Abstract
Background
Limitations in mobility are common among older adults with cardiovascular and cardiometabolic disorders and have profound effects on health and well-being. With the growing population of older adults in the United States, effective and scalable public health approaches are needed to address this problem. Our goal was to determine the effects of a physical activity and weight loss intervention on 18-month change in mobility among overweight or obese older adults in poor cardiovascular health.
Methods
The study design was a translational, randomized controlled trial of physical activity (PA) and weight loss (WL) on mobility in overweight or obese older adults with cardiovascular disease (CVD) or at risk for CVD. The study was conducted within the community infrastructure of Cooperative Extension Centers. Participants were randomized to 1 of 3 interventions: PA, WL+PA, or a successful aging (SA) education control arm. The primary outcome was time to complete a 400-m walk in seconds (400MWT).
Results
A significant treatment effect (P=.002) and follow-up testing revealed that the WL+PA group improved their 400MWT (adjusted mean [SE], 323.3 [3.7] seconds) compared with both PA (336.3 [3.9] seconds; P=.02) and SA (341.3 [3.9] seconds; P<.001). Participants with poorer mobility at baseline benefited the most (P<.001).
Conclusion
Existing community infrastructures can be effective in delivering lifestyle interventions to enhance mobility in older adults in poor cardiovascular health with deficits in mobility; attention should be given to intervening on both weight and sedentary behavior since weight loss is critical to long-term improvement in mobility.
There is substantial burden among older adults from cardiovascular disease (CVD)1 and the cardiometabolic dys-function caused by obesity thatposesahighriskforCVD.2,3 The 10-year incidence rate of coronary heart disease in the Cardiovascular Health Study among older adults aged 65 to 74 years was 39.6 and 22.3 per 1000 person-years for men and women, respectively.4 In this same study, the rates for stroke were also high, and there was a 9% increase in congestive heart failure with each year of aging in this cohort; more than 40% of older adults ages 65 to 74 years have the metabolic syndrome.2 Diseases of the heart and circulatory system are second only to arthritis as a major cause of physical disability among people 60 years or older,5 and obesity compromises mobility in aging.6 With an expanding older adult population in the United States7 and the epidemic of obesity,6 effective and scalable public health measures are needed to confront this challenge.
One potential solution is to foster partnerships between experts in preventive medicine and a community organization known to be an unbiased, reliable resource with expertise in educational outreach and dissemination.8-10 Creating a partnership with the North Carolina Cooperative Extension (NCCE), we designed a randomized controlled clinical trial to evaluate the effects of weight loss (WL) and physical activity (PA) on improvement in mobility among physically limited older adults who were over-weight or obese and had either CVD or were at risk for CVD owing to cardiometabolic dysfunction.
We used the 400-m walk test (400MWT) as a measure of mobility because loss of the ability to walk 400 m predicts multiple adverse outcomes such as morbidity, worsening disability, CVD, institution-alization, and mortality.11-14 Clearly, the ability to walk without assistance is a critical factor in an older person's capacity to function independently in the community.15 Our primary aim was to compare the effects of 3 treatment arms on 18-month change in time (in seconds) to complete the 400MWT. The 3 treatments included a successful aging (SA) control arm, PA, and WL+PA. Our secondary aims included examining the effects of the treatments on WL, level of PA, and adverse events.
METHODS
OVERVIEW
The study recruited 288 participants ages 60 to 79 years from 3 counties (Forsyth, Davidson, and Guilford Counties) in North Carolina. After baseline assessments and randomization to SA, PA, or WL+PA, participants returned for assessments at 6, 12, and 18 months. Participants were treated in 8 waves, with all sessions being conducted indoors at the NCCE centers. Each wave within counties consisted ofapproximately 39 participants with approximately 13 in each treatment arm. A data safety and monitoring board routinely evaluated the execution of the study protocol and adverse events. Electronic copies of the intervention manuals are available on request from the corresponding author (W.J.R.).
All interventionists had degrees in the health sciences and were trained by the study investigators. A registered dietician provided oversight for the dietary intervention arm. The SA arm and portions of the WL+PA arm were delivered by Cooperative Extension agents—also known as Family and Consumer Sciences (FCS) educators—who are field faculty from North Carolina State University. These educators have degrees in Home Economics and/or Nutrition Education. Cooperative extension centers are located in all counties and on the Cherokee Reservation in North Carolina and in most counties nationwide. Cooperative extension specialists provide unbiased, research-based information to the public in such areas as agriculture, human nutrition, diet and health, food safety, gerontology, and human development.
ELIGIBILITY
The eligibility criteria identified ambulatory, overweight or obese, community-dwelling older adults who either had CVD or cardiometabolic dysfunction and evidence of self-reported limitations in mobility using the following inclusion criteria: (1) age 60 to 79 years; (2) having less than 60 minutes/week of moderate, structured PA; (3) having a body mass index (BMI), calculated as weight in kilograms divided by height in meters squared, greater than 28; (4) having evidence of a myocardial infarction, percutaneous transluminal angioplasty, chronic stable angina, or cardiovascular surgery in the past 6 months or an Adult Treatment Panel III diagnosis of the metabolic syndrome16; (5) having a self-reported mobility limitation; and (6) willingness to sign an informed consent and a Health Insurance Portability and Accountability Act authorization form.
Exclusions included (1) a baseline BMI of 40 or higher; (2) bipolar depression or schizophrenia; (3) unstable angina, symptomatic congestive heart failure, or exercise-induced complex ventricular arrhythmias; (4) resting blood pressure greater than 160/100 mm Hg; (5) diagnosis of systemic diseases that precluded participants from safely participating in the interventions; (6) a fasting blood glucose level higher than 140 mg/dL (7.77 mmol/L), type 1 diabetes mellitus (DM), or type 2 DM with insulin therapy; (7) active treatment for cancer; (8) clinically significant visual or hearing impairment; (9) dementia, delirium, or impaired cognitive function; (10) participation in another medical intervention study; (11) having more than 21 alcoholic drinks per week; (12) inability to walk unassisted; and (13) inability to speak or read English.
RECRUITMENT, ENROLLMENT, AND RANDOMIZATION
Recruitment occurred over 2.5 years. The first person was enrolled on January 17, 2005, and the last person was closed out on April 6, 2010. Recruitment strategies included newspaper advertisements and direct mailings. Each participant was randomized to treatment using a permuted block randomization scheme with stratification by wave.
MEASURES
Demographics, medical history, and comorbidities were collected by self-report. Height without shoes was measured to the nearest 0.1 cm using a stadiometer and weight to the nearest 0.1 kg using a calibrated electronic scale.
Mobility
The 400MWT was used to assess mobility.17 In a study of middle-to older-aged women, the 400MWT had excellent stability (intraclass correlation [ICC], 0.95) and was significantly related to measured V̇o2peak (the highest oxygen value achieved during an exercise test to exhaustion), PA, body composition, and balance.18 Moreover, prospective data analyses in healthy older adults have shown that performance on the 400MWT is predictive of mortality, incident cardiovascular disease, and disability.14
Physical Activity
The Lifecorder-EX accelerometer (New-Lifestyles Inc, Lees Summit, Missouri) was used to assess PA.19,20 Intensity levels 3 to 9 were classified as moderate to vigorous (M-V); this is consistent with the metabolic demands of activity for this age group.20 At baseline and at the 6-month and the 18-month follow-up assessments, all participants were asked to wear the accelerometer for 7 days. More than 97% of the activity for M-V was of a moderate intensity, and the data were processed consistent with established protocols.19,20
PA INTERVENTION
The PA intervention (48 total sessions) was based on an evidence-based program for older adults that is conceptually driven by principles from Bandura's social cognitive theory and the group dynamics literature.21 A primary goal was to gradually increase or shape PA in a home-based environment to more than 30 minutes of moderately intense activity on most days of the week for a total of more than 150 minutes/wk. Participants walked at a moderate intensity of “somewhat hard—13” as assessed by the Borg Rating of Perceived Exertion scale.22 Weekly trackers (written self-monitoring logs) were used to document walking behavior.
The PA intervention involved 2 phases: intensive and maintenance. The 6-month intensive phase involved counseling sessions in a mix of 3 group sessions and 1 individual session per month. Group sessions lasted 90 minutes, and individual sessions lasted 30 minutes. Each group session started with a 30- to 45-minute period of walking followed by an interactive, group-mediated, behavioral-focused session.
During the first 2 months of the intensive phase, participants were asked to identify their primary motivations for becoming more active, and group leaders emphasized the risk of disability with aging. In addition, participants were introduced to the concepts of goal setting and self-monitoring, documenting minutes of walking in activity logs, and learning how to adjust goals when warranted.
During months 3 and 4, discussions focused on creating a PA program that had the flexibility to accommodate the multiple barriers that inevitably occur. During this period, strategies focused on the development of self-regulatory skills and a network of social support.
During months 5 and 6, discussions focused on the concept of participants perceiving themselves as physically active, independent older adults. They were taught how to use environmental cues to facilitate activity goals and how to avoid or deal with relapses when they occurred.
Months 7 to 18 formed the maintenance phase with a reduction in the frequency of contact to 2 times per month. One contact was a group session and the second was a telephone contact that lasted approximately 10 to 20 minutes. Discussion mirrored the check-in during the intensive phase in that PA goals were discussed, specific plans of action were implemented, and self-regulatory skills were reinforced.
WL AND PA INTERVENTION
The combined treatment arm (48 total sessions) involved the PA program in conjunction with dietary WL, using the same conceptual model as PA.21 The WL goal was to reduce caloric intake to produce a WL ofapproximately 0.3 kg per week for the first 6 months for a total loss in mass of 7% to 10%. During maintenance, participants were encouraged to continue WL as long as their BMI was 20 or greater; however, the primary focus was on weight maintenance. At program inception, participants were assigned a daily energy intake goal based on their baseline weight. A 1200- to 1500-kcal goal was used for those weighing less than 250 lbs (113.4 kg), and a 1500- to 1800-kcal goal for those weighing 250 lbs or more. Recommendations for choices of foods were based on the MyPyramid Food Guidance System.23 Participants were given food tracking booklets and, at the end of each week, an average was calculated for their calorie and fat consumption. The intervention was codelivered by a trained interventionist and FCS agents in the NCCE centers.
As with PA, the intensive phase lasted 90 minutes. The first segment reviewed participants’ progress from the previous session. After a private weigh-in, participants provided a confidential progress update and identified problems encountered. Progress was highlighted with strong positive feedback. Reported difficulties were dealt with through group support and advice. The second segment involved a group-mediated session that focused on skill training related to cognitive behavioral self-management skills, nutritional training, and topics in exercise science. Each month there was a cooking demonstration or food tasting that illustrated the preparation of palatable, low-fat, low-calorie foods. The final component of each session consisted of setting individual goals for the coming week.
The interventionists placed a major focus on increasing participants’ abilities to self-regulate eating behavior. This involved (1) promoting awareness of how different internal and external factors promote eating,24 (2) having participants track and discuss personal triggers to eating, (3) teaching participants to stop and think before eating, (4) using a 5-stage problem-solving model to develop specific action plans for difficult situations,25 (5) nor-malizing slips and relapses, and (6) using the individual sessions to provide feedback and reinforcement to participants. As in the PA arm, maintenance involved 2 contacts each month: 1 group contact and 1 telephone counseling call.
SA EDUCATION INTERVENTION
The SA treatment was developed by faculty at North Carolina State University, Raleigh, who serve as extension specialists for NCCE. Each scripted lesson was taught by an FCS agent in each county and was tailored for older adults. The purpose and structure of the SA group was to (1) control for general levels of staff and participant interactions, (2) optimize recruitment and to ensure participants’ ongoing cooperation and retention, (3) select a control intervention that would have minimal effects on the primary outcome, and (4) use an intervention that had tangible benefit. Participants in the SA arm met weekly for the first 2 months, monthly through the sixth month, and then bimonthly until the end of the study—a total of 18 sessions.
In the SA treatment, participants were taught how to actively “take charge” of their health. Examples of topics covered included the following: how the body changes with aging, preventing or delaying disease, eating for good health, positive attitudes toward aging, family relationships and care giving, and talking to health care providers. The SA intervention differed from the other 2 arms of the study in that participants did not receive a progressive, supervised program of PA or diet for WL; however, both PA and nutrition for aging were addressed as separate and distinct topics.
STATISTICAL ANALYSES
Using a 2 df test for our primary outcome, we calculated that 300 participants would provide 94% power to detect a difference in the 400MWT of 13 seconds between SA and PA and 26 seconds between SA and WL+PA. This allowed for a 25% dropout rate and the ICC due to the group-based intervention. Descriptive statistics were used to describe the sample. The primary analysis used a linear mixed model with covariates, including the baseline 400MWT, county, wave within county, visit (at 6, 12, and 18 months), and sex. “Participant” was included as a random effect to account for the within-participant correlation. Adjusted means were used to account for the variables in the primary model. Fisher exact test was used to compare adverse event rates by treatment group. The potential impact of missing data on our conclusions was examined by comparing the proportion of missing primary outcome data between treatments using a generalized linear mixed model similar to the primary analysis model and by use of multiple imputation. Analyses were conducted using SAS statistical software (version 9.2; SAS Institute, Cary, North Carolina).
RESULTS
INCLUSION, RETENTION/ADHERENCE, AND DESCRIPTIVE CHARACTERISTICS
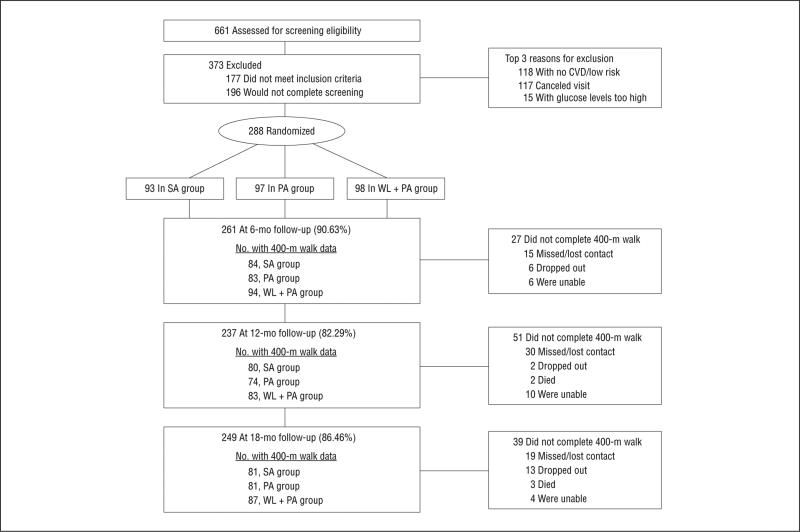
The Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 1) shows that 86.5% of the participants completed the 18-month follow-up; using a mixed-model in SAS we were able to conduct analyses for the primary outcome on 93.4% of those randomized. Participants in SA attended a mean (SD) of 70.9% (26.5%) of the scheduled sessions, whereas for PA it was 79.8% (24.6%), and for WL+PA it was 88.2% (25.2%). Women made up 67.0% of this cohort, and 81.9% were white. As shown in Table 1, the participants were socioeconomically diverse, had multiple comorbidities, and all were overweight or obese.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram (for “Did not complete 400-m walk,” the participants had dropped out, died, missed follow-up or contact with them was lost [“missed/lost contact”], or they were unable to attend the screening visit or could not be located).
Table 1.
Characteristics of Participants
| Characteristic | Treatment Group, No. (%) |
||
|---|---|---|---|
| SA (n=93) | PA (n=97) | WL + PA (n=98) | |
| Age, mean (SD), y | 67.2 (4.8) | 67.2 (5.1) | 66.8 (4.6) |
| Sex | |||
| Male | 31 (33.3) | 33 (34.0) | 31 (31.6) |
| Female | 62 (66.7) | 64 (66.0) | 67 (68.4) |
| Race | |||
| White | 76 (81.7) | 76 (78.4) | 84 (85.7) |
| Black | 15 (16.1) | 19 (19.6) | 14 (14.3) |
| Other | 2 (2.2) | 2 (2.1) | 0 |
| Education | |||
| < HS | 3 (3.2) | 2 (2.1) | 1 (1.0) |
| HS/HS and some college | 43 (46.2) | 47 (48.5) | 47 (48.0) |
| ≥ Associate's degree | 47 (50.5) | 48 (49.5) | 50 (51.0) |
| BMI, mean (SD) | 32.6 (3.5) | 32.8 (3.9) | 33.1 (4.1) |
| Comorbidities | |||
| Myocardial infarction | 10 (10.8) | 4 (4.1) | 7 (7.1) |
| Angina | 11 (11.8) | 17 (17.5) | 13 (13.3) |
| Hypertension | 60 (64.5) | 65 (67.0) | 73 (74.5) |
| Diabetes mellitus | 18 (19.4) | 15 (15.5) | 16 (16.3) |
| Arthritis | 61 (65.6) | 49 (50.5) | 61 (62.2) |
| Cancer | 17 (18.3) | 21 (21.6) | 19 (19.4) |
| Metabolic syndrome | 49 (52.7) | 54 (55.7) | 58 (59.2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HS, high school; PA, physical activity; SA, successful aging education control; WL + PA, weight loss + physical activity.
400-m WALK TIME
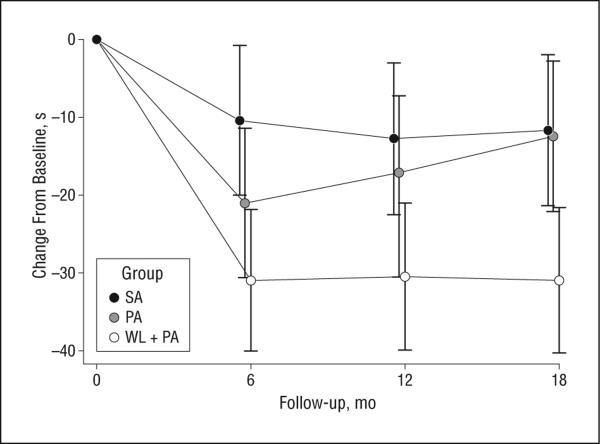
Table 2 and Figure 2 provide descriptive data for the primary outcome by treatment group. A statistically significant overall treatment effect (P=.002) was observed, and follow-up testing revealed that the WL+PA group improved their 400MWT performance over time compared with both SA (P<.001) and PA (P=.02). Baseline 400MWT was a strong predictor of follow-up change in 400MWT such that those who were the most compromised at baseline experienced the largest improvement. Although the PA group improved their performance at 6 months in comparison to SA (Figure 2), this benefit disappeared by 18 months. The mean adjusted mean difference (95% confidence intervals [CIs]) for the SA vs WL+PA comparison was 18.0 seconds (95% CI, 7.5-28.5), whereas for the PA vs WL+PA comparison it was 13.0 seconds (95% CI, 2.5-23.5). Clearly, WL+PA resulted in improved walking performance compared with either SA or PA. There was no evidence of differential missingness of the primary outcome (P=.13). The multiple imputation analyses confirmed the complete case analyses.
Table 2.
400-m Walk Time: Raw Data for Each Time Point and Average Adjusted Values From the Linear Modela
| Time of Assessment, mo | Treatment Group |
||
|---|---|---|---|
| SA | PA | WL + PA | |
| Baseline | 352.5 (72.6) | 352.5 (67.6) | 351.1 (84.0) |
| 6-mo | 339.4 (59.3) | 327.5 (57.0) | 320.9 (57.7) |
| 12-mo | 337.1 (56.8) | 327.2 (70.2) | 321.4 (56.6) |
| 18-mo | 338.9 (62.1) | 336.5 (75.4) | 320.8 (65.3) |
| Average adjusted | 341.3 (3.9) | 336.3 (3.9) | 323.3 (3.7) |
| 95% CI for adjusted, mean | (333.7-348.9) | (328.6-343.9) | (316.0-330.6) |
Abbreviations: CI, confidence interval; PA, physical activity; SA, successful aging education control; WL + PA, weight loss + physical activity.
Data are given as mean (SD) 400-m walk time results in seconds.
Figure 2.
Adjusted means of the change from baseline in 400-m walk time and 95% confidence intervals by treatment condition (SA, successful aging; PA, physical activity; and WL+PA, weight loss+physical activity).
WL, LEVELS OF PA, AND ADVERSE EVENTS
Participants in SA and PA experienced very small decreases in weight, approximately 1.0% after randomization, whereas the WL+PA group had lost 8.5% at 6 months and essentially retained this level at 18 months (7.7%) (P<.001) (see Table 3 for means and 95% CIs for adjusted means).
Table 3.
Body Mass: Raw Data at Each Time Point and Mean Adjusted Values From the Linear Modela
| Treatment Group |
|||
|---|---|---|---|
| Time of Assessment, mo | SA | PA | WL + PA |
| Baseline | 91.2 (15.1) | 91.7 (13.1) | 92.8 (16.1) |
| 6 | 90.4 (15.4) | 91.2 (13.7) | 84.9 (15.2) |
| 18 | 90.3 (16.0) | 90.9 (14.4) | 85.7 (15.5) |
| Average adjusted | 91.9 (0.5) | 90.8 (0.5) | 84.5 (0.5) |
| 95% CI for adjusted mean | (89.8-91.9) | (89.7-91.8) | (83.5-85.5) |
Abbreviations: CI, confidence interval; PA, physical activity; SA, successful aging education control; WL + PA, weight loss + physical activity.
Data are given as mean (SD) body mass in kilograms.
There were significant group differences (P<.01) for minutes of M-V PA (see Table 4 for means and 95% CIs for adjusted means), with those in the PA and WL+PA group increasing their minutes of M-V over time compared with those in the SA group (P<.02). In addition, adverse events are presented in Table 5. There were 28 people with adverse events in the WL+PA group, 16 in the PA group, and 14 in the SA group (P=.04). The pattern was similar for serious adverse events (17 in the PA+WL group, 13 in the PA group, and 9 in the SA group), although the differences were not statistically significant (P=.33). It is important to note that most adverse events in the WL+PA group and PA group involved musculoskeletal complaints that were transient, whereas only 2 of the serious adverse events were definitely related to treatment.
Table 4.
Moderate to Vigorous (M-V) Physical Activity (PA): Raw Data at Each Time Point and Average Adjusted Values From the Linear Modela
| Outcome and Time of Assessment | M-V Physical Activity per Treatment Group, Mean (SD) |
||
|---|---|---|---|
| SA | PA | WL + PA | |
| Minimum of M-V, mo | |||
| Baseline | 107.6 (69.2) | 115.8 (84.1) | 122.5 (77.4) |
| 6 | 117.8 (114.5) | 189.8 (121.8) | 227.8 (144.9) |
| 18 | 118.1 (111.2) | 140.7 (128.8) | 165.9 (102.1) |
| Average adjusted | 120.4 (10.7) | 156.4 (10.8) | 189.4(10.0) |
| 95% CI for adjusted mean | (99.3-141.5) | (138.0-180.7) | (169.6-209.3) |
Abbreviations: CI, confidence interval; SA, successful aging education control; WL + PA, weight loss + physical activity.
Data are given as mean (SD) minutes of M-V PA per week except where noted.
Table 5.
Adverse Events and Serious Adverse Events (SAEs) by Treatment Groupa
| Parameter | Treatment Group |
P Value | ||
|---|---|---|---|---|
| SA | PA | WL + PA | ||
| Adverse events | 14 (18) | 16 (34) | 28 (35) | .04 |
| SAEs | 9 (10) | 13 (19) | 17 (21) | .33 |
| SAEs definitely related to Tx | 0 | 0 | 2 (2) | .33 |
| SAEs possibly related to Tx | 0 | 2 (3) | 4 (4) | .17 |
| Systems | ||||
| Circulatory | 3 (4) | 3 (5) | 3 (3) | >.99 |
| Genitourinary | 1 (1) | 2 (2) | 3 (3) | .87 |
| Musculoskeletal | 7 (7) | 12 (18) | 16 (17) | .19 |
| Nervous | 1 (1) | 3 (3) | 2 (2) | .79 |
| Respiratory | 0 | 0 | 2 (2) | .33 |
| Digestive | 3 (3) | 0 | 4 (4) | .13 |
| Skin | 0 | 1 (1) | 0 | .66 |
| Unknown | 0 | 1 (1) | 0 | .66 |
| Other | 0 | 4 (5) | 7 (8) | .02 |
Abbreviations: PA, physical activity; SA, successful aging control group; WL + PA, weight loss + physical activity; Tx, treatment.
Data are given as number of patients (number of events).
COMMENT
There were 2 major findings in this study. First, the results illustrate that to improve mobility in older, functionally compromised, obese adults with either CVD or cardiometabolic dysfunction, PA must be coupled with WL. And second, the WL+PA intervention was successfully translated into a community setting with results for WL and increased PA comparable with those observed in the best randomized controlled trials conducted in academic health centers.26,27 Although PA was effective at improving 400MWT performance at 6 months compared with SA, this benefit disappeared at 18 months. We surmise that the benefit of WL+PA over PA alone was due either to the increased motivation to be physically active when one has lost weight, and/or that being physically active is perceptually or objectively less demanding once one has lost weight.
As evident from the 95% CI of the 400MWT data, the magnitude of the treatment difference between WL+PA and SA was substantial. Of course, readers may question the clinical meaningfulness of this difference, given that Kwon et al,28 using data from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study, proposed that a 20-second change in 400MWT represents the lower end of the range for clinical significance. There are 2 important points to consider. First, LIFE-P29 targeted older adults (>70 years) who had poor mobility. As observed in the current study, change in function is affected dramatically by baseline performance such that those who perform worse at baseline experience greater improvement. Thus, a clinically significant change in 400MWT for more functionally able older adults has yet to be determined but will clearly fall below what Kwon et al28 observed. Second, it is likely that the clinical significance of a change in 400MWT depends on the outcome of interest. Interestingly, the 12-month outcome data for the 400MWT in LIFE-P yielded a change in gait speed of 0.003 m/s for the PA group (from 0.854 m/s to 0.857 m/s), which equates to an improvement of 1.64 seconds, whereas those in the SA group experienced a decline in gait speed of 0.031 m/s (from 0.854 m/s to 0.823 m/s)—17.56 seconds worse. Moreover, the functional changes observed in LIFE-P and power estimates based of failure to complete the 400MWT led to the largest PA trial of older adults ever funded by the National Institute on Aging. Thus, it would be premature to dismiss an 18-second treatment change for the 400MWT in the current study as clinically irrelevant, particularly recognizing that this value was obtained while adhering to the principle of intent-to-treat. Further research on this question is warranted.
Compared with the SA group, both the PA and WL+PA treatment groups experienced statistically significant increases in PA, whereas the WL+PA group lost considerable weight—7.7% at 18 months—compared with either the SA or PA groups. In addition, there was little evidence that the interventions posed a safety risk for participants. It is now well known that older adults with various existing comorbidities respond favorably to PA interventions.29-32 Although the number of trials focusing on the management of obesity in older adults has been limited,6 the Obesity Society has underscored the need for research in this area.33 The current study is unique given the target population investigated, the interventions, and the fact that the research was conducted within a translational context partnering with the NCCE and faculty at North Carolina State University who serve as extension specialists for this organization.
In summary, this investigation revealed that a community-based WL+PA intervention can have a favorable effect on preserving the mobility of older, obese adults who are at risk for or have CVD. The magnitude of change that we observed in both the WL and PA groups was comparable with data from highly successful, center-based intervention research.6,26,27 Future studies are needed to expand this line of investigation to other community jurisdictions to examine the generalizability of these findings.
Acknowledgments
Funding/Support: Support for this study was provided by National Heart, Lung, and Blood Institute grant HL076441-01A1, National Institutes for Aging grant P30 AG021332, and General Clinical Research Center grant, M01-RR007122.
Role of the Sponsors: The National Institutes of Health had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Rejeski had full access to all of the data, and Dr Ambrosius takes responsibility for the integrity and accuracy of all data analyses. Study concept and design: Rejeski, Bearon, McClelland, Perri, and Ambrosius. Acquisition of data: Rejeski, Brubaker, Goff, and Ambrosius. Analysis and interpretation of data: Rejeski, Brubaker, Goff, Bearon, McClelland, and Ambrosius. Drafting of the manuscript: Rejeski and Ambrosius. Critical revision of the manuscript for important intellectual content: Rejeski, Brubaker, Goff, Bearon, McClelland, Perri, and Ambrosius. Statistical analysis: Ambrosius. Obtained funding: Rejeski, Goff, Bearon, McClelland, Perri, and Ambrosius. Administrative, technical, and material support: Rejeski, Brubaker, and Ambrosius. Study supervision: Rejeski, Brubaker, Perri, and Ambrosius.
Financial Disclosure: None reported.
Additional Contributions: John M. Jakicic, PhD, David Kelley, MD, and Mark R. Conaway, PhD, served on the study's Data and Safety Monitoring Board. We are also indebted to Lawton S. Cooper, MD, from the National Heart Lung and Blood Institute. We thank the participants, the NCCE, and the assessment and intervention staff: Geissler Baker, MEd; Christie Fain, BS; Martha Isenberg, MS; Jill Gaukstern, MS; Jeannie Leonard, BS; and Beverly Nesbitt, MA.
Trial Registration: clinicaltrials.gov Identifier: NCT00119795
REFERENCES
- 1.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25(4):563–577, vii. doi: 10.1016/j.cger.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeill AM, Katz R, Girman CJ, et al. Metabolic syndrome and cardiovascular disease in older people: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54(9):1317–1324. doi: 10.1111/j.1532-5415.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 3.Denys K, Cankurtaran M, Janssens W, Petrovic M. Metabolic syndrome in the elderly: an overview of the evidence. Acta Clin Belg. 2009;64(1):23–34. doi: 10.1179/acb.2009.006. [DOI] [PubMed] [Google Scholar]
- 4.Arnold AM, Psaty BM, Kuller LH, et al. Incidence of cardiovascular disease in older Americans: the cardiovascular health study. J Am Geriatr Soc. 2005;53(2):211–218. doi: 10.1111/j.1532-5415.2005.53105.x. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky JL, Jette AM, Branch LG, Kannel WB, Feinleib M. The Framingham Disability Study: relationship of various coronary heart disease manifestations to disability in older persons living in the community. Am J Public Health. 1990;80(11):1363–1367. doi: 10.2105/ajph.80.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11(9):671–685. doi: 10.1111/j.1467-789X.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manton KG, Vaupel JW. Survival after the age of 80 in the United States, Sweden, France, England, and Japan. N Engl J Med. 1995;333(18):1232–1235. doi: 10.1056/NEJM199511023331824. [DOI] [PubMed] [Google Scholar]
- 8.Franklin BA, Hall L, Timmis GC. Contemporary cardiac rehabilitation services. Am J Cardiol. 1997;79(8):1075–1077. doi: 10.1016/s0002-9149(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 9.Gordon NF, English CD, Contractor AS, et al. Effectiveness of three models for comprehensive cardiovascular disease risk reduction. Am J Cardiol. 2002;89(11):1263–1268. doi: 10.1016/s0002-9149(02)02323-8. [DOI] [PubMed] [Google Scholar]
- 10.Eves ND, Plotnikoff RC. Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes Care. 2006;29(8):1933–1941. doi: 10.2337/dc05-1981. [DOI] [PubMed] [Google Scholar]
- 11.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272(13):1036–1042. [PubMed] [Google Scholar]
- 12.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48(5):493–498. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 13.Lampinen P, Heikkinen E. Reduced mobility and physical activity as predictors of depressive symptoms among community-dwelling older adults: an eight-year follow-up study. Aging Clin Exp Res. 2003;15(3):205–211. doi: 10.1007/BF03324501. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life, I: demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137(8):845–857. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 16.Cleeman JI, Grundy SM, Becker D, et al. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 18.Pettee Gabriel KK, Rankin RL, Lee C, Charlton ME, Swan PD, Ainsworth BE. Test-retest reliability and validity of the 400-meter walk test in healthy, middle-aged women. J Phys Act Health. 2010;7(5):649–657. doi: 10.1123/jpah.7.5.649. [DOI] [PubMed] [Google Scholar]
- 19.Ayabe M, Brubaker PH, Mori Y, et al. Self-monitoring moderate-vigorous physical activity versus steps/day is more effective in chronic disease exercise programs. J Cardiopulm Rehabil Prev. 2010;30(2):111–115. doi: 10.1097/HCR.0b013e3181be7c80. [DOI] [PubMed] [Google Scholar]
- 20.Ayabe M, Brubaker PH, Dobrosielski D, et al. The physical activity patterns of cardiac rehabilitation program participants. J Cardiopulm Rehabil. 2004;24(2):80–86. doi: 10.1097/00008483-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Rejeski WJ, Brawley LR, Ambrosius WT, et al. Older adults with chronic disease: benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003;22(4):414–423. doi: 10.1037/0278-6133.22.4.414. [DOI] [PubMed] [Google Scholar]
- 22.Borg G. Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL: 1998. [Google Scholar]
- 23. [October 13, 2010];US Department of Agriculture Web site. http://www.mypyramid.gov.
- 24.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991;59(5):739–744. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 25.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69(4):722–726. [PubMed] [Google Scholar]
- 26.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13(6):538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pahor M, Blair SN, Espeland M, et al. LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 30.Ettinger WH, Jr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis: the Fitness Arthritis and Seniors Trial (FAST). JAMA. 1997;277(1):25–31. [PubMed] [Google Scholar]
- 31.Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23(1):60–68. doi: 10.1097/00008483-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in over-weight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 33.Villareal DT, Apovian CM, Kushner RF, Klein S. American Society for Nutrition; NAASO, the Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, the Obesity Society. Obes Res. 2005;13(11):1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]