Abstract
Vitamin D is a direct and indirect regulator of T cells. The mechanisms by which vitamin D directly regulates T cells are reviewed and new primary data on the effects of 1,25 dihydroxyvitamin D (1,25(OH)2D) on human invariant natural killer (iNK)T cells is presented. The in vivo effects of vitamin D on murine T cells include inhibition of T cell proliferation, inhibition of IFN-γ, IL-17 and induction of IL-4. Experiments in mice demonstrate that the effectiveness of 1,25(OH)2D requires NKT cells, IL-10, the IL-10R and IL-4. Comparisons of mouse and human T cells show that 1,25(OH)2D inhibits IL-17 and IFN-γ, and induces T regulatory cells and IL-4. IL-4 was induced by 1,25(OH)2D in mouse and human iNKT cells. Activation for 72h was required for optimal expression of the vitamin D receptor (VDR) in human and mouse T and iNKT cells. In addition, T cells are potential autocrine sources of 1,25(OH)2D but again only 48–72h after activation. Together the data support the late effects of vitamin D on diseases like inflammatory bowel disease and multiple sclerosis where reducing IL-17 and IFN-γ, while inducing IL-4 and IL-10, would be beneficial.
Keywords: vitamin D, T cells, vitamin D receptor
1. Introduction
Vitamin D is a fat soluble vitamin that is either consumed in the diet or produced in the skin following sunlight exposure of the skin. Vitamin D is inactive and is hydroxylated twice, once in the liver and once in the kidney to make the active form of the vitamin, 1,25 dihydroxyvitamin D (1,25(OH)2D) [1,2]. Production of 1,25(OH)2D in the kidney is tightly regulated by serum calcium, parathyroid hormone and 1,25(OH)2D levels [3]. Vitamin D as 1,25(OH)2D functions by binding to a nuclear vitamin D receptor (VDR) and retinoid X receptor to regulate gene transcription [4]. The classic roles of vitamin D are in the regulation of calcium uptake and homeostasis, bone metabolism, and cell growth and division. The VDR has been identified in many other tissues including the immune system and it is now accepted that 1,25(OH)2D and vitamin D are important immune system regulators [5]. All cells of the immune system have been shown to express the VDR including T cells [6].
2. T Cells
Several different types of T cells exist to provide defense from a variety of different insults to the body. CD4+ T cells provide help for B cell antibody production and to other cell types to engulf and kill pathogens. T helper (h) cell responses are heterogeneous although it has been shown that specific cytokine patterns are critical for control of susceptibility to infection. Th1 cells are important for the control of intracellular infections with Mycobacterium tuberculosis, Listeria monocytogenes, and a number of viruses [7,8,9]. Th2 cells that produce IL-4, IL-5, and IL-13 are required for host defense against parasitic helminthes [10]. Th17 cells produce IL-17, IL-22, and granulocyte macrophage-colony stimulating factor (GM-CSF) [11] and are essential for resistance to extracellular pathogens [12]. Additional T cells include the innate natural killer (NK)T cells. NKT cells recognize lipid antigens instead of protein antigens and produce large amounts of cytokines early during infection [13]. CD8+ T cells are cytotoxic T cells and are important for killing virally and bacterially infected cells. Each of these T cells are critical for immune protection from infection.
Dysregulation of T cell responses can cause pathology. Immune mediated diseases occur as a result of chronically activated T cells. T regulatory (reg) cells produce IL-10 and serve to inhibit effector T cell responses in an antigen specific and non-specific manner [14]. Deficiency of T reg cells results in multi-organ immune mediated disease [14]. Th1 and/or Th17 cells transfer experimental models of immune mediated diseases such as inflammatory bowel disease and multiple sclerosis. Experimental asthma and allergy models occur in animals that have Th2 cells, and in some cases NKT cells that overproduce IL-13 and/or IL-17 [15]. Control of the T cell response is therefore required for robust elimination of infection and the resolution of inflammation to prevent pathology due to chronic T cell activation.
3. Vitamin D and T Cells
The effects of vitamin D and 1,25(OH)2D as inhibitors of T cells have been well described. There are direct and indirect effects of 1,25(OH)2D on T cells but this review will focus on the direct effects. Since 1983 it has been described that 1,25(OH)2D inhibited T cell proliferation and the secretion of select cytokines after mitogen stimulation [16,17]. Moreover, 1,25(OH)2D directly inhibited IL-2 and IFN-γ transcription [17,18]. More recently 1,25(OH)2D has also been shown to inhibit IL-17 secretion by Th17 cells [19,20]. The effects of 1,25(OH)2D on Th2 cells is more controversial with evidence that 1,25(OH)2D inhibits IL-4 transcriptionally as well as evidence that 1,25(OH)2D upregulates IL-4 in mouse and human T cells [20,21,22,23]. In vitro, 1,25(OH)2D3 treatments induced IL-10 and T regulatory cell development [24]. In addition, 1,25(OH)2D upregulated the gut homing receptor CCR9 and inhibited CXCR3 on T cells potentially changing the homing properties of the Th cells [25]. Vitamin D and 1,25(OH)2D inhibited Th1 and Th17 responses, induced T reg responses, and controlled proliferation and Th cell localization.
CD8+ T cells and iNKT cells are also vitamin D targets. In vitro, 1,25(OH)2D treatment inhibited CD8 T cell proliferation and VDR knockout (KO) CD8+ T cells proliferated without antigen stimulation due in part to over-production of IL-2 [26,27]. VDR KO CD8 T cells had altered homing patterns, a reduction in granzyme B production and more rapid contraction in an infection model [28]. Other defects in VDR KO CD8+ T cells included the ability, when transferred to leucopenic hosts, to develop into IL-17 and IFN-γ secreting cells that produced colitis symptoms [26]. In the gastrointestinal tract, CD8αα/TCRαβ T cells important in maintaining homeostasis, required vitamin D to maintain their homeostatic proliferation [29]. Development and function of iNKT cells depends on expression of the VDR and vitamin D [30,31]. In vitro, 1,25(OH)2D inhibited iNKT cell derived IL-17 and induced IL-4 and IL-5 [32]. The requirement for the VDR in the development of iNKT cells was traced to regulation of the survival of maturing iNKT cells in the thymus [31]. Vitamin D controls CD8 proliferation, IL-2 production and the potential to develop into effector T cells that produce IFN-γ, IL-17 and granzyme B. Vitamin D also controls iNKT cell expansion during development. Lastly vitamin D changes early cytokine production by iNKT cells that could shape later T cell responses.
The effectiveness of vitamin D and 1,25(OH)2D treatments on animal models of T cell mediated diseases has been informative. Th1 and Th17 cells cause experimental autoimmune encephalomyelitis (EAE, murine model of multiple sclerosis), inflammatory bowel disease and type-1 diabetes. In vivo, 1,25(OH)2D treatments suppressed the development and progression of these Th1/Th17 mediated diseases [33,34,35]. In addition, vitamin D and VDR deficiency exacerbated experimental type-1 diabetes and inflammatory bowel disease in mice [33,35,36]. In EAE the effectiveness of 1,25(OH)2D has been shown to require iNKT cells, IL-4, IL-10, and L-10R [32,37,38]. In addition, the 1,25(OH)2D-mediated inhibition of IL-17 and IFN-γ with the induction of IL-10 and T reg cells have been suggested as mechanisms to explain suppression of experimental EAE, IBD and diabetes [24,39,40,41].
Vitamin D has also been proposed as a regulator of Th2 mediated disease such as allergy and asthma. In vitro, 1,25(OH)2D treatment of T cells has been shown to increase IL-4 secretion by human and mouse Th cells [21,42,43]. IL-13 has been shown to be induced [44] or decreased [45] by 1,25(OH)2D treatment of human T cells. VDR KO Th2 cells made less IL-4 on the C57BL/6 background and less IL-4, IL-5 and IL-13 on the Balb/c background [46]. In addition, VDR KO mice on either the Balb/c or C57BL/6 background failed to develop experimental allergic asthma [46]. However, cell transfer and bone marrow transplantation showed that VDR KO T cells could induce asthma in the WT host and therefore VDR expression in the lung might account for the increased resistance of the VDR KO mice to allergic asthma [47]. iNKT cells also contribute to asthma development and 1,25(OH)2D induced IL-4, and IL-5 from iNKT cells [32,48]. The data on the effects of 1,25(OH)2D treatments of experimental allergic asthma show contradictory results with no effects, worsening of symptoms and symptom amelioration [49,50,51]. The effects of 1,25(OH)2D on T reg cells and IL-10, that also suppress the Th2 response, could explain some of the beneficial effects of vitamin D in experimental asthma [52,53,54]. Overall the data support that vitamin D and 1,25(OH)2D induce production of Th2 cytokines. Other vitamin D targets in the lung, T reg cells and/or other immune cells are likely the explanation for the contradictory results in the experimental allergic asthma models.
4. Vitamin D Regulation of Mouse versus Human T Cells
Much of the work describing the basic mechanisms of vitamin D, the VDR and 1,25(OH)2D on T cells in vivo have been done in mice. These in vivo experiments are difficult to replicate in humans. However, since the goal is to use mice to model the effects of vitamin D and 1,25(OH)2D in humans, it is important to determine which of the effects of vitamin D in murine T cells can also be observed in human T cells. It should be noted however, that much of the work using human T cells is done with peripheral blood mononuclear cells (PBMC). In the mouse the T cells studied come from different tissues (usually not the blood) and the functions of the T cells depend to a large extent on where they are located.
The early work utilized human PBMC to demonstrate that T cells expressed the VDR and were vitamin D targets. Human CD8 and CD4 T cells were inhibited from proliferating in the presence of 1,25(OH)2D [18,27,55]. In addition, 1,25(OH)2D inhibited IL-2, IFN-γ, and IL-17 in human and mouse T cells [21,42,43,56]. Freshly isolated PBMC were stimulated with CD3 and CD28 antibodies or αGalCer in the presence of 0-50nM 1,25(OH)2D. Confirming the literature, our experiments also showed 1,25(OH)2D inhibited IFN-γ and T cell proliferation and induced IL-4 production from PBMC stimulated with CD3/CD28 (data not shown). Activation of both human and mouse T cells induced expression of the VDR and it took 48-72h to induce VDR protein in the T cells [6,21,57]. Human Th1, Th2 and Th17 cells expressed similar and high amounts of the VDR protein 72 hours after activation [57]. The amount of 1,25(OH)2D addition to activated T cells protected the VDR protein from proteasomal degradation and 1,25(OH)2D has been shown to stabilize VDR protein in other cell types as well [57,58]. In addition, activation of mouse CD8+ T cells and human CD4+ T cells for 48-72 hours induced expression of the vitamin D 1-alpha hydroxylase (Cyp27B1) suggesting that T cells might be able to locally produce 1,25(OH)2D [59,60]. In human PBMC 1,25(OH)2D induced the expression of IL-4 when added in vitro and 1,25(OH)2D induced human T reg development and IL-10 production [42,43,61]. Collectively the effects of vitamin D, production of Cyp27B1 and 1,25(OH)2D on mouse T cells in vitro reflect the effects of 1,25(OH)2D on human T cells from the PBMC.
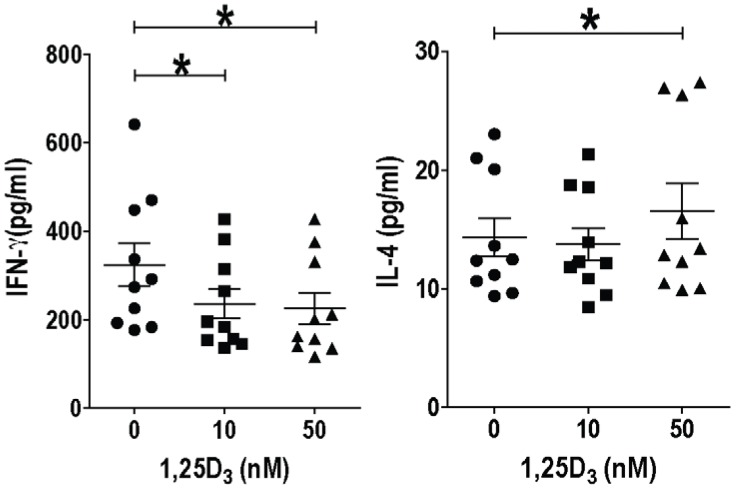
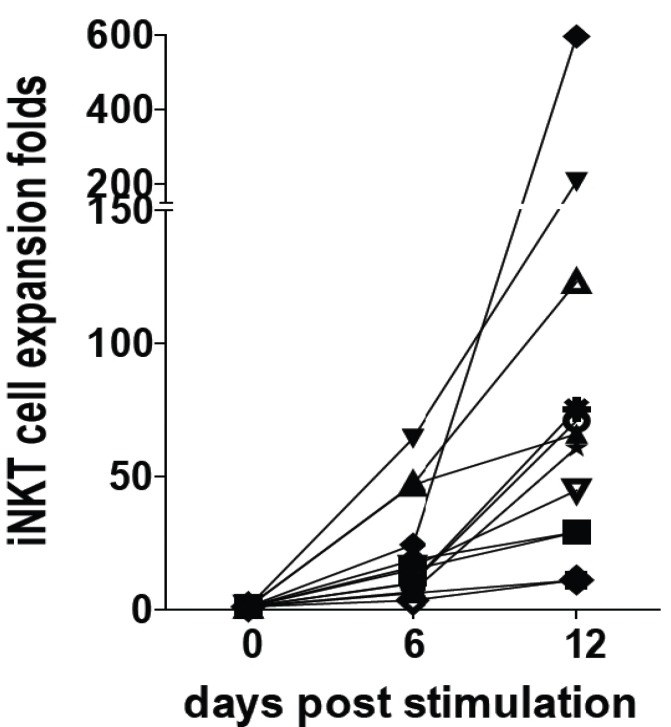
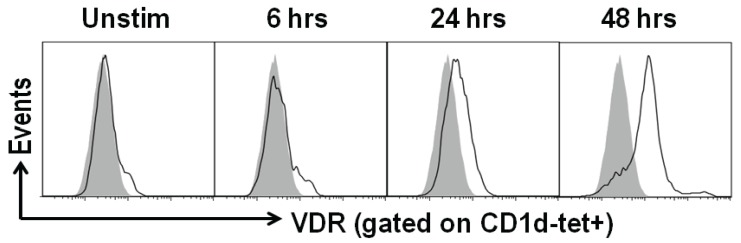
PBMC are readily accessible sources of human immune cells including iNKT cells. However, the frequencies of iNKT cells (CD1d tetramer+/CD3+) in the PBMC is very low and ranged from 0.008%–0.292% of the cells (data not shown). α-Galactoceramide (GalCer), an iNKT cell specific ligand, was used to stimulate freshly isolated human PBMC. IFN-γ was inhibited by 10 and 50 nM of 1,25(OH)2D addition to αGalCer stimulated PBMC (Figure 1). IL-4 went up with 50nM but not 10 nM 1,25(OH)2D (Figure 1). Cultures were set up to expand and purify the iNKT cells. The ability to expand iNKT cells from some donors was low (11–29 fold expansion) while iNKT cell expansion from 3 individuals was high (123–596 fold expansion, Figure 2). Adding 10 and 50 nM 1,25(OH)2D at the start of the 12 day culture reduced the iNKT cells that could be recovered from the cultures (data not shown). Cell lines were generated utilizing magnetic bead purified iNKT cells (95% CD1d tetramer+/CD3+) for experiments. Resting iNKT cell lines had very low expression of the VDR that was upregulated with 48h of αGalCer and irradiated PBMC incubations (Figure 3). There was not an effect of 1,25(OH)2D on the proliferation of three different human iNKT cell lines (data not shown). The data we provide here suggest that there are important differences between the effects of 1,25(OH)2D on tissue iNKT cells in the mouse and PBMC iNKT cells in the human. Human iNKT cells, like T cells, optimally express the VDR after several days of activation. Induction of IL-4 by 1,25(OH)2D in murine and human iNKT cells was the same. The reason for the difference between the effects of 1,25(OH)2D on mouse and human iNKT cells might be a species difference, the location of the cells, the low frequencies of the iNKT cells in the freshly isolated PBMC or changes that occur in vitro when culturing and expanding human iNKT cells.
Figure 1.
Fresh peripheral blood mononuclear cells (PBMCs) stimulated with α-Galcer were cultured for 72 h with or without 1,25(OH)2D treatment and supernatants were analyzed by ELISA for IFN-γ and IL-4 production. n = 10 different PBMC donors. * P < 0.05. Experiments using humans were done with the approval of the Pennsylvania State University, University Park, PA, Institutional Review Board: #32141.
Figure 2.
iNKT cell expansion ex vivo. PBMCs were stimulated with 100 ng/mL α-GalCer for 12 days to expand iNKT cells. n = 12 individual donors were used.
Figure 3.
Vitamin D receptor (VDR) expression is up regulated with activation. iNKT cell lines were restimulated with α-GalCer and pulsed irradiated PBMCs in vitro. Cells were harvested at 6, 24 and 48 h post stimulation. iNKT cells (gated on CD1d-tet+ cells) were stained with anti-VDR antibodies (Clone H4537, R&D Systems) The isotype control staining is the grey histogram. Data shown is one representative of two experiments performed done with iNKT cell lines from 2 different donors.
5. Conclusions
T cells become vitamin D targets by expressing the VDR and inducing autocrine 1,25(OH)2D following activation. The 1,25(OH)2D inhibits murine and human T cells from proliferating, producing IFN-γ, and IL-17 while inducing IL-4. In animal models these effects correspond to the vitamin D mediated inhibition of experimental immune mediated diseases caused by Th1 and Th17 cells. Further, 1,25(OH)2D induced IL-4 in both mouse and human iNKT cells. The effects of 1,25(OH)2D on proliferation and IFN-γ production in mouse and human iNKT cells was different. Overall the effects of vitamin D on both iNKT cells and T cells seem to require 48–72 h of activation and suggest that vitamin D is important late during the T cell response. Together the data suggest an important role for vitamin D and 1,25(OH)2D in regulating T cells to limit immune mediated diseases where IL-17 and IFN-γ, are pathogenic.
Acknowledgements
This work was supported in part by the National Institutes of Neurologic and Stroke Grant NS067563 and National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements AT005378.
Author Contributions
L.Y. and M.T.C. designed the human experiments, L.Y. conducted research, L.Y., L.S., Y.L. and M.T.C. analyzed research, L.S., Y.L. and M.T.C. wrote the manuscript; M.T.C. had primary responsibility for final content.
Conflicts of Interests
The authors have no conflicts of interest to declare.
References
- 1.Reeve L., Tanaka Y., DeLuca H.F. Studies on the site of 1,25-dihydroxyvitamin d3 synthesis in vivo. J. Biol. Chem. 1983;258:3615–3617. [PubMed] [Google Scholar]
- 2.Christakos S., Ajibade D.V., Dhawan P., Fechner A.J., Mady L.J. Vitamin D: Metabolism. Rheum. Dis. Clin. N. Am. 2012;38:1–11,vii. doi: 10.1016/j.rdc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 3.DeLuca H.F. Overview of general physiologic features and functions of vitamin d. Am. J. Clin. Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 4.Pike J.W., Meyer M.B. Fundamentals of vitamin d hormone-regulated gene expression. J. Steroid Biochem. Mol. Biol. 2014;144:5–11. doi: 10.1016/j.jsbmb.2013.11.004. (Pt. A) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant W.B., Holick M.F. Benefits and requirements of vitamin d for optimal health: A review. Altern. Med. Rev. 2005;10:94–111. [PubMed] [Google Scholar]
- 6.Veldman C.M., Cantorna M.T., DeLuca H.F. Expression of 1,25-dihydroxyvitamin d(3) receptor in the immune system. Arch. Biochem. Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 7.Swain S.L., McKinstry K.K., Strutt T.M. Expanding roles for cd4(+) t cells in immunity to viruses. Nat. Rev. Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh C.S., Macatonia S.E., Tripp C.S., Wolf S.F., O’Garra A., Murphy K.M. Development of th1 cd4+ t cells through il-12 produced by listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 9.Haanen J.B., de Waal Malefijt R., Res P.C., Kraakman E.M., Ottenhoff T.H., de Vries R.R., Spits H. Selection of a human t helper type 1-like t cell subset by mycobacteria. J. Exp. Med. 1991;174:583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maizels R.M., Hewitson J.P., Smith K.A. Susceptibility and immunity to helminth parasites. Curr. Opin. Immunol. 2012;24:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver C.T., Elson C.O., Fouser L.A., Kolls J.K. The th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. Differential roles of interleukin-17a and -17f in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda J.L., Mallevaey T., Scott-Browne J., Gapin L. Cd1d-restricted inkt cells, the ‘swiss-army knife’ of the immune system. Curr. Opin. Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratz I.K., Campbell D.J. Organ-specific and memory treg cells: Specificity, development, function, and maintenance. Front. Immunol. 2014;5:333. doi: 10.3389/fimmu.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwamura C., Nakayama T. Role of nkt cells in allergic asthma. Curr. Opin. Immunol. 2010;22:807–813. doi: 10.1016/j.coi.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Rigby W.F., Stacy T., Fanger M.W. Inhibition of t lymphocyte mitogenesis by 1,25-dihydroxyvitamin d3 (calcitriol) J. Clin. Investig. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Provvedini D.M., Tsoukas C.D., Deftos L.J., Manolagas S.C. 1,25-dihydroxyvitamin d3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 18.Tsoukas C.D., Provvedini D.M., Manolagas S.C. 1,25-dihydroxyvitamin d3: A novel immunoregulatory hormone. Science. 1984;224:1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- 19.Palmer M.T., Lee Y.K., Maynard C.L., Oliver J.R., Bikle D.D., Jetten A.M., Weaver C.T. Lineage-specific effects of 1,25-dihydroxyvitamin d(3) on the development of effector cd4 t cells. J. Biol. Chem. 2011;286:997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F., O’Garra A. 1alpha,25-dihydroxyvitamin d3 has a direct effect on naive cd4(+) t cells to enhance the development of th2 cells. J. Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 21.Mahon B.D., Wittke A., Weaver V., Cantorna M.T. The targets of vitamin d depend on the differentiation and activation status of cd4 positive t cells. J. Cell. Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 22.Pichler J., Gerstmayr M., Szepfalusi Z., Urbanek R., Peterlik M., Willheim M. 1alpha,25(oh)2d3 inhibits not only th1 but also th2 differentiation in human cord blood t cells. Pediatr. Res. 2002;52:12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Staeva-Vieira T.P., Freedman L.P. 1,25-dihydroxyvitamin d3 inhibits ifn-gamma and il-4 levels during in vitro polarization of primary murine cd4+ t cells. J. Immunol. 2002;168:1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 24.Korf H., Wenes M., Stijlemans B., Takiishi T., Robert S., Miani M., Eizirik D.L., Gysemans C., Mathieu C. 1,25-dihydroxyvitamin d(3) curtails the inflammatory and t cell stimulatory capacity of macrophages through an il-10-dependent mechanism. Immunobiology. 2012;217:1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Sigmundsdottir H., Pan J., Debes G.F., Alt C., Habtezion A., Soler D., Butcher E.C. Dcs metabolize sunlight-induced vitamin d3 to ‘program’ t cell attraction to the epidermal chemokine ccl27. Nat. Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Bruce D., Cantorna M.T. Vitamin d receptor expression controls proliferation of naive cd8+ t cells and development of cd8 mediated gastrointestinal inflammation. BMC Immunol. 2014;15:6. doi: 10.1186/1471-2172-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigby W.F., Yirinec B., Oldershaw R.L., Fanger M.W. Comparison of the effects of 1,25-dihydroxyvitamin d3 on t lymphocyte subpopulations. Eur. J. Immunol. 1987;17:563–566. doi: 10.1002/eji.1830170420. [DOI] [PubMed] [Google Scholar]
- 28.Yuzefpolskiy Y., Baumann F.M., Penny L.A., Studzinski G.P., Kalia V., Sarkar S. Vitamin d receptor signals regulate effector and memory cd8 t cell responses to infections in mice. J. Nutr. 2014;144:2073–2082. doi: 10.3945/jn.114.202895. [DOI] [PubMed] [Google Scholar]
- 29.Bruce D., Cantorna M.T. Intrinsic requirement for the vitamin d receptor in the development of cd8alphaalpha-expressing t cells. J. Immunol. 2011;186:2819–2825. doi: 10.4049/jimmunol.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu S., Cantorna M.T. The vitamin d receptor is required for inkt cell development. Proc. Natl. Acad. Sci. USA. 2008;105:5207–5212. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S., Cantorna M.T. Epigenetic reduction in invariant nkt cells following in utero vitamin d deficiency in mice. J. Immunol. 2011;186:1384–1390. doi: 10.4049/jimmunol.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waddell A., Zhao J., Cantorna M.T. Natural killer t cells can help mediate the protective effects of 1,25-dihydroxyvitamin d3 in experimental autoimmune encephalomyelitis in mice. Int. Immunol. 2015 doi: 10.1093/intimm/dxu147. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zella J.B., McCary L.C., DeLuca H.F. Oral administration of 1,25-dihydroxyvitamin d3 completely protects nod mice from insulin-dependent diabetes mellitus. Arch. Biochem. Biophys. 2003;417:77–80. doi: 10.1016/S0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 34.Gregori S., Giarratana N., Smiroldo S., Uskokovic M., Adorini L. A 1alpha,25-dihydroxyvitamin d(3) analog enhances regulatory t-cells and arrests autoimmune diabetes in nod mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 35.Cantorna M.T., Munsick C., Bemiss C., Mahon B.D. 1,25-dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J. Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 36.Froicu M., Zhu Y., Cantorna M.T. Vitamin d receptor is required to control gastrointestinal immunity in il-10 knockout mice. Immunology. 2006;117:310–318. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantorna M.T., Humpal-Winter J., DeLuca H.F. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin d(3) Arch. Biochem. Biophys. 2000;377:135–138. doi: 10.1006/abbi.2000.1765. [DOI] [PubMed] [Google Scholar]
- 38.Spach K.M., Nashold F.E., Dittel B.N., Hayes C.E. Il-10 signaling is essential for 1,25-dihydroxyvitamin d3-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 39.Cantorna M.T. Vitamin d, multiple sclerosis and inflammatory bowel disease. Arch. Biochem. Biophys. 2012;523:103–106. doi: 10.1016/j.abb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantorna M.T., McDaniel K., Bora S., Chen J., James J. Vitamin d, immune regulation, the microbiota, and inflammatory bowel disease. Exp. Biol. Med. (Maywood) 2014;239:1524–1530. doi: 10.1177/1535370214523890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ooi J.H., Chen J., Cantorna M.T. Vitamin d regulation of immune function in the gut: Why do t cells have vitamin d receptors? Mol. Aspects Med. 2012;33:77–82. doi: 10.1016/j.mam.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colin E.M., Asmawidjaja P.S., van Hamburg J.P., Mus A.M., van Driel M., Hazes J.M., van Leeuwen J.P., Lubberts E. 1,25-dihydroxyvitamin d3 modulates th17 polarization and interleukin-22 expression by memory t cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–142. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 43.Van der Eerden B.C., van der Heyden J.C., van Hamburg J.P., Schreuders-Koedam M., Asmawidjaja P.S., de Muinck Keizer-Schrama S.M., Boot A.M., Lubberts E., Drop S.L., van Leeuwen J.P. A human vitamin d receptor mutation causes rickets and impaired th1/th17 responses. Bone. 2014;69:6–11. doi: 10.1016/j.bone.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Keating P., Munim A., Hartmann J.X. Effect of vitamin d on t-helper type 9 polarized human memory cells in chronic persistent asthma. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2014;112:154–162. doi: 10.1016/j.anai.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Zhong H., Zhou X.J., Hong J.G. The effects of vitamin d on allergen-induced expression of interleukin-13 and interleukin-17 in cord blood cd4(+)t cells. Iran. J. Allergy Asthma Immunol. 2014;13:93–97. [PubMed] [Google Scholar]
- 46.Yu S., Zhao J., Cantorna M.T. Invariant nkt cell defects in vitamin d receptor knockout mice prevents experimental lung inflammation. J. Immunol. 2011;187:4907–4912. doi: 10.4049/jimmunol.1101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wittke A., Chang A., Froicu M., Harandi O.F., Weaver V., August A., Paulson R.F., Cantorna M.T. Vitamin d receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Arch. Biochem. Biophys. 2007;460:306–313. doi: 10.1016/j.abb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akbari O., Stock P., Meyer E., Kronenberg M., Sidobre S., Nakayama T., Taniguchi M., Grusby M.J., DeKruyff R.H., Umetsu D.T. Essential role of nkt cells producing il-4 and il-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 49.Wittke A., Weaver V., Mahon B.D., August A., Cantorna M.T. Vitamin d receptor-deficient mice fail to develop experimental allergic asthma. J. Immunol. 2004;173:3432–3436. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 50.Matheu V., Back O., Mondoc E., Issazadeh-Navikas S. Dual effects of vitamin d-induced alteration of th1/th2 cytokine expression: Enhancing ige production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 2003;112:585–592. doi: 10.1016/S0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 51.Topilski I., Flaishon L., Naveh Y., Harmelin A., Levo Y., Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin d3 on th2 cells in vivo are due in part to the control of integrin-mediated t lymphocyte homing. Eur. J. Immunol. 2004;34:1068–1076. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 52.Gorman S., Judge M.A., Burchell J.T., Turner D.J., Hart P.H. 1,25-dihydroxyvitamin d3 enhances the ability of transferred cd4+ cd25+ cells to modulate t helper type 2-driven asthmatic responses. Immunology. 2010;130:181–192. doi: 10.1111/j.1365-2567.2009.03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorman S., Judge M.A., Hart P.H. Topical 1,25-dihydroxyvitamin d3 subverts the priming ability of draining lymph node dendritic cells. Immunology. 2010;131:415–425. doi: 10.1111/j.1365-2567.2010.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taher Y.A., van Esch B.C., Hofman G.A., Henricks P.A., van Oosterhout A.J. 1alpha,25-dihydroxyvitamin d3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: Role for il-10 and tgf-beta. J. Immunol. 2008;180:5211–5221. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- 55.Rigby W.F., Noelle R.J., Krause K., Fanger M.W. The effects of 1,25-dihydroxyvitamin d3 on human t lymphocyte activation and proliferation: A cell cycle analysis. J. Immunol. 1985;135:2279–2286. [PubMed] [Google Scholar]
- 56.Joshi S., Pantalena L.C., Liu X.K., Gaffen S.L., Liu H., Rohowsky-Kochan C., Ichiyama K., Yoshimura A., Steinman L., Christakos S., et al. 1,25-dihydroxyvitamin d3 ameliorates th17 autoimmunity via transcriptional modulation of interleukin-17a. Mol. Cell. Biol. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kongsbak M., von Essen M.R., Boding L., Levring T.B., Schjerling P., Lauritsen J.P., Woetmann A., Odum N., Bonefeld C.M., Geisler C. Vitamin d up-regulates the vitamin d receptor by protecting it from proteasomal degradation in human cd4+ t cells. PLoS ONE. 2014;9:e96695. doi: 10.1371/journal.pone.0096695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Healy K.D., Frahm M.A., DeLuca H.F. 1,25-dihydroxyvitamin d3 up-regulates the renal vitamin d receptor through indirect gene activation and receptor stabilization. Arch. Biochem. Biophys. 2005;433:466–473. doi: 10.1016/j.abb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Ooi J.H., McDaniel K.L., Weaver V., Cantorna M.T. Murine cd8+ t cells but not macrophages express the vitamin d 1alpha-hydroxylase. J. Nutr. Biochem. 2014;25:58–65. doi: 10.1016/j.jnutbio.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kongsbak M., von Essen M.R., Levring T.B., Schjerling P., Woetmann A., Odum N., Bonefeld C.M., Geisler C. Vitamin d-binding protein controls t cell responses to vitamin d. BMC Immunol. 2014;15:35. doi: 10.1186/s12865-014-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrat F.J., Cua D.J., Boonstra A., Richards D.F., Crain C., Savelkoul H.F., de Waal-Malefyt R., Coffman R.L., Hawrylowicz C.M., O’Garra A. In vitro generation of interleukin 10-producing regulatory cd4(+) t cells is induced by immunosuppressive drugs and inhibited by t helper type 1 (th1)- and th2-inducing cytokines. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]