubMed] [Google Scholar]
- 57.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 59.Remely M., Aumueller E., Merold C., Dworzak S., Hippe B., Zanner J., Pointner A., Brath H., Haslberger A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537:85–92. doi: 10.1016/j.gene.2013.11.081. [DOI] [PubMed] [Google Scholar]