Abstract
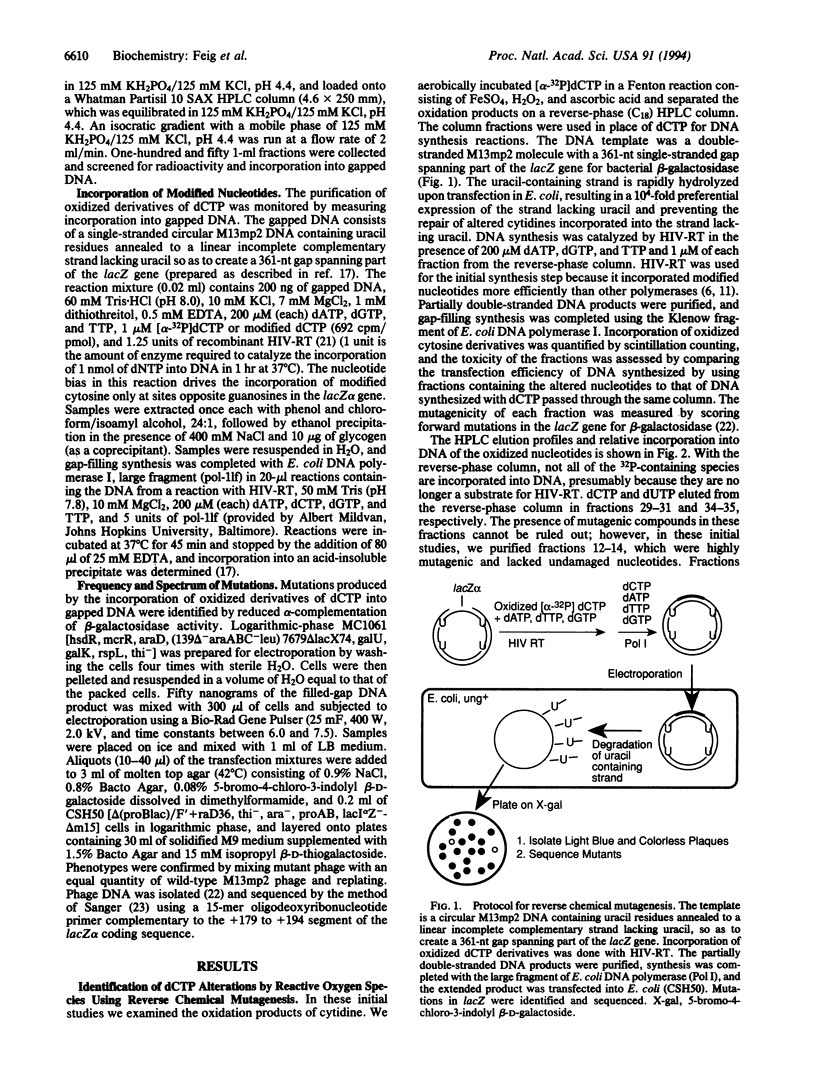
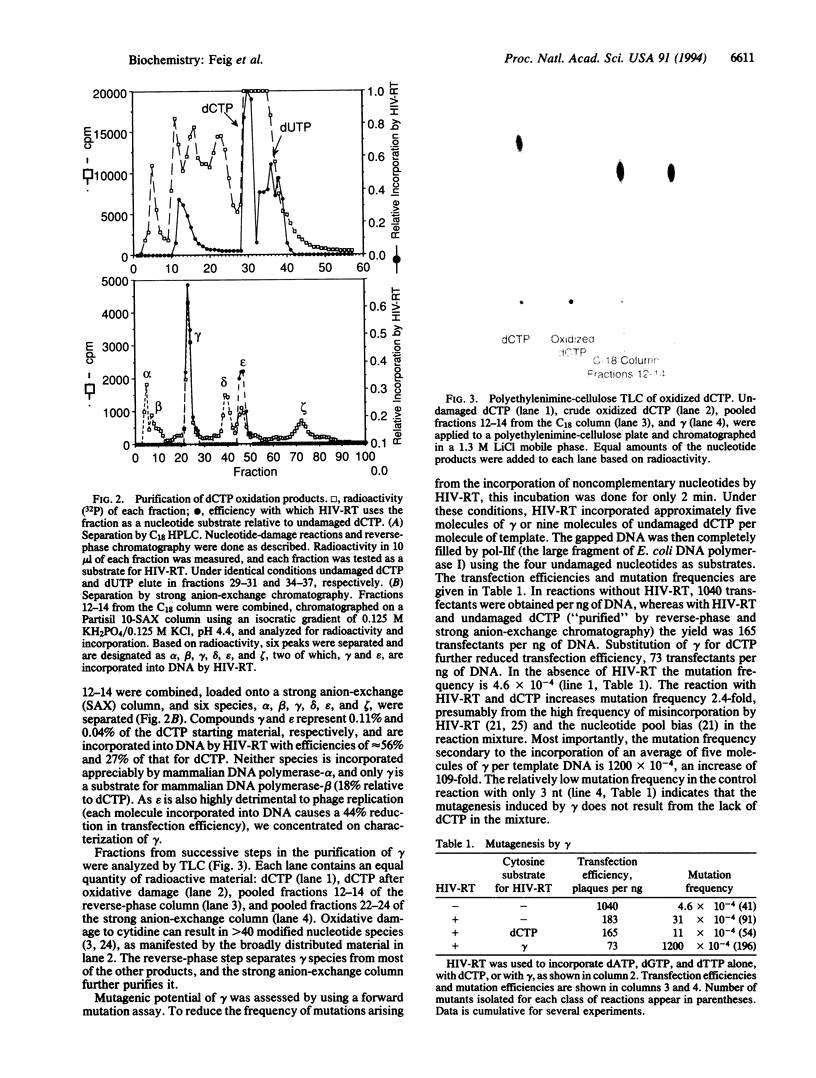
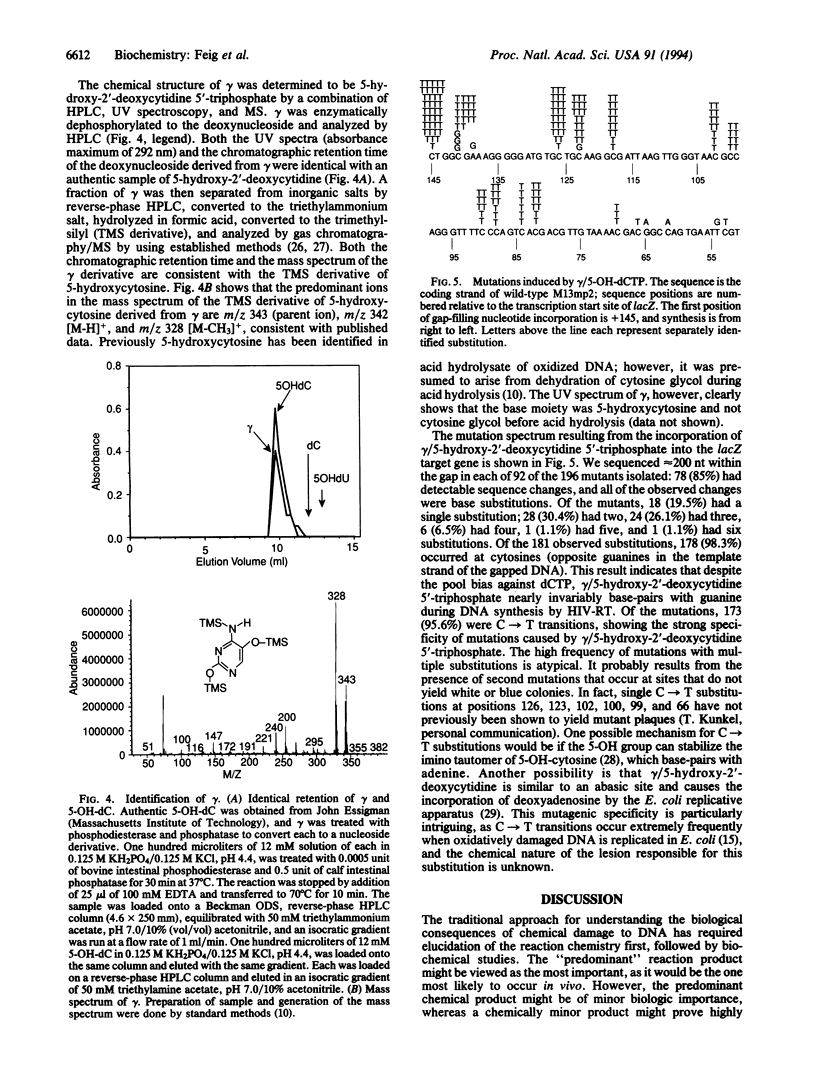
An understanding of the contribution of reactive oxygen species to mutagenesis has been hampered by the vast number of different chemical modifications they cause in DNA. Even though many of these DNA alterations have been catalogued, the identification of specific lesions that cause mutations has depended on testing one modification at a time. In this study we present another approach to identify key mutagenic lesions from a pool of oxidatively modified nucleotides. dCTP was treated with an oxygen radical-generating system containing FeSO4, H2O2, and ascorbic acid. The modification products were separated by reverse-phase and anion-exchange HPLC and then incorporated by human immunodeficiency virus reverse transcriptase into a DNA that contains a target gene for scoring for mutations. One of the mutagenic species isolated was identified as 5-hydroxy-2'-deoxycytidine. It is incorporated efficiently into DNA and causes C-->T transitions in Escherichia coli at a frequency of 2.5%, which is more mutagenic than any previously identified oxidative DNA lesion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. K., Christensen R. B., Lawrence C. W., LeClerc J. E. Frequency and spectrum of mutations produced by a single cis-syn thymine-thymine cyclobutane dimer in a single-stranded vector. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8141–8145. doi: 10.1073/pnas.85.21.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J., Berger M. Radiation-induced decomposition of the purine bases within DNA and related model compounds. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):127–143. doi: 10.1080/09553008514550201. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Cheng K. C., Preston B. D., Cahill D. S., Dosanjh M. K., Singer B., Loeb L. A. The vinyl chloride DNA derivative N2,3-ethenoguanine produces G----A transitions in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9974–9978. doi: 10.1073/pnas.88.22.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M. The use of capillary gas chromatography-mass spectrometry for identification of radiation-induced DNA base damage and DNA base-amino acid cross-links. J Chromatogr. 1984 Jul 6;295(1):103–121. doi: 10.1016/s0021-9673(01)87602-0. [DOI] [PubMed] [Google Scholar]
- Feig D. I., Loeb L. A. Mechanisms of mutation by oxidative DNA damage: reduced fidelity of mammalian DNA polymerase beta. Biochemistry. 1993 Apr 27;32(16):4466–4473. doi: 10.1021/bi00067a040. [DOI] [PubMed] [Google Scholar]
- Feig D. I., Loeb L. A. Oxygen radical induced mutagenesis is DNA polymerase specific. J Mol Biol. 1994 Jan 7;235(1):33–41. doi: 10.1016/s0022-2836(05)80009-9. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel K., Chrzan K., Troll W., Teebor G. W., Steinberg J. J. Radiation-like modification of bases in DNA exposed to tumor promoter-activated polymorphonuclear leukocytes. Cancer Res. 1986 Nov;46(11):5533–5540. [PubMed] [Google Scholar]
- Gajewski E., Rao G., Nackerdien Z., Dizdaroglu M. Modification of DNA bases in mammalian chromatin by radiation-generated free radicals. Biochemistry. 1990 Aug 28;29(34):7876–7882. doi: 10.1021/bi00486a014. [DOI] [PubMed] [Google Scholar]
- Hayes R. C., Petrullo L. A., Huang H. M., Wallace S. S., LeClerc J. E. Oxidative damage in DNA. Lack of mutagenicity by thymine glycol lesions. J Mol Biol. 1988 May 20;201(2):239–246. doi: 10.1016/0022-2836(88)90135-0. [DOI] [PubMed] [Google Scholar]
- Huber H. E., McCoy J. M., Seehra J. S., Richardson C. C. Human immunodeficiency virus 1 reverse transcriptase. Template binding, processivity, strand displacement synthesis, and template switching. J Biol Chem. 1989 Mar 15;264(8):4669–4678. [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Preston B. D. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride T. J., Preston B. D., Loeb L. A. Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry. 1991 Jan 8;30(1):207–213. doi: 10.1021/bi00215a030. [DOI] [PubMed] [Google Scholar]
- Moriya M., Takeshita M., Johnson F., Peden K., Will S., Grollman A. P. Targeted mutations induced by a single acetylaminofluorene DNA adduct in mammalian cells and bacteria. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1586–1589. doi: 10.1073/pnas.85.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino F. W., Mekosh H. L. Incorporation of cytosine arabinoside monophosphate into DNA at internucleotide linkages by human DNA polymerase alpha. J Biol Chem. 1992 Nov 15;267(32):23043–23051. [PubMed] [Google Scholar]
- Polverelli M., Teoule R. Gamma irradiation of cytosine in an aerated aqueous solution. I. Identification of radiolysis products of cytosine resulting from the deamination pathway. Z Naturforsch C. 1974 Jan-Feb;29(1):12–15. [PubMed] [Google Scholar]
- Preston B. D., Poiesz B. J., Loeb L. A. Fidelity of HIV-1 reverse transcriptase. Science. 1988 Nov 25;242(4882):1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8501–8505. doi: 10.1073/pnas.83.22.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall S. K., Eritja R., Kaplan B. E., Petruska J., Goodman M. F. Nucleotide insertion kinetics opposite abasic lesions in DNA. J Biol Chem. 1987 May 15;262(14):6864–6870. [PubMed] [Google Scholar]
- Rhaese H. J. Chemical analysis of DNA alterations. 3. Isolation and characterization of adenine oxidation products obtained from oligo- and monodeoxyadenylic acids treated with hydroxyl radicals. Biochim Biophys Acta. 1968 Sep 24;166(2):311–326. [PubMed] [Google Scholar]
- Roberts J. D., Bebenek K., Kunkel T. A. The accuracy of reverse transcriptase from HIV-1. Science. 1988 Nov 25;242(4882):1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Singer B. N-nitroso alkylating agents: formation and persistence of alkyl derivatives in mammalian nucleic acids as contributing factors in carcinogenesis. J Natl Cancer Inst. 1979 Jun;62(6):1329–1339. [PubMed] [Google Scholar]
- Tkeshelashvili L. K., McBride T., Spence K., Loeb L. A. Mutation spectrum of copper-induced DNA damage. J Biol Chem. 1991 Apr 5;266(10):6401–6406. [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- Wagner J. R., Hu C. C., Ames B. N. Endogenous oxidative damage of deoxycytidine in DNA. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3380–3384. doi: 10.1073/pnas.89.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]




