Abstract
IMPORTANCE
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive neuromodulation technique that has been closely examined as a possible treatment for Parkinson disease (PD). However, results evaluating the effectiveness of rTMS in PD are mixed, mostly owing to low statistical power or variety in individual rTMS protocols.
OBJECTIVES
To determine the rTMS effects on motor dysfunction in patients with PD and to examine potential factors that modulate the rTMS effects.
DATA SOURCES
Databases searched included PubMed, EMBASE, Web of Knowledge, Scopus, and the Cochrane Library from inception to June 30, 2014.
STUDY SELECTION
Eligible studies included sham-controlled, randomized clinical trials of rTMS intervention for motor dysfunction in patients with PD.
DATA EXTRACTION AND SYNTHESIS
Relevant measures were extracted independently by 2 investigators. Standardized mean differences (SMDs) were calculated with random-effects models.
MAIN OUTCOMES AND MEASURES
Motor examination of the Unified Parkinson’s Disease Rating Scale.
RESULTS
Twenty studies with a total of 470 patients were included. Random-effects analysis revealed a pooled SMD of 0.46 (95%CI, 0.29–0.64), indicating an overall medium effect size favoring active rTMS over sham rTMS in the reduction of motor symptoms (P < .001). Subgroup analysis showed that the effect sizes estimated from high-frequency rTMS targeting the primary motor cortex (SMD, 0.77; 95%CI, 0.46–1.08; P < .001) and low-frequency rTMS applied over other frontal regions (SMD, 0.50; 95%CI, 0.13–0.87; P = .008) were significant. The effect sizes obtained from the other 2 combinations of rTMS frequency and rTMS site (ie, high-frequency rTMS at other frontal regions: SMD, 0.23; 95% CI, −0.02 to 0.48, and low primary motor cortex: SMD, 0.28; 95%CI, −0.23 to 0.78) were not significant. Meta-regression revealed that a greater number of pulses per session or across sessions is associated with larger rTMS effects. Using the Grading of Recommendations, Assessment, Development, and Evaluation criteria, we characterized the quality of evidence presented in this meta-analysis as moderate quality.
CONCLUSIONS AND RELEVANCE
The pooled evidence suggests that rTMS improves motor symptoms for patients with PD. Combinations of rTMS site and frequency as well as the number of rTMS pulses are key modulators of rTMS effects. The findings of our meta-analysis may guide treatment decisions and inform future research.
Parkinson disease (PD) is a progressive neurodegenerative disorder characterized by resting tremor, bradykinesia, rigidity, gait disorder, and postural instability. It is estimated that 6 to 10 million people worldwide have PD, affecting all races and ethnicities. The incidence of PD rises rapidly with age, affecting approximately 1% of the population older than 60 years and approximately 4%of those older than 80 years.1 As the average age of the population increases, the prevalence of PD worldwide is expected to more than double by 2030.2
Medical therapy substantially improves quality of life and functional capacity in PD; however, most patients develop complications after 5 years of treatment, including dyskinesia and motor fluctuations.3 Surgical techniques, including deep brain stimulation, improve advanced symptoms above the best medical therapy, although less than 5% of the PD population may be eligible for the procedure.4 During the past 2 decades, repetitive transcranial magnetic stimulation (rTMS) has been closely examined as a possible treatment for PD.5–7 As a noninvasive procedure, rTMS does not require surgery or anesthesia. It delivers repeated magnetic pulses to a specific brain area within a short time through a stimulation coil placed over the scalp. The repeated magnetic pulses not only alter excitability at the site of stimulation but also influence brain regions anatomically connected to the stimulation site.8 Because rTMS can produce changes in neural activity and behavior that last well after stimulation, this technique has generated much interest as a potential therapeutic intervention for patients with PD.
Accumulating studies investigating the effectiveness of rTMS have yielded mixed results, possibly owing to low statistical power and wide variation in treatment protocols. Thus, it is critical to integrate and arrange these findings based on rTMS-related factors to more accurately estimate the effects of rTMS on PD. The objectives of this meta-analysis were to (1) systematically evaluate the efficacy of rTMS intervention compared with sham controls for motor dysfunction in PD from randomized clinical trials (RCTs) and (2) identify factors of rTMS protocols that may moderate the rTMS effects. The objectives were defined in terms of population, interventions, comparators, outcomes, and study designs.
Methods
Study Design and Registration
Our meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement9 and is registered with PROSPERO (registration number CRD42014013372).
Search Strategy
To identify studies for inclusion in this meta-analysis, we searched PubMed, EMBASE, Web of Knowledge, Scopus, and the Cochrane Library from inception through June 30, 2014. Databases were searched using combinations of the following terms: Parkinson disease and repetitive transcranial magnetic stimulation or rTMS or repetitive TMS. The complete documentation of the search procedure is included in the eAppendix in the Supplement. We also searched the reference lists of general reviews on rTMS for PD10–12 and of meta-analyses6,7 to identify additional relevant articles.
Inclusion Criteria for Study Selection
Studies identified through database searches were first screened on the basis of their title and abstract. Studies were excluded if it was clear from the article title or abstract that the trial was not relevant or did not meet the inclusion criteria. If relevance was unclear, we assessed the article in its entirety. We included trials that met the following criteria: population (patients with a diagnosis of idiopathic PD), intervention (rTMS), comparators (sham-controlled group or condition), outcome measure (motor examination of the Unified Parkinson’s Disease Rating Scale [UPDRS-III]), study design (parallel or crossover RCTs that used a sham-controlled group or condition), and language (articles written in English). Studies were excluded if they did not have data available for effect size estimation or were a conference abstract or presentation.
Data Extraction
Two authors (Y.-h.C. and M.S.) independently performed data extraction, with disagreement resolved by discussion. Extracted data included sample size, sample characteristics, study design, rTMS protocol, statistical data on the UPDRS-III score for effect size estimation, medication state during assessment (“on” or “off”), and timing of outcome measurements (short-term, <1 week; or long-term, ≥1 week). When reported data were insufficient for data analysis, we contacted the study author to request access to additional data.
Statistical Analysis
Effect Size Calculation
We used standardized mean difference (SMD [Cohen d]) to express the size of the rTMS effect on motor symptoms measured with the UPDRS-III. A random-effects model was used to calculate pooled effect sizes and test whether the mean effect size was significantly different from zero (P ≤ .05, 2-tailed). The mean effect was expressed as SMD with 95%CIs. If a study had multiple effect sizes from the same patient group (eg, short term and long-term rTMS effects), we obtained one mean effect size across multiple effect sizes within this study. For total effect size estimation, the unit of analysis was study.
Heterogeneity Analysis
Multiple types of heterogeneity were present in the included studies: (1) variability in the participants, intervention characteristics, and the timing of outcome measurements (clinical heterogeneity); (2) variability in study design and risk of bias (methodologic heterogeneity); and (3) variability in treatment effects (statistical heterogeneity).13 Methodologic and clinical sources of heterogeneity contribute to the magnitude and presence of statistical heterogeneity13; we used the Q statistic and the I2 index to assess the statistical heterogeneity. A probability value of P ≤ .05 and an I2 value of greater than 40% are indicative of heterogeneity between included studies as the values exceed what is expected by chance.14
Publication or Selection Bias
Publication or selection bias was evaluated with the Egger test of asymmetry15 and the Orwin fail-safe N approach.16 In the absence of publication or selection bias, effect sizes are symmetrically distributed around the overall mean effect size, since the sampling error is random. The Egger test evaluated whether the amount of asymmetry was significant. In addition, studies that demonstrated a lack of benefit might not have been published or submitted for publication. Therefore, we used the Orwin fail-safe N test to estimate the number of missing studies that we would need to retrieve and incorporate in our meta-analysis to make the summary effect become trivial.
Subgroup Analysis
For subgroup analysis, the unit of analysis was trial (ie, effect size that was reported or could be estimated from a study). Our prespecified comparisons included rTMS site (primary motor cortex [M1] vs other frontal regions), rTMS frequency (low [≤1 Hz] vs high [≥5 Hz]), interaction between rTMS site and rTMS frequency, timing of outcome measurement (short-term [<1 week] vs long-term [≥1 week]), medication state during assessment (on-state vs off-state), and type of sham-rTMS approach.
Meta-regression
We used meta-regression to identify the major sources of between-study variation in the results by using the SMD from each study or trial as a dependent variable and rTMS variables as predictors. All values from predictor variables were z transformed for meta-regression.
Sensitivity Analysis
The process of undertaking a meta-analysis involves making decisions about inclusion criteria. We performed a sensitivity analysis to examine whether our results would have differed if we had included non-RCTs into the meta-analysis.
Risk of Bias Assessment in Individual Studies
We assessed the risk of bias using the Cochrane risk of bias assessment tool outlined in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0.13 The Cochrane tool classifies studies as having low, high, or unclear risk of bias in the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and carryover effect. In addition, we used the Physiotherapy Evidence Database (PEDro) scale17,18 to quantify the quality of included studies. The PEDro scale scored 11 items (eTable 1 in the Supplement) as either present or absent. The final score is the number of positive answers on all questions. We considered a PEDro score of 11 to represent an excellent-quality study, scores of 8 to 10 a good-quality study, scores of 6 and 7 a fair-quality study, and scores of 5 or lower a low-quality study.19
Risk of Bias Assessment Across Studies
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.20 Four levels of quality of evidence are specified: high, moderate, low, and very low. An initially assumed high level of evidence would be downgraded for meeting any of the following criteria21: (1) risk of bias (downgrade once if less than 75% of the included studies are at low risk of bias across all risk of bias domains), (2) heterogeneity (downgrade once if heterogeneity between the included studies is significant and the I2 value is greater than 40%), (3) indirectness (downgrade once if more than 50% of the participants were outside the target group), (4) imprecision (downgrade once if fewer than 400 participants),22 and (5) publication/selection bias (downgrade once if the publication/selection bias is significant).
Results
Search Results
Our initial search of all databases retrieved 2203 studies (eAppendix in the Supplement), yet many of these were identified as duplicates. After screening for title and abstract, the full texts of 60 articles were obtained for examination. Of these, 40 studies were excluded and 20 studies23–42 that met the inclusion criteria were evaluated in this meta-analysis (eFigure 1 in the Supplement). Two studies presented different phenomena (eg, on or off state) from the same group of patients in 2 articles37,38; therefore, the SMDs from these 2 studies were averaged for total effect size estimation.
Study Characteristics
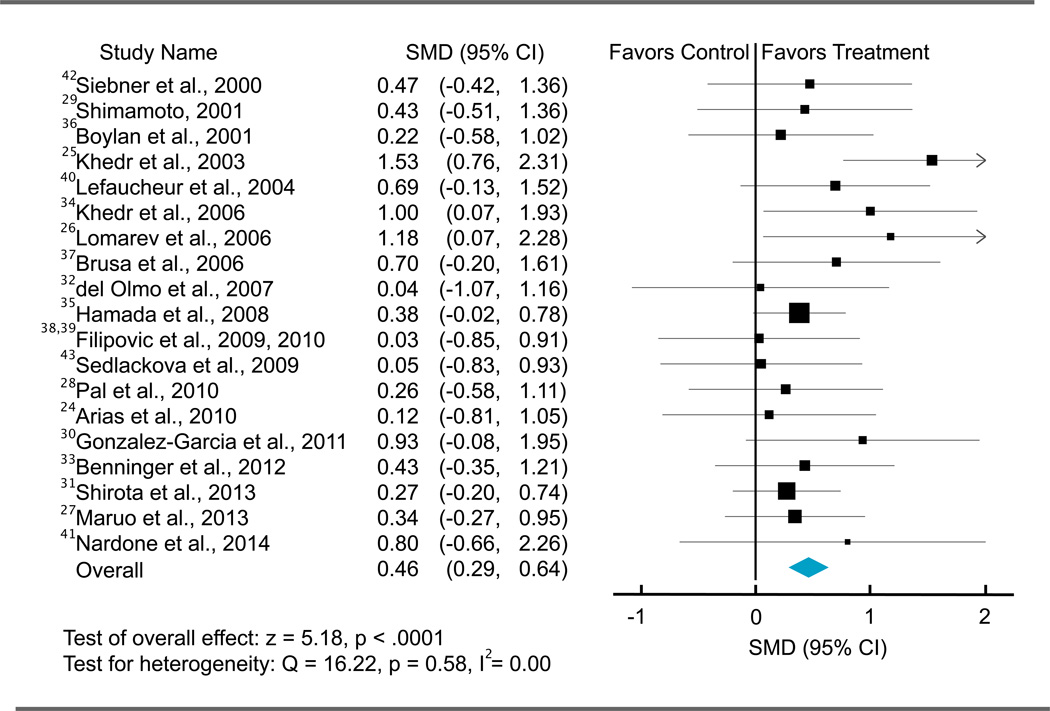
The 20 eligible studies included 470 participants (mean [SD] age, 63.59 [8.24] years; 57% men). The total effect size of rTMS on UPDRS-III score was 0.46 (95% CI, 0.29 to 0.64), indicating a medium effect size favoring active rTMS over sham rTMS (z = 5.18; P < .001). The mean score change in the UPDRS-III following active rTMS intervention was −6.42 (5.79), corresponding to a moderate clinically important difference.43 The main characteristics of the included studies are described in Tables 1, 2, and 3, and the distribution of effect sizes is illustrated in Figure 1.
Table 1.
Characteristics of included studies: participants
| Source | Sample Size, No. |
Age, Mean (SD), y |
Sex (M/F) |
DD, Mean (SD), y |
H and Y Stage |
|---|---|---|---|---|---|
| Siebner et al,42 2000 | 10 | 57 (11) | 7/3 | 6 (3) | 1–2 |
| Boylan et al,35 2001 | 8 | 63.5a | 6/4a | 8.6a | 2–3 |
| Shimamoto et al,28 2001 | 18 | 65 (7) | 12/6 | 7 (5) | 1.5–4 |
| Khedr et al,24 2003 | 36b | 58 (9) | 24/12 | 3 (2) | 2–3 |
| Lefaucheur et al,39 2004 | 12 | 64 (9) | 7/5 | 11 (5) | 2.5–4 |
| Khedr et al,33 2006 | 20 | 60 (10) | NA | 4 (2) | 3–5 |
| Lomarev et al,25 2006 | 16 | 65 (10) | 14/2 | 12 (5) | 2–4 |
| Brusa et al,36 2006 | 10 | 61 (8) | 6/4 | 16 (5) | NA |
| del Olmo et al,31 2007 | 13 | 62 (5) | 6/7 | 8 (5) | 1–3 |
| Hamada et al,34 2008 | 98 | 66 (9) | 54/44 | 8 (5) | 2–4 |
| Sedlácková et al,41 2009 | 9 | 64 (7)a | 9/1a | 8 (7)a | NA |
| Filipović et al,37,38 2010, 2009 | 10 | 65 (10) | 5/5 | 16 (6) | 2–4 |
| Pal et al,27 2010 | 22 | 69 (8) | 11/11 | 6c | NA |
| Arias et al,23 2010 | 18 | 66 (9) | 9/9 | 7 (3) | 2–4 |
| González-García et al,29 2011 | 17 | 65 (5) | 11/6 | NA | 2–3 |
| Benninger et al,32 2012 | 26 | 64 (9) | 20/6 | 9 (5) | 2–4 |
| Maruo et al,26 2013 | 21 | 63 (11) | 11/10 | 12 (6) | 2–4 |
| Shirota et al,30 2013 | 102d | 67 (8)d | 43/59d | 5 (6)d | 2–4d |
| Nardone et al,40 2014 | 4 | 66 (4) | 3/1 | 10 (5) | 2–4 |
Abbreviations: DD, disease duration H and A, Hoehn and Yahr; NA, not available.
Data from enrolled participants.
Participants who completed the repetitive transcranial magnetic stimulation sessions and the evaluation 1 hour after the session.
Median disease duration.
Data analyzed.
Table 2.
Characteristics of Included Studies: rTMS Variables
| Source | rTMS site |
rTMS frequency |
Intensity, % | No. of pulsesa | Treatment duration |
Sham-rTMS |
|---|---|---|---|---|---|---|
| Siebner et al,42 2000 | M1 | 5 Hz | 90 RMT | 2250 (2250 × 1) | 1 d | Tilted coil |
| Boylan et al,35 2001 | SMA | 10 Hz | Approximately 96 MT | 2000 (2000 × 1) | 1 d | Tilted coil |
| Shimamoto et al,28 2001 | Frontal | 0.2 Hz | 700 V | 480 (60 × 8) | 2 mo | Inactive coil and sound |
| Khedr et al,24 2003 | M1 | 5 Hz | 120 RMT | 20 000 (2000 × 10) | 10 d | Tilted coil |
| Lefaucheur et al,39 2004 | M1 | 0.5 and 10 Hz | 80 RMT | 600 (600 × 1); | 1 d | Sham coil |
| Khedr et al,33 2006 | M1 | 10 Hz | 100 MT | 2000 (2000 × 1) | 6 d | Occipital stimulation |
| Lomarev et al,25 2006 | M1/DLPFC | 25 Hz | 100 MT | 18 000 (3000 × 6) | 4 wk | Coil back surface |
| Brusa et al,36 2006 | SMA | 1 Hz | 90 RMT | 9600 (1200 × 8) | 5 d | Tilted coil |
| del Olmo et al,31 2007 | DLPFC | 10 Hz | 90 RMT | 4500 (900 × 5) | 10 d | Tilted coil |
| Hamada et al,34 2008 | SMA | 5 Hz | 110 AMT | 4500 (450 × 10) | 8 wk | Realistic sham |
| Sedlácková et al,41 2009 | PMd/DLPFC | 10 Hz | 100 RMT | 8000 (1000 × 8) | 1 db | Occipital stimulation |
| Filipović et al,37,38 2009, 2010 | M1 | 1 Hz | Approximately 90 RMT | 1350 (1350 × 1) | 4 d | Sham coil |
| Pal et al,27 2010 | DLPFC | 5 Hz | 90 RMT | 7200 (1800 × 4) | 10 d | Tilted coil |
| Arias et al,23 2010 | Vertex | 1 Hz | 90 RMT | 6000 (600 × 10) | 10 d | Inactive and active coils |
| González-García et al,29 2011 | M1 | 25 Hz | 80 RMT | 1000 (100 × 10) | 12 wk | Occipital stimulation |
| Benninger et al,32 2012 | M1 | 50 Hz | 80 AMT | 30 000 (2000 × 15) | 2 wk | Inactive and active coils |
| Maruo et al,26 2013 | M1 | 10 Hz | 100 RMT | 4800 (600 × 8) | 3 d | Realistic sham |
| Shirota et al,30 2013 | SMA | 1 and 10 Hz | 110 RMT | 3000 (1000 × 3) | 8 wk | Realistic sham |
| Nardone et al,40 2014 | DLPFC | 1Hz | Below AMT | 8000 (1000 × 8) | 1 d | Sham coil |
Abbreviations: AMT, active motor threshold (MT); DLPFC, dorsolateral prefrontal cortex; M1, primary motor cortex; PMd, dorsal premotor cortex; RMT, resting MT; rTMS, repetitive transcranial magnetic stimulation; SMA, supplementary motor area.
Total number of pulses (number of pulses per session times number of sessions).
Treatment was 1 day for each region; these treatment days were separated by at least 1 day.
Table 3.
Characteristics of Included Studies: Outcome Measurements
| Source | On/Offa | Post-rTMS Evaluation |
rTMS Changeb | |
|---|---|---|---|---|
| Active | Sham | |||
| Siebner et al,42 2000 | Off | 1 h | −7.40 | −2.90 |
| Boylan et al,35 2001 | Off | Immediately | NA | NA |
| Shimamoto et al,28 2001 | On and off | Immediately | −8.35 | −0.80 |
| Khedr et al,24 2003 | Off | 1 h and 1 mo | −14.3 | −0.62 |
| Lefaucheur et al,39 2004 | Off | 20 min | −6.50 | −2.00 |
| Khedr et al,33 2006 | Off | Immediately | NA | NA |
| Lomarev et al,25 2006 | On and off | 1 d and 1 mo | −3.50 | 1.08 |
| Brusa et al,36 2006 | On | 1 h | −23.90 | −20.80 |
| del Olmo et al,31 2007 | On | 1 d | NA | NA |
| Hamada et al,34 2008 | On | 4 wk | −4.78 | 0.01 |
| Sedlácková et al,41 2009 | Off | Immediately | 0.05 | 0.80 |
| Filipović et al,37,38 2009, 2010 | On and off | 1 d | −1.75 | −1.40 |
| Pal et al,27 2010 | On | 1 d and 1 mo | −5.00 | −1.50 |
| Arias et al,23 2010 | On and off | Immediately and 1 wk | −6.78 | −3.83 |
| González-García et al,29 2011 | On | 15 min | −7.50 | −0.80 |
| Benninger et al,32 2012 | On and off | 1 d and 1 mo | −2.10 | −0.88 |
| Maruo et al,26 2013 | Off | 1 h | −5.90 | −0.40 |
| Shirota et al,30 2013 | On | 1 and 12 wk | −3.91 | −3.20 |
| Nardone et al,40 2014 | On | 1, 12, and 24 h | −1.17 | −0.75 |
Abbreviations: NA, not available; rTMS, repetitive transcranial magnetic stimulation.
Medication state during assessment.
Changes in the motor section of the Unified Parkinson’s Disease Rating Scale-III score (post-rTMS score minus pre-rTMS score). More negative values (ie, greater decrements in the score) represent greater improvement in motor symptoms following rTMS sessions.
Figure 1.
Overall Forest Plot
Individual and pooled repetitive transcranial magnetic stimulation effect sizes (standardized mean differences [SMDs]) for the motor section of Unified Parkinson’s Disease Rating Scale score in patients with Parkinson disease. The size of the squares increases with increasing sample size.
Heterogeneity between the included studies did not exceed that expected by chance (Q18 = 16.22; P = .58; I2 = 0.00), implying that the results across the included studies were statistically homogeneous. Publication bias was evaluated using the Egger test of asymmetry and Orwin fail-safe N approach. The Egger test did not reveal significant asymmetry across included studies (intercept17 = 0.77; t = 1.12; 2-tailed P = .28) (eFigure 2 in the Supplement). The Orwin fail-safe N analysis showed that 157 studies with a mean effect size of 0 would be needed to change our conclusion (ie, from determining that active rTMS is more effective than the sham rTMS in reducing motor symptoms to determining that active rTMS is not more effective than the sham rTMS in reducing motor symptoms). The results demonstrate that our findings are robust without significant concerns regarding publication bias.
Subgroup Analysis
rTMS Site and rTMS Frequency
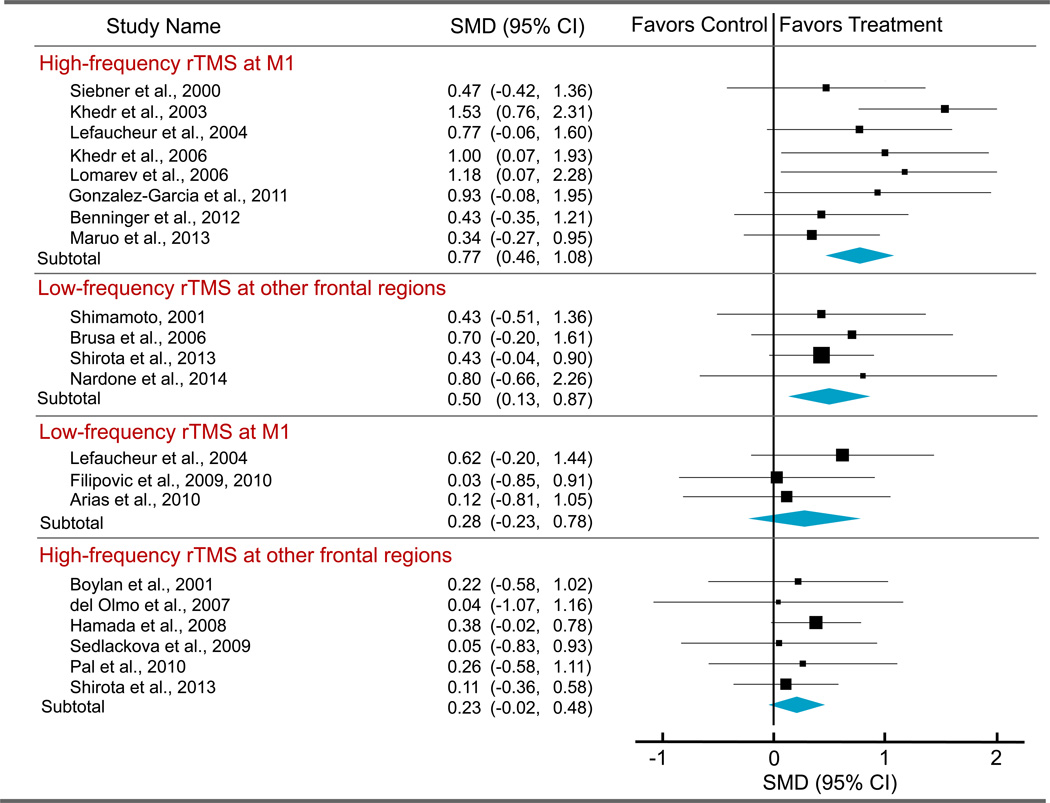
Our subgroup analysis revealed that there were no significant differences in effect size between rTMS sites (M1 vs other frontal regions) and between high-frequency and low-frequency rTMS. However, there was a significant difference in effect size among different combinations of rTMS frequency and rTMS site (Q3 = 7.82; P = .05). The effect sizes estimated from high-frequency rTMS targeting M1 (SMD, 0.77; P < .001) and from low-frequency rTMS applied over other frontal regions (SMD, 0.50; P = .008) were significant (Figure 2). The effect sizes obtained from the other 2 combinations of rTMS frequency and rTMS site (ie, high-others: SMD, 0.23, and low-M1: SMD, 0.28) were not significant.
Figure 2.
Forest Plot for Subgroups
Individual and pooled repetitive transcranial magnetic stimulation (rTMS) effect sizes (standardized mean differences [SMDs]) for the Unified Parkinson’s Disease Rating Scale score in patients with Parkinson disease. The size of the squares increases with increasing sample size. M1 indicates primary motor cortex.
Timing of Outcome Measurements
Seventeen trials23–29,31–33,35–42 assessed UPDRS-III at the short-term stage (<1 week), and 7 trials23–25,27,30,32,34 reported collecting long-term outcome (≥1 week). The short-term assessment was administered immediately after, 15 or 20 minutes after, 1 hour after, 12 hours after, or 1 day after rTMS sessions; the long-term assessment occurred 1 week, 1 month, or 12 weeks after rTMS sessions (Table 3). There was no significant difference between short-term rTMS effect (SMD, 0.50; 95% CI, 0.29–0.71) and long-term rTMS effect (SMD, 0.59; 95% CI, 0.19–1.00).
Medication State During Assessment
The UPDRS-III was assessed in 12 trials* during the off state and in 12 trials23,25,27–32,34,36–38,40 during the on state (Table 3). The estimated effect sizes were not significantly different between the off-state (SMD, 0.53; 95% CI, 0.27–0.79) and on-state (SMD, 0.39; 95% CI, 0.17–0.60) evaluations.
Sham-rTMS Conditions/Group
Different sham rTMS approaches were used in the included studies (Table 2). The rTMS effects for different sham rTMS approaches are summarized in eTable 2 in the Supplement. The subgroup analysis did not reveal a significant difference in effect sizes between different sham rTMS conditions/groups (P = .73).
Meta-regression
We performed meta-regression to examine whether effect sizes varied with rTMS variables. The mean (SD) number of rTMS treatment sessions was 5.95 (4.16) (range, 1–15), the mean number of rTMS pulses per session was 1285.50 (790.10) (range, 60–3000), and the mean number of rTMS pulses across treatment sessions (ie, number of pulses per session times the number of sessions) was 6754.00 (7649.99) (range, 480–30 000). The mean intensity was 95% (11%) of the active/resting motor threshold (range, 96%–120%), and the mean adjusted total number of rTMS pulses by intensity (ie, total number of pulses across sessions times intensity) was 7110.00 (7486.60) (range, 480–30 000).
The meta-regression analysis across trials showed that the number of pulses per session (r = 0.20; P = .05), the total number of pulses across sessions (r = 0.25; P = .02), and the adjusted total number of pulses across sessions by intensity (r = 0.27; P = .01) were significant predictors of the rTMS effect, suggesting that an increased number of pulses per session or across sessions yields enhanced the effects of rTMS. The number of sessions and intensity were not significant predictors.
Adverse Events
Thirteen studies24–27,30,32,33,36–40,42 evaluated the incidence of adverse events. Of these, 9 studies24,26,32,36–40,42 did not observe any adverse events. Shirota et al30 and Pal et al27 reported that 2 patients in the active-rTMS group in each one of studies experienced mild headache that did not require medical treatment. Khedr et al33 reported an occasional mild, transient headache in some patients. Lomarev et al25 reported that 1 patient in the active-rTMS group could not tolerate the pain under the coil and so withdrew from the study.
Sensitivity Analysis
We included 14 non-RCTs (18 trials in total; eReferences in the Supplement) into our meta-analysis to examine whether the results would change if we used a different inclusion criterion for study design. Inclusion of non-RCTs did not significantly alter findings. The pooled rTMS effect from both RCTs and non-RCTs remained moderate and significant (SMD, 0.50; 95%CI, 0.35–0.65; z = 6.64; P < .001), as did the pooled effect from RCTs.
Risk-of-Bias Assessment in Individual Studies
Ten studies† had incomplete data for risk-of-bias assessment. We were unable to locate one group of authors. Of the remaining 9 groups of authors, 5 individuals (56%) responded to information requests. The assessment of risk of bias for all included studies is summarized in eTable 3 in the Supplement. Overall, 11 of 19 included studies were at low risk of bias across all 6 domains. Similar to the risk-of-bias assessment, our quantitative analysis showed that 8 studies were of excellent methodologic quality (PEDro scale, 11 of 11), 10 studies were of good methodological quality (8–10), and 1 study was of fair methodologic quality (7). Additional analyses regarding whether the quality of studies would influence the overall rTMS effect size are described in the eResults and eTable 3 in the Supplement.
Risk-of-Bias Assessment Across Studies
Using the GRADE criteria, we characterized the quality of evidence presented in this meta-analysis as moderate. An initially assumed high level of evidence was downgraded once because less than 75%of the included studies were at low risk of bias across all domains. Despite the risk of bias, our meta-analysis exhibited homogeneity, directedness (ie, all participants were patients with PD), and precision (ie, >400 participants), and it is free from publication/selection bias across included studies.
Discussion
There are several relevant general conclusions one can draw based on the findings of this meta-analysis. First, the random effects across 20RCTs revealed a significant medium effect size (SMD, 0.46) favoring active rTMS over sham rTMS in the reduction of motor symptoms for patients with PD. Second, the rTMS effects were significant when high-frequency (≥5 Hz) rTMS was targeted at M1 (SMD,0.77; P < .001) or when low-frequency (≤1 Hz) rTMS was applied over other frontal regions (SMD, 0.50; P = .008). The effect sizes estimated from the other 2 combinations of rTMS frequency and site (high-others: SMD, 0.23; low-M1: SMD, 0.28) were not significant. Third, the meta-regression analysis showed that the number of pulses per session (r = 0.20; P = .05), the total number of pulses across sessions (r = 0.25;P = .02), and the adjusted total number of pulses across sessions by intensity (r = 0.27; P = .01) were significant predictors of rTMS effect, suggesting that a greater number of pulses per session or across sessions is associated with larger rTMS effects. Fourth, no significant differences in rTMS effects were found between short-term (≤1 week: SMD, 0.50) and long-term (>1 week: SMD, 0.59) outcome or between off-state (SMD, 0.53) and on-state (SMD, 0.39) during assessment. In addition, 13 studies evaluated the incidence of adverse events and, of these, no severe adverse events were reported. Finally, using the GRADE criteria, we characterized the quality of evidence of our meta-analysis as moderate.
One of the most important findings emerging from our meta-analysis is that the rTMS effects were stronger and significant when high-frequency rTMS was targeted at M1 or when low-frequency rTMS was applied over other frontal regions. We speculate that the results could be attributed to the state of brain activity (eg, degree of brain activation or interregional connectivity) at the time of stimulation. The state-dependent effects have been explained in terms of homeostatic plasticity, in which the form of synaptic plasticity—long-term potentiation or long-term depression—can be flexibly adjusted to stabilize cortico-spinal excitability depending on the state of brain activity.44,45 According to Siebner et al,46 a brain region with a prolonged reduction in postsynaptic activity (thereby decreasing levels of activity) would reduce a “modification threshold,” favoring the induction of long-term potentiation. Conversely, a brain region with a prolonged increase in postsynaptic activity (thereby increasing levels of activity) would raise the modification threshold, favoring the induction of long-term depression. Increased levels of activity in the supplementary motor area (SMA) and prefrontal cortex have been found in patients with PD and animal models of PD relative to healthy controls.47,48 The increased levels of activity in these frontal regions may result in the inhibition of action via a hyper direct pathway that connects several frontal regions (including the SMA, dorsolateral prefrontal cortex, and inferior frontal gyrus) and the subthalamic nucleus.49 Successful therapies, such as deep brain stimulation or medication, are associated with reductions in activity of the SMA and prefrontal cortex.50 A direct comparison between high-frequency (10 Hz) and low-frequency (1 Hz) rTMS over the SMA was conducted recently by Shirota et al30 in patients with PD. Their results demonstrated a long-lasting beneficial effect of low-frequency rTMS targeted at the SMA, whereas this effect was not significant for high-frequency rTMS targeting the same brain region. Coupled with previous findings, the results of our meta-analysis suggest that the stimulation site or, more precisely, the state of brain activity of the stimulated brain region, will be critical for choosing an optimal rTMS frequency.
This article updates preceding meta-analyses with the inclusion of new RCTs, and our estimated medium rTMS effect size is in agreement with the effect sizes previously reported by Fregni et al7 and Elahi et al.6 In addition, our findings highlight the importance of considering the interplay between rTMS frequency and the state of brain activity of the rTMS site, as well as the number of pulses per session and across sessions when developing a treatment protocol. Regarding the limitations of the present meta-analysis, our results could be constrained by the unclear risk of bias on certain domains owing to incomplete data in a few studies. In addition, several uncontrolled variables, such as medication use, disease stage, side of onset, side of rTMS stimulation, age, and sex, exist that could confound the results and must be acknowledged.
Conclusions
To date, evidence-based alterations in rTMS protocols for patients with PD have been only partially explored, and better rTMS protocols created from these findings have the potential to be more effective and more potent than current options. Future multimodal rTMS studies that combine the use of rTMS with different neuroimaging techniques are needed to better understand how brain activities are modulated by rTMS intervention, to more accurately and consistently target stimulation site, and to guide future rTMS treatment protocols.8 In addition, future research should attempt to establish a more precise relationship between rTMS effect and patients’ clinical and demographic characteristics, such as medication use, stage of disease, side of onset, dominant motor symptoms (eg, bradykinesia, resting tremor, rigidity, postural instability, or levodopa-induced dyskinesia), nonmotor symptoms (eg, depression or cognitive dysfunction), age, or sex. Establishing specificity in rTMS intervention and elucidating the role these potential factors could play in moderating rTMS effects will be necessary to guide the optimization of rTMS intervention in the near future.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported in part by research grant R01-NS074045 from the National Institutes of Health (Dr Chen).
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Chou had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chou, Hickey, Song, Chen.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Chou, Chen.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Chou.
Obtained funding: Song, Chen.
Administrative, technical, or material support: Sundman, Chen.
Study supervision: Song, Chen.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord. 2008;23(suppl 3):S497–S508. doi: 10.1002/mds.22028. [DOI] [PubMed] [Google Scholar]
- 4.Morgante L, Morgante F, Moro E, et al. How many parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? results of a questionnaire. Parkinsonism Relat Disord. 2007;13(8):528–531. doi: 10.1016/j.parkreldis.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Pascual-Leone A, Valls-Solé J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. Akinesia in Parkinson’s disease, II: effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology. 1994;44(5):892–898. doi: 10.1212/wnl.44.5.892. [DOI] [PubMed] [Google Scholar]
- 6.Elahi B, Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function—systematic review of controlled clinical trials. Mov Disord. 2009;24(3):357–363. doi: 10.1002/mds.22364. [DOI] [PubMed] [Google Scholar]
- 7.Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for Parkinson’s disease: a systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry. 2005;76(12):1614–1623. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reithler J, Peters JC, Sack AT. Multimodal transcranial magnetic stimulation: using concurrent neuroimaging to reveal the neural network dynamics of noninvasive brain stimulation. Prog Neurobiol. 2011;94(2):149–165. doi: 10.1016/j.pneurobio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmich RC, Siebner HR, Bakker M, Münchau A, Bloem BR. Repetitive transcranial magnetic stimulation to improve mood and motor function in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):84–96. doi: 10.1016/j.jns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695–707. doi: 10.1016/j.parkreldis.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Lefaucheur JP, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125(11):2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. chap 8. Oxford, England: Cochrane Collaboration; 2011. [Accessed December 22, 2014]. Assessing risk of bias in included studies. http://www.cochrane.org/handbook. Updated March 2011. [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Publication Bias: Introduction to Meta-analysis. Chichester, England: John Wiley & Sons; 2009. [Google Scholar]
- 17.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 18.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 19.Franssen M, Winward C, Collett J, Wade D, Dawes H. Interventions for fatigue in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1675–1678. doi: 10.1002/mds.26030. [DOI] [PubMed] [Google Scholar]
- 20.Atkins D, Best D, Briss PA, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;4:CD008208. doi: 10.1002/14651858.CD008208.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6: rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Arias P, Vivas J, Grieve KL, Cudeiro J. Controlled trial on the effect of 10 days low-frequency repetitive transcranial magnetic stimulation (rTMS) on motor signs in Parkinson’s disease. Mov Disord. 2010;25(12):1830–1838. doi: 10.1002/mds.23055. [DOI] [PubMed] [Google Scholar]
- 24.Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur J Neurol. 2003;10(5):567–572. doi: 10.1046/j.1468-1331.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- 25.Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord. 2006;21(3):325–331. doi: 10.1002/mds.20713. [DOI] [PubMed] [Google Scholar]
- 26.Maruo T, Hosomi K, Shimokawa T, et al. High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in Parkinson’s disease. Brain Stimul. 2013;6(6):884–891. doi: 10.1016/j.brs.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Pal E, Nagy F, Aschermann Z, Balazs E, Kovacs N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: a randomized, double-blind, placebo-controlled study. Mov Disord. 2010;25(14):2311–2317. doi: 10.1002/mds.23270. [DOI] [PubMed] [Google Scholar]
- 28.Shimamoto H, Takasaki K, Shigemori M, Imaizumi T, Ayabe M, Shoji H. Therapeutic effect and mechanism of repetitive transcranial magnetic stimulation in Parkinson’s disease. J Neurol. 2001;248(suppl 3):III48–III52. doi: 10.1007/pl00007826. [DOI] [PubMed] [Google Scholar]
- 29.González-García N, Armony JL, Soto J, Trejo D, Alegría MA, Drucker-Colín R. Effects of rTMS on Parkinson’s disease: a longitudinal fMRI study. J Neurol. 2011;258(7):1268–1280. doi: 10.1007/s00415-011-5923-2. [DOI] [PubMed] [Google Scholar]
- 30.Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y Research Committee on rTMS Treatment of Parkinson’s Disease. Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology. 2013;80(15):1400–1405. doi: 10.1212/WNL.0b013e31828c2f66. [DOI] [PubMed] [Google Scholar]
- 31.del Olmo MF, Bello O, Cudeiro J. Transcranial magnetic stimulation over dorsolateral prefrontal cortex in Parkinson’s disease. Clin Neurophysiol. 2007;118(1):131–139. doi: 10.1016/j.clinph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Benninger DH, Iseki K, Kranick S, Luckenbaugh DA, Houdayer E, Hallett M. Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of Parkinson disease. Neurorehabil Neural Repair. 2012;26(9):1096–1105. doi: 10.1177/1545968312445636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov Disord. 2006;21(12):2201–2205. doi: 10.1002/mds.21089. [DOI] [PubMed] [Google Scholar]
- 34.Hamada M, Ugawa Y, Tsuji S Effectiveness of rTMS on Parkinson’s Disease Study Group, Japan. High-frequency rTMS over the supplementary motor area for treatment of Parkinson’s disease. Mov Disord. 2008;23(11):1524–1531. doi: 10.1002/mds.22168. [DOI] [PubMed] [Google Scholar]
- 35.Boylan LS, Pullman SL, Lisanby SH, Spicknall KE, Sackeim HA. Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol. 2001;112(2):259–264. doi: 10.1016/s1388-2457(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 36.Brusa L, Versace V, Koch G, et al. Low frequency rTMS of the SMA transiently ameliorates peak-dose LID in Parkinson’s disease. Clin Neurophysiol. 2006;117(9):1917–1921. doi: 10.1016/j.clinph.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Filipović SR, Rothwell JC, Bhatia K. Low-frequency repetitive transcranial magnetic stimulation and off-phase motor symptoms in Parkinson’s disease. J Neurol Sci. 2010;291(1–2):1–4. doi: 10.1016/j.jns.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Filipović SR, Rothwell JC, van de Warrenburg BP, Bhatia K. Repetitive transcranial magnetic stimulation for levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2009;24(2):246–253. doi: 10.1002/mds.22348. [DOI] [PubMed] [Google Scholar]
- 39.Lefaucheur JP, Drouot X, Von Raison F, Ménard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol. 2004;115(11):2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Nardone R, De Blasi P, Höller Y, et al. Repetitive transcranial magnetic stimulation transiently reduces punding in Parkinson’s disease: a preliminary study. J Neural Transm. 2014;121(3):267–274. doi: 10.1007/s00702-013-1100-3. [DOI] [PubMed] [Google Scholar]
- 41.Sedlácková S, Rektorová I, Srovnalová H, Rektor I. Effect of high frequency repetitive transcranial magnetic stimulation on reaction time, clinical features and cognitive functions in patients with Parkinson’s disease. J Neural Transm. 2009;116(9):1093–1101. doi: 10.1007/s00702-009-0259-0. [DOI] [PubMed] [Google Scholar]
- 42.Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B. Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson’s disease. J Neurol Sci. 2000;178(2):91–94. doi: 10.1016/s0022-510x(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 43.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67(1):64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 44.Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21(1):1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sejnowski TJ. Statistical constraints on synaptic plasticity. J Theor Biol. 1977;69(2):385–389. doi: 10.1016/0022-5193(77)90146-1. [DOI] [PubMed] [Google Scholar]
- 46.Siebner HR, Lang N, Rizzo V, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24(13):3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang DP, Min HK, Lee SY, et al. Functional neuroimaging of the 6-OHDA lesion rat model of Parkinson’s disease. Neurosci Lett. 2012;513(2):187–192. doi: 10.1016/j.neulet.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 48.Pinto S, Thobois S, Costes N, et al. Subthalamic nucleus stimulation and dysarthria in Parkinson’s disease: a PET study. Brain. 2004;127(pt 3):602–615. doi: 10.1093/brain/awh074. [DOI] [PubMed] [Google Scholar]
- 49.Nambu A, Tokuno H, Inase M, Takada M. Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neurosci Lett. 1997;239(1):13–16. doi: 10.1016/s0304-3940(97)00877-x. [DOI] [PubMed] [Google Scholar]
- 50.Hershey T, Revilla FJ, Wernle AR, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61(6):816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.