Abstract
Substantial evidence suggests that catechol estrogen-3,4-quinones react with DNA to form predominantly the depurinating adducts 4-hydroxyestrone (estradiol)-1-N3Ade [4-OHE1(E2)-1-N3Ade] and 4-OHE1(E2)-1-N7Gua. Apurinic sites resulting from these adducts generate critical mutations that can initiate cancer. The paradigm of cancer initiation is based on an imbalance in estrogen metabolism between activating pathways that lead to estrogen–DNA adducts and deactivating pathways that lead to estrogen metabolites and conjugates. This imbalance can be improved to minimize formation of adducts by using antioxidants, such as resveratrol (Resv) and N-acetylcysteine (NAcCys). To compare the ability of Resv and NAcCys to block formation of estrogen–DNA adducts, we used the human breast epithelial cell line MCF-10F treated with 4-OHE2. Resv and NAcCys directed the metabolism of 4-OHE2 toward protective pathways. NAcCys reacted with the quinones and reduced the semiquinones to catechols. This pathway was also carried out by Resv. In addition, Resv induced the protective enzyme quinone reductase, which reduces E1(E2)-3,4-quinones to 4-OHE1(E2). Resv was more effective at increasing the amount of 4-OCH3E1(E2) than NAcCys. Inhibition of estrogen–DNA adduct formation was similar at lower doses, but at higher doses Resv was about 50% more effective than NAcCys. Their combined effects were additive. Therefore, these two antioxidants provide an excellent combination to protect catechol estrogens from oxidation to catechol quinones.
Keywords: Cancer prevention, Catechol estrogen quinones, Depurinating estrogen–DNA adducts, Balanced estrogen metabolism, Free radicals
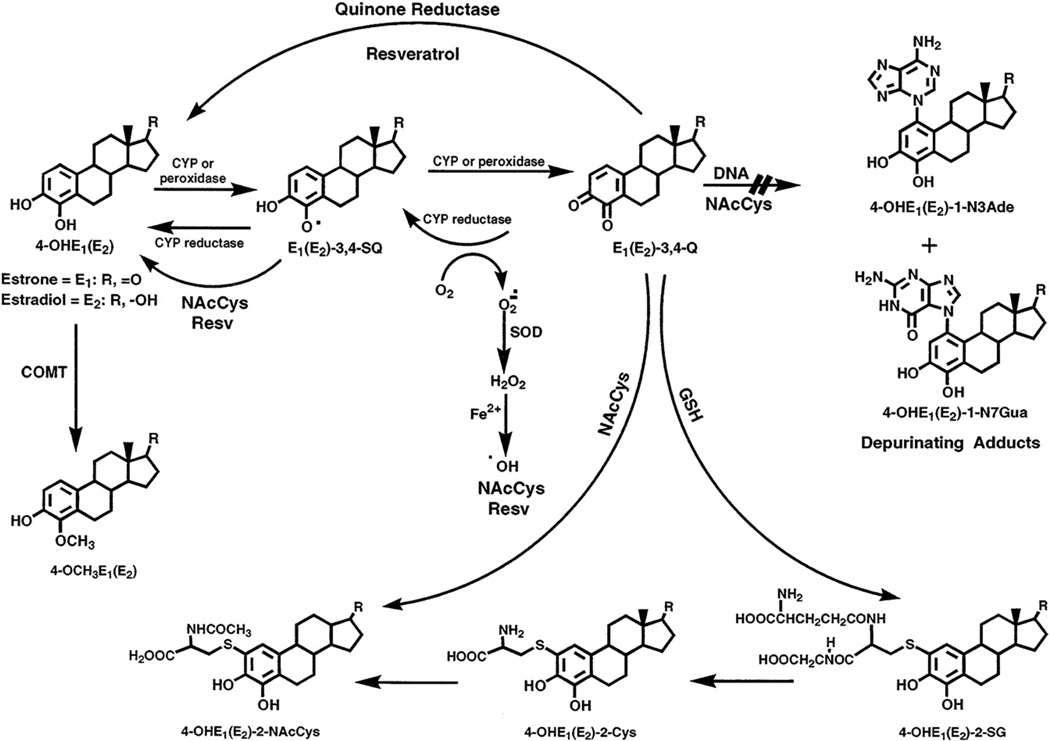
Substantial evidence from experiments on estrogen metabolism [1–3], formation of DNA adducts [4–8], mutagenicity [9–13], cell transformation [14–16], and carcinogenicity [17–20] suggests that certain estrogen metabolites, particularly catechol estrogen-3,4-quinones, react with DNA to form predominantly the depurinating adducts 4-hydroxyestrone (estradiol)-1-N3Ade [4-OHE1(E2)-1-N3Ade]1 and 4-OHE1(E2)-1-N7Gua [4–6] (Fig. 1). Apurinic sites resulting from formation of these adducts can undergo error-prone repair to generate critical mutations that can initiate cancer [9–11,21]. This paradigm of cancer initiation is based on disruption of the homeostatic balance in estrogen metabolism. The balance is between activating pathways that can lead to estrogen–DNA adducts and deactivating pathways that lead to estrogen metabolites and conjugates (Fig. 1). This hypothesis has been supported by results obtained in women in which the level of estrogen–DNA adducts in urine from healthy women is low and the levels of estrogen conjugates are high. In contrast, higher levels of adducts and lower levels of conjugates are present in the urine of women with breast cancer or at high risk for the disease [7,22].
Fig. 1.
Metabolic activation of 4-OHE1(E2) to form DNA adducts and its inhibition by NAcCys and Resv.
The oxidation of catechol estrogens to quinones can be reduced by the intervention of phase II enzymes. For example, catechol-O-methyltransferase (COMT) catalyzes the methylation of catechol estrogens, thereby preventing their conversion to estrogen semiquinones and quinones (Fig. 1). In fact, we have obtained increased formation of estrogen–DNA adducts when the activity of COMT was inhibited [23,24]. A second conjugation that prevents the reaction of catechol estrogen quinones with DNA is provided by the cellular antioxidant glutathione (GSH), either nonenzymatically or, more efficiently, with the catalytic action of glutathione S-transferase. Other phase II enzymes, such as quinone reductases (NQO1 and NQO2), are also involved in the detoxification of the quinones by their reduction to catechol estrogens [25–27] (Fig. 1).
The balance between activating and deactivating pathways leading to estrogen–DNA adducts or estrogen conjugates, respectively, can be further improved to minimize formation of adducts and maximize formation of conjugates. The use of specific antioxidant compounds can potentially achieve this improved balance of estrogen homeostasis. In a preliminary in vitro study, we tested several antioxidants, cysteine, N-acetylcysteine (NAcCys), GSH, melatonin, resveratrol (Resv), and reduced lipoic acid [28]. The best inhibitors of the formation of depurinating estrogen–DNA adducts were Resv and NAcCys.
NAcCys is an acetylamino thiol. Its hydrolytic product, cysteine, is one of the three amino acids present in the intracellular antioxidant GSH [29]. Changes in GSH homeostasis have been implicated in the etiology and progression of a variety of human diseases, including cancer [30]. NAcCys has been used as a mucolytic drug [31] and as an antidote for paracetamol poisoning [32]. Later it was shown to have antioxidant and anticoagulant properties [33–36].
Resv (3,5,4′-trihydroxystilbene) is a natural antioxidant present in grapes and many other plants [37] and has anticarcinogenic effects in diverse in vitro and in vivo systems [38,39].
Using the human breast epithelial cell line MCF-10F (estrogen receptor-α negative and aryl hydrocarbon receptor positive), we have investigated the effects of Resv [16,40] and NAcCys [41] on estrogen metabolism and formation of estrogen–DNA adducts. In this study, we have compared the ability of Resv and NAcCys to block formation of estrogen–DNA adducts, using the two compounds individually and mixed together.
Materials and methods
Chemicals and reagents
4-OHE2 and all standards were synthesized in our laboratory, as previously described [4,5,42,43]. All other chemicals were purchased from Sigma (St. Louis, MO, USA) and used without further purification.
Cell culture and treatment
MCF-10F cells were obtained from the ATCC (Rockville, MD, USA) and cultured in DMEM and Ham's F12 medium (Mediatech) with 20 ng/ml epidermal growth factor, 0.01 mg/ml insulin, 500 ng/ml hydrocortisone, 5% horse serum, and 100 µg/ml penicillin/streptomycin mixture. Estrogen-free medium was prepared in phenol red-free DME/F12 medium with charcoal-stripped FBS (HyClone, Logan, UT, USA). Cell viability was determined by the MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide; Sigma] assay [44].
Cytotoxicity assay
Cells were seeded at a density of 3000 cells/well in 96-well plates. After 8 h (day 0), cells in one 96-well plate were counted using the MTT assay (see below), whereas other cells were treated with 4-OHE2 (10 µM) with or without the presence of various doses of NAcCys (0.6–60 µM) and Resv (0.37–36 µM) alone or combined together. The cells were further incubated for 24–72 h. In the MTT assay, the medium in the 96-well plate cultures was replaced with 100 µl fresh medium containing 25 µl of MTT (5 mg/ml in PBS) and incubated for 2 h at 37 °C to allow the reduction of MTT by metabolically active cells to form a purple formazan precipitate. The precipitate was then solubilized by adding 20% SDS in 1:1 DMF:H2O, pH 4.7 (100 µl), and incubating overnight at 37 °C. The purple color was read at 570 nm in a µQuant microplate spectrophotometer (Bio-Tek Instruments) and analyzed by the KCjunior (version 1.41) software. The absorbance values were converted into percentage of viable cells with respect to the blank control absorbance value.
Metabolism
The MCF-10F cells (0.75×105 cells) were seeded for 24 h in estrogen-containing medium. The medium was changed to estrogen-free medium and the cells were grown for another 48 h. To see the effect of antioxidants on estrogen metabolism, cells were first preincubated for 48 h with selected doses of Resv or NAcCys alone or in combined form, washed with PBS, and, after adding fresh medium, treated with 10 µM 4-OHE2 for 24 h with or without antioxidant. To keep the concentration of ethanol (0.001%) the same in all experiments, different stock solutions of NAcCys (0.6–60mM) and Resv (0.37–36 mM) were prepared. Media from five T-150 flasks of MCF-10F cells treated with equal amounts of organic solvent were used as controls.
Once the medium was removed from five flasks, each flask was incubated at 37 °C with 1 ml of trypsin. After 10 min, 10 ml of double-serum medium was used to combine the cells from all five flasks. Cell numbers were estimated using a Coulter counter (Beckman Coulter).
Analysis of estrogen–DNA adducts
After the treatments, the media were harvested, supplemented with 2 mM ascorbic acid (to prevent possible decomposition of the compounds), and processed immediately. Sample preparation and analysis by HPLC with electrochemical detection (HPLC-ECD) was carried out as follows.
Sample preparation
Culture media from five flasks (50 ml) were processed by passage through C8 Certify II cartridges (Varian, Harbor City, CA, USA), which were equilibrated by sequentially passing 1 ml of methanol, distilled water, and potassium phosphate buffer (100 mM, pH 8) through them. The harvested media (50 ml) were adjusted to pH 8.0 and passed through these cartridges. The retained analytes in the cartridges were washed with the above phosphate buffer, eluted with methanol:acetonitrile:water:trifluoroacetic acid (8:1:1:0.1), and processed as described previously [15].
HPLC-ECD
Analyses of all samples were conducted on an HPLC system equipped with dual ESA Model 580 solvent delivery modules, an ESA Model 540 autosampler, and a 12-channel CoulArray electrochemical detector (ESA, Chelmsford, MA, USA) using the method previously described [15]. Three-point calibration curves were run for each standard. Triplicate samples were analyzed for each data point. The analytes were identified by their retention time and peak height ratios between the dominant peak and the peaks in the two adjacent channels. The analytes were quantified by comparison with known amounts of standards. The percentage recovery of each standard was used to normalize the data. Detailed information, along with a chromatogram, was reported previously [41].
Statistical analysis
Cytotoxicity
We used a repeated-measures multivariate analysis of variance to examine whether cell viability significantly differed between 4-OHE2-treated and untreated cells at increasing doses of NAcCys at 24, 48, and 72 h. The dependent variables were the nine doses, each treated as an independent variable. Because the sample size was small for each dose, and treatment group and time were both assessed, the Huynh–Feldt epsilon correction factor was applied to the univariate p values because the residuals were not normally distributed within each treatment group [45]. Multivariate results are valid despite the violation of this assumption. Pillai's trace test was used for the F statistics and p values were calculated for overall significance between models.
Metabolism
All experiments were independently replicated at least three times. Data are presented as means ± standard deviation. Methoxy conjugates, quinone conjugates, and DNA adducts were plotted separately for Resv alone, NAcCys alone, and Resv plus NAcCys at nine dose combinations.
The individual dose–response plots of Resv and NAcCys on methoxy conjugate, quinone conjugate, and DNA adduct levels showed that the dose–response curves were not consistently parallel, indicating more complex relationships. Therefore, we tested for independence and synergism/antagonism using the Bliss equations.
Nonlinear regression was used to model dose response, and independence or synergism/antagonism was tested using Bliss models [46]. The dose pairs were grouped into four categories as follows: (1) no antioxidant treatment; (2) NAcCys>0 and Resv = 0; (3) NAcCys = 0 and Resv>0; and (4) NAcCys>0 and Resv>0. Bliss independence assumes that two agents each contribute to an effect, but do not interfere with one another's activities, an assumption that applies based on the mechanisms of action of Resv and NAcCys. Bliss synergism occurs if at certain doses the combined effect creates greater potency than the effects of each agent alone. Antagonism occurs when the combination of the two agents results in a less potent effect than the two agents acting alone. The Bliss model is an extension of a four-parameter logistic regression model that introduces a parameter called "ν" that, when equal to 1, means two agents are independent; values between 0 and 1 represent antagonism and values greater than 1 mean synergy. The ν parameter is added to the dose–response curves of the single agents to determine whether the model improves after accounting for the interaction between the two compounds.
We used these three models to examine the relationship of each agent, and the combination of agents, on methoxy conjugate, quinone conjugate, and DNA adduct levels. The models allow quantification of antagonism/additivity or synergism with a statistical test of significance for model fit. All analyses were done in SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results and discussion
NAcCys and Resv reverse 4-OHE2 toxicity in MCF-10F cells
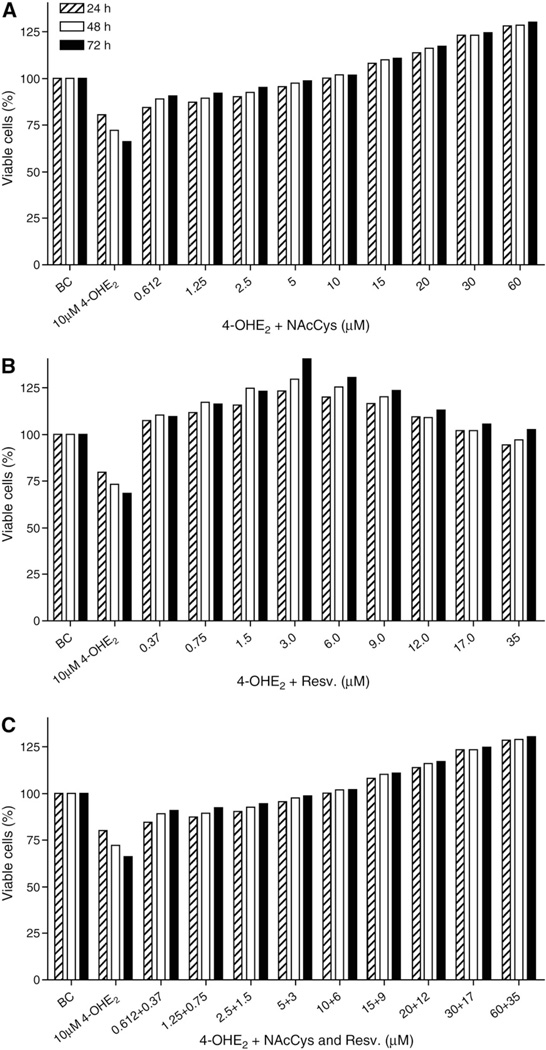
In this study, we examined the cytotoxic effect of 4-OHE2 on MCF-10F cells using the MTT assay. We observed some cytotoxicity in MCF-10F cells exposed to 10 µM 4-OHE2 over 72 h compared with control cells (Fig. 2), with the treated cells showing 42% cell mortality after 72 h of incubation.
Fig. 2.
Protective effects of NAcCys and Resv against the cytotoxicity of 10 µM4-OHE2 in MCF-10F cells. The cells were incubated for 24, 48, or 72 h with 4-OHE2 plus increasing doses of (A) NAcCys, (B) Resv, or (C) NAcCys + Resv.
Plots of percentage of viable cells for examining the effects of NAcCys and Resv on 4-OHE2 cytotoxicity were constructed by converting the MTT absorbance values of treated cells to ratios (percentage viable cells) at days 1, 2, and 3 with respect to those in the control wells, and they were plotted as a time course. We treated MCF-10F cells with a mixture of 4-OHE2 (10 µM) plus increasing concentrations of NAcCys (0.6–60 µM) and/or Resv (0.37–36 µM) and examined viable cells for 0–72 h (Fig. 2).
The results indicate that NAcCys can protect cells from the cytotoxic effects of 4-OHE2 (Fig. 2A). For example, the addition of NAcCys increased the number of viable MCF-10F cells treated with 10 µM 4-OHE2 by 24% after 72 h (66 to 90%). Interestingly, 10 µM NAcCys abolished the toxic effect of 4-OHE2 at 24, 48, and 72 h. Higher doses of NAcCys (15–60 µM) did not just overcome the toxicity of 4-OHE2, but promoted cell growth to higher levels than in the untreated control cultures (e.g., at 72 h, 110–129% viable cells, Fig. 2A). Statistically significant differences were noted at both dose and time point (all p<0.0001), indicating that increasing concentrations of NAcCys improved cell viability at later time points.
The incubations with Resv showed that this compound is slightly more effective than NAcCys, and even the lowest dose of Resv (0.37 µM) completely eliminated the cytotoxic insult from 10 µM 4-OHE2 in 72 h (Fig. 2B). The protective effect of Resv against 4-OHE2 was more prominent as the dose increased. Similar data were observed when both compounds (NAcCys and Resv) were combined (Fig. 2C). Therefore, the two antioxidants were not cytotoxic, but protected the cells.
Inhibition of estrogen–DNA adduct formation by Resv and NAcCys
In our experiments with MCF-10F cells, we divided the metabolic products of 4-OHE2 into three categories of compounds: (1) methoxy conjugates [4-OCH3E1(E2)], (2) quinone conjugates [4-OHE1(E2)-2-SG; 4-OHE1(E2)-2-NAcCys and 4-OHE1(E2)-2-Cys], and (3) depurinating estrogen–DNA adducts [4-OHE1(E2)-1-N3Ade and 4-OHE1 (E2)-1-N7Gua]. In previous studies we reported that the formation of methoxy and quinone conjugates and estrogen–DNA adducts increases with higher doses of estrogen catechols or quinones [23,24]. In the experiments reported here, we decided to use a minimally cytotoxic dose of 4-OHE2 (10 µM) with or without different doses of antioxidant (Resv or NAcCys) alone or in combination. Resv was always at a level 60% that of NAcCys.
Under our experimental conditions, we observed that after treatment, free 4-OHE2 was undetectable. A major portion was converted to the methoxy conjugate [4-OCH3E1(E2)] by the abundant presence of COMT in the cells [23,24], and the remaining catechols were oxidized to form the E1(E2)-3,4-Q.
The quinones have three major fates (Fig. 1): first, they can be reduced by quinone reductase (NQO1 or NQO2) [25–27] back to catechols, which can again be methylated by COMT or reoxidized to quinones. Second, the quinones can react with endogenous GSH to form GSH conjugates. Third, the quinones can react with DNA to form estrogen–DNA adducts. Treatment of cells with 10 µM 4-OHE2 alone showed the expected result (Figs. 3–5), i.e., the formation of methoxy and quinone conjugates, as well as depurinating estrogen–DNA adducts.
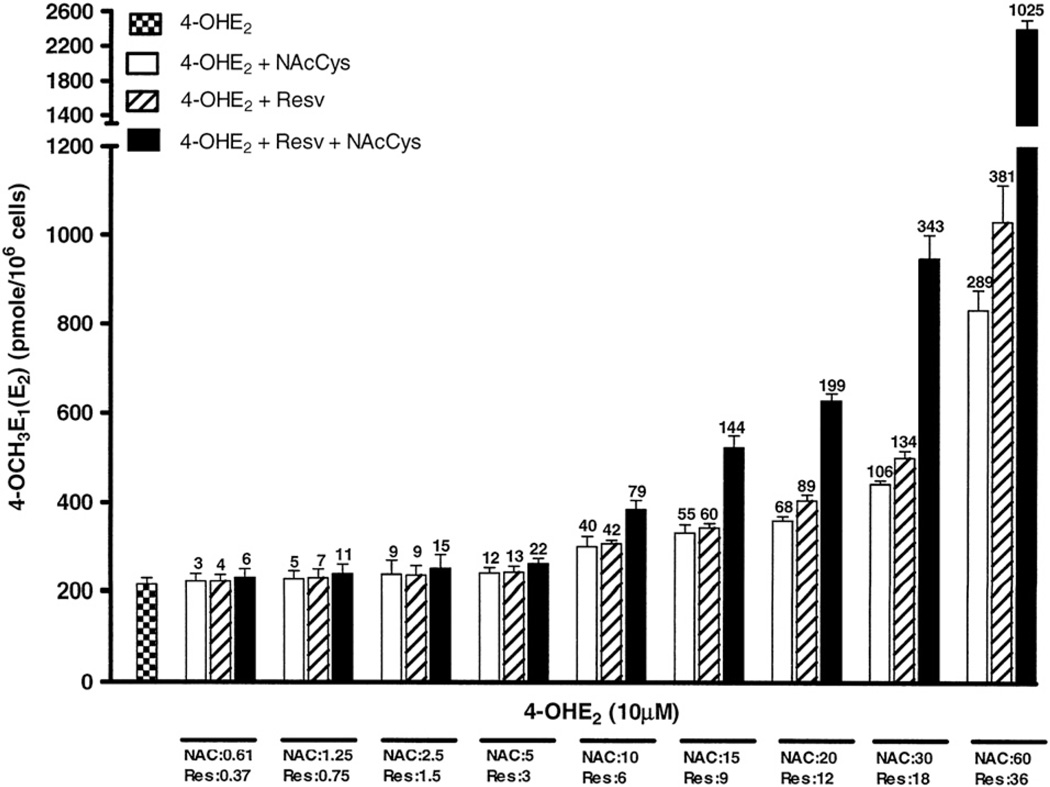
Fig. 3.
Effects of NAcCys, Resv, or NAcCys + Resv on the formation of 4-methoxy conjugates in MCF-10F cells treated with 4-OHE2. The compounds were identified and quantified by HPLC-ECD. The bars represent the means of triplicate cultures from three experiments ± standard deviation. The value on each bar shows the percentage increase or decrease with respect to the control, in which cells were treated only with 10 µM 4-OHE2.
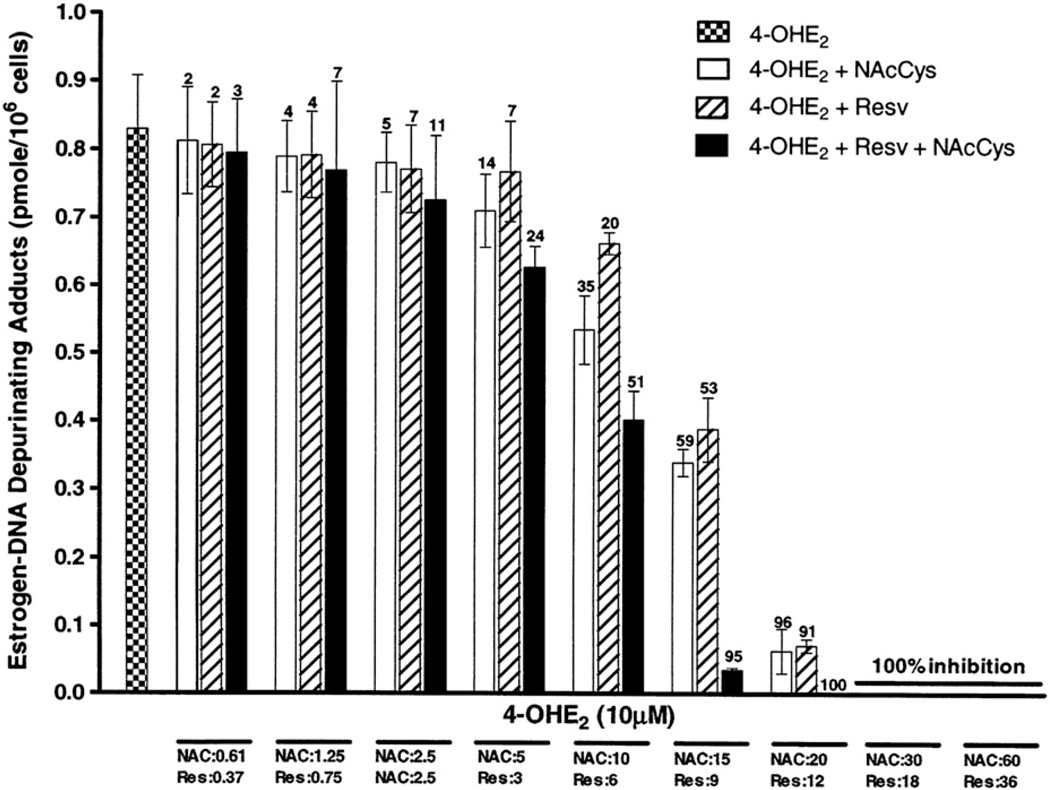
Fig. 5.
Effects of NAcCys, Resv, or NAcCys + Resv on the formation of depurinating estrogen–DNA adducts in MCF-10F cells treated with 4-OHE2. The methods are as in Fig. 3.
Methoxy conjugates
The addition of NAcCys and/or Resv substantially directed estrogen metabolism toward the protective pathways. At the low doses of NAcCys, this effect was not prominent, but when NAcCys reached a 1:1 ratio with 4-OHE2, it significantly increased the level of methoxy conjugates from 214 ± 25 to 299 ± 40 pmol/million cells (40% increase, Fig. 3). A similar effect was observed with 6 µM Resv, in that the methoxy conjugate level increased 42%, corresponding to 305 ± 14 pmol/million cells. On the other hand, when both antioxidant compounds were added together, this effect was greater and the methoxy conjugate level increased 79%. Thus, the level of methoxy compounds increased with higher ratios of antioxidants either alone or mixed together. At the highest dose, in which NAcCys was at a ratio of 1:6 and Resv at 1:3.6 with respect to 4-OHE2, the methoxy conjugates were greater with Resv, 381% increase, whereas with NAcCys the increase was 298%.With the mixture, the increase was 1025%, which is threefold more than the individual antioxidant compounds themselves (Fig. 3). This result may be explained by the observation from previous experiments that NAcCys and Resv reduce estrogen semiquinones produced in these cells during the oxidation of 4-OHE2 back to the catechols [16,28,47]. The large amount of COMT in the cells ensures that the 4-OHE2 is converted to 4-OCH3E1(E2) [23,24]. Therefore, Resv was more effective in increasing the amount of 4-OCH3E1(E2) than NAcCys. However, attempts at nonlinear modeling of the methoxy conjugate dose–response curve resulted in nonconvergent models due to nonsingular Hessian matrices. Therefore statistical tests for antagonism, additivity, or synergism could not be conducted.
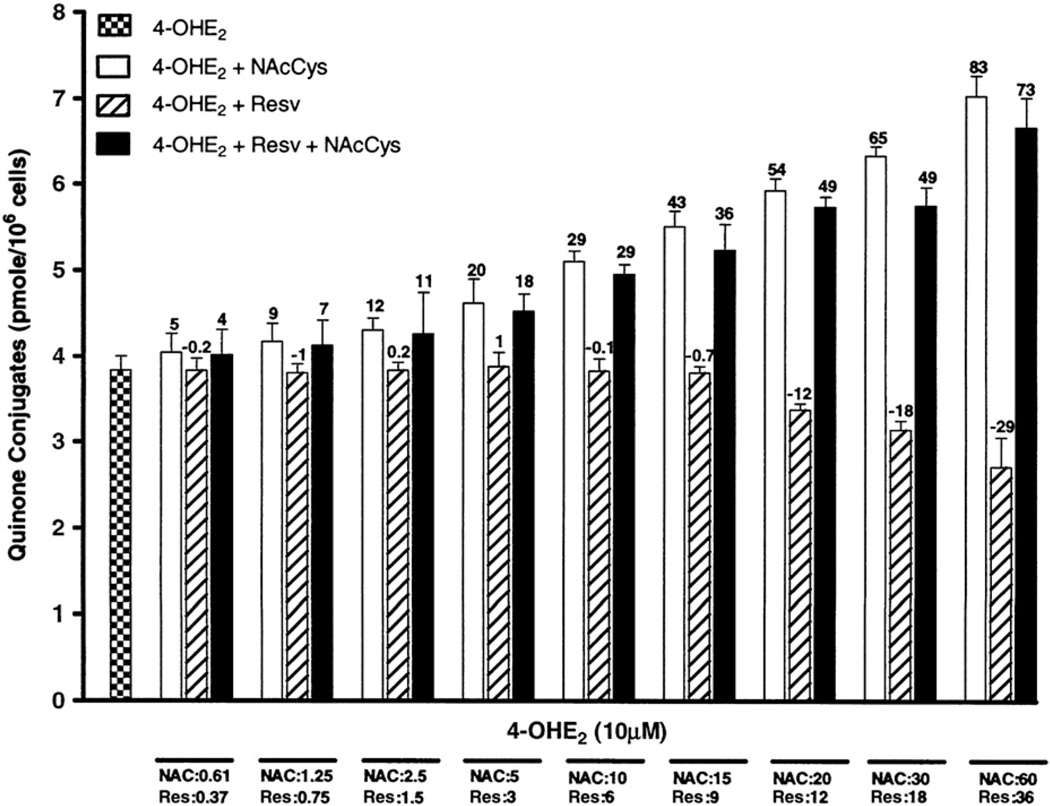
Quinone conjugates
Interesting results were observed for the quinone conjugates, which increased with higher doses of NAcCys (Fig. 4). At the highest dose, this increase was approximately twofold. This is understandable, as NAcCys can react directly with the catechol quinones to form 4-OHE1(E2)-2-NAcCys [43]. Similarly, NAcCys is a precursor of GSH, which, in turn, reacts itself with E1(E2)-3,4-Q to form GSH conjugates. These convert by the mercapturic acid pathway to 4-OHE1(E2)-2-Cys and 4-OHE1(E2)-2-NAcCys (Fig. 1). On the other hand, Resv did not show any effect on the formation of quinone conjugates at low doses, whereas at the two highest doses (18 and 36 µM), the quinone conjugate level decreased noticeably (Fig. 4). The Resv effect on quinone conjugates can be explained by our previous finding that Resv does not react with E2-3,4-Q to form any conjugated compound [28]. On the other hand, Resv shows stronger potential in reducing the semiquinone to catechol than NAcCys, and it also induces the enzyme NQO1, which reduces the quinone back to catechol [16,25,28,40]. These processes all reduce the level of quinones available to form conjugates. In the experiments in which both antioxidants were used together, the conjugate level is moderately increased, but at all the selected doses the level reflects the contribution of NAcCys (Fig. 4).
Fig. 4.
Effects of NAcCys, Resv, or NAcCys + Resv on the formation of quinone conjugates in MCF-10F cells treated with 4-OHE2. The results are as in Fig. 3.
Looking at Fig. 4, it is apparent that Resv and NAcCys have opposite effects on the formation of quinone conjugates. The dose–response curve for the two agents was shown to be parallel, but three of the four logistic regression parameters were in the negative direction. The data adequately fit the Bliss model (p<0.0001), but the ν parameter was 0.82 (95% confidence interval 0.70–0.97), indicating an antagonistic relationship between NAcCys and Resv on quinone conjugate levels. These results confirm that the major effect of NAcCys on quinone conjugate levels is to react itself with the quinones.
Estrogen–DNA adducts
Thus, NAcCys and Resv affect estrogen metabolism in MCF-10F cells, minimizing quinone levels chemically (reducing semiquinones) and biologically (inducing protective phase II enzymes). The quinones are essential for the reaction with DNA to form estrogen–DNA adducts. A drastic reduction in the level of depurinating estrogen–DNA adducts, which are predominantly formed (99%) compared to stable adducts (1%) [28], was observed. At the doses in which NAcCys is 15 µM and Resv is 9 µM, the level of adducts was reduced from 0.83±0.14 to 0.34±0.03 (59% decrease) and 0.39±0.08 pmol/million cells (53% decrease), respectively (Fig. 5). When mixed together, these doses worked additively, and the adduct level decreased to 0.03±0.001 (95% reduction). The doses of 20 µM NAcCys and 12µM Resv reduced the adduct level 96 and 91%, respectively, whereas the mixture showed complete inhibition. The two highest doses showed 100% inhibition either alone or mixed together (Fig. 5). Statistical tests showed that a four-parameter logistic regression model significantly fit the data when the interaction term (ν) was included in the model (p<0.0001). The ν parameter under the Bliss synergism/antagonism model was 1.33 (95% confidence interval 0.87–2.02),which, being slightly greater than unity, showed that at the higher doses there was evidence of synergism, but the result was not statistically significant. However, the Bliss independence model was independent of the ν parameter and a better fit was found after it was dropped from the model, suggesting that overall Resv and NAcCys were acting in an additive fashion on methoxy conjugates, quinone conjugates, and DNA adduct formation.
In summary, inhibition of estrogen–DNA adduct formation was similar at lower doses of NAcCys and Resv, but at higher doses the effect of Resv was about 50% greater than that of NAcCys.
The depurinating estrogen–DNA adduct levels are considered the starting point that generates mutations that lead to breast cancer. These two antioxidants (NAcCys and Resv) may be useful for probing the link between the oxidative metabolism of estrogens and the initiation of cancer.
Conclusion
Estrogen metabolism is characterized by two major pathways. The first is the 16α-hydroxylation, and the second is the formation of catechols by hydroxylation of the 2- and 4-positions [1,2]. 16α-Hydroxylation is not involved in any specific reaction with DNA, whereas oxidation of 2-and 4-catechols leads to their respective quinones, which can react with DNA. The most effective reaction of these quinones with DNA occurs when the 3,4-quinones are formed. In this case, a very effective protonated 1,4-Michael addition [46] leads to the predominant formation (99%) of depurinating estrogen–DNA adducts [6,7]. Several lines of evidence indicate that this reaction with DNA to form predominantly the depurinating N3Ade and N7Gua adducts could initiate the series of events that lead to the development of cancer [21]. This reaction with DNA occurs more efficiently when the metabolism of estrogens is unbalanced, namely, excessive formation of quinones, which may react with DNA, occurs. The reaction of catechol estrogen quinones with DNA can be minimized by the specific antioxidants NAcCys and Resv. NAcCys exerts its protective effects chemically. In fact, NAcCys reacts directly with the catechol estrogen quinones and is hydrolyzed to Cys, which is used in the biosynthesis of the cellular antioxidant GSH (Fig. 1). In addition, NAcCys reduces estrogen semiquinones to catechols (Fig. 1), thereby decreasing the amount of quinones formed. The reduction of semiquinones to catechols is also obtained with Resv [16,40]. Resv, however, also exerts enzymatic protective effects, namely, induction of NQO1, which reduces the quinones to catechols (Fig. 2), and it modulates the expression of CYP1B1, which specifically metabolizes E2 to 4-OHE2 (Fig. 1). In conclusion, these two antioxidants provide an excellent combination to minimize the metabolism of estrogens from catechols to catechol quinones. Through these effects, the combination of NAcCys and Resv is expected to inhibit the initiation of cancer by estrogens.
Acknowledgments
This research was supported by Prevention LLC. Core support at the Eppley Institute was provided by Grant CA P30 36727 from the National Cancer Institute.
Abbreviations
- COMT
catechol-O-methyltransferase
- CYP
cytochrome P450
- E2-3,4-Q
estradiol-3,4-quinone
- GSH
glutathione
- HPLC-ECD
high-performance liquid chromatography with electrochemical detection
- MTT
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
- NAcCys
N-acetylcysteine
- NQO1 and NQO2
NAD(P) H quinone oxidoreductase 1 and 2
- 4-OCH3E1(E2)
4-methoxyestrone (estradiol)
- 4-OHE1(E2)
4-hydroxyestrone (estradiol)
- Resv
resveratrol
- –SG
glutathione moiety.
References
- 1.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Cavalieri EL, Kumar S, Todorovic R, Higginbotham S, Badawi AF, Rogan EG. Imbalance of estrogen homeostasis in kidney and liver of hamsters treated with estradiol: implications for estrogen-induced initiation of renal tumors. Chem. Res. Toxicol. 2001;14:1041–1050. doi: 10.1021/tx010042g. [DOI] [PubMed] [Google Scholar]
- 3.Rogan EG, Badawi AF, Devanesan PD, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 4.Cavalieri EL, Stack DE, Devanesan PD, et al. Molecular origin of cancer: catechol estrogen-3, 4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K-M, Todorovic R, Devanesan P, et al. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3, 4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25:289–297. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 6.Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E. The greater reactivity of estradiol-3, 4-quinone versus estradiol-2, 3-quinone with DNA in the formation of depurinating DNA adducts: implications for tumor-initiating activity. Chem. Res. Toxicol. 2006;19:164–172. doi: 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- 7.Gaikwad NW, Yang L, Muti P, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int. J. Cancer. 2008;122:1949–1957. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Gaikwad N, Meza J, et al. Novel biomarkers for risk of prostate cancer: results from a case–control study. Prostate. 2009;69:41–48. doi: 10.1002/pros.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarti D, Mailander P, Li K-M, et al. Evidence that a burst of DNA depurination in SENCAR mouse skin induces error-prone repair and forms mutations in the H-ras gene. Oncogene. 2001;20:7945–7953. doi: 10.1038/sj.onc.1204969. [DOI] [PubMed] [Google Scholar]
- 10.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T to G.C mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J. Steroid Biochem. Mol. Biol. 2006;101:204–215. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Z, Kosinska W, Khmelnitsky M, et al. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem. Res. Toxicol. 2006;19:475–479. doi: 10.1021/tx0502645. [DOI] [PubMed] [Google Scholar]
- 12.Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. BBA Rev. Cancer. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int. J. Cancer. 2006;118:1862–1868. doi: 10.1002/ijc.21590. [DOI] [PubMed] [Google Scholar]
- 14.Russo J, Fernandez SV, Russo PA, et al. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 15.Venugopal D, Zahid M, Mailander PC, et al. Reduction of estrogen-induced transformation of mouse mammary epithelial cells by N-acetylcysteine. J. Steroid Biochem. Mol. Biol. 2008;109:22–30. doi: 10.1016/j.jsbmb.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, Rogan EG. Resveratrol prevents estrogen–DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catecholestrogens in Syrian hamsters. J. Steroid Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 18.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissue: role of metabolism. Fed. Proc. 1987;46:1858–1863. [PubMed] [Google Scholar]
- 19.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- 20.Yue W, Santen RJ, Wang JP, et al. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J. Steroid Biochem. Mol. Biol. 2003;86:477–486. doi: 10.1016/s0960-0760(03)00377-7. [DOI] [PubMed] [Google Scholar]
- 21.Cavalieri EL, Rogan EG. Depurinating estrogen–DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaikwad NW, Yang L, Pruthi S, et al. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer: Basic Clin. Res. 2009;3:1–8. doi: 10.4137/bcbcr.s2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu F, Zahid M, Saeed M, Cavalieri EL, Rogan EG. Estrogen metabolism and formation of estrogen–DNA adducts in estradiol-treated MCF-10F cells: the effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin induction and catechol-O-methyltransferase inhibition. J. Steroid Biochem. Mol. Biol. 2007;105:150–158. doi: 10.1016/j.jsbmb.2006.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahid M, Saeed M, Lu F, Gaikwad N, Cavalieri EL, Rogan EG. Inhibition of catechol-O-methyltransferase increases estrogen–DNA adduct formation. Free Radic. Biol. Med. 2007;43:1534–1540. doi: 10.1016/j.freeradbiomed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaikwad NW, Rogan EG, Cavalieri EL. Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic. Biol. Med. 2007;43:1289–1298. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montano MM, Chaplin LJ, Deng H, et al. Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene. 2007;26:3587–3590. doi: 10.1038/sj.onc.1210144. [DOI] [PubMed] [Google Scholar]
- 27.Gaikwad NW, Yang L, Rogan EG, Cavalieri EL. Evidence for NQO2-mediated reduction of the carcinogenic estrogen ortho-quinones. Free Radic. Biol. Med. 2009;46:253–262. doi: 10.1016/j.freeradbiomed.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahid M, Gaikwad N, Cavalieri EL, Rogan EG. Inhibition of depurinating estrogen–DNA adduct formation by natural compounds. Chem. Res. Toxicol. 2007;20:1947–1953. doi: 10.1021/tx700269s. [DOI] [PubMed] [Google Scholar]
- 29.Bonanomi L, Gazzaniga A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur. J. Respir. Dis. Suppl. 1980;111:45–51. [PubMed] [Google Scholar]
- 30.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human diseases. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb WR. New mucolytic agents for sputum liquefaction. Postgrad. Med. 1964;36:449–453. doi: 10.1080/00325481.1964.11695324. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan RJ. The role of acetylcysteine in clinical toxicology. Med. Toxicol. 1987;2:93–104. doi: 10.1007/BF03260008. [DOI] [PubMed] [Google Scholar]
- 33.Doelman CJ, Bast A. Oxygen radicals in lung pathology. Free Radic. Biol. Med. 1990;9:381–400. doi: 10.1016/0891-5849(90)90015-b. [DOI] [PubMed] [Google Scholar]
- 34.De Flora S, Cesarone CF, Balansky RM, et al. Chemopreventive properties and mechanisms of N-acetylcysteine: the experimental background. J. Cell. Biochem. Suppl. 1995;22:33–41. doi: 10.1002/jcb.240590806. [DOI] [PubMed] [Google Scholar]
- 35.Grandjean EM, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 36.De Flora S, Izzotti A, D'Agostini F, Cesarone CF. Antioxidant activity and other mechanisms of thiols involved in chemoprevention of mutation and cancer. Am. J. Med. 1991;91:122S–130S. doi: 10.1016/0002-9343(91)90295-9. [DOI] [PubMed] [Google Scholar]
- 37.Jang M, Cai L, Udeani GO. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 38.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 39.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms. Int. J. Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 40.Zahid M, Gaikwad N, Ali MF, et al. Prevention of estrogen–DNA adducts in MCF-10F cells by resveratrol. Free Radic. Biol. Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahid M, Saeed M, Ali MF, Rogan EG, Cavalieri EL. N-acetylcysteine blocks formation of cancer-initiating estrogen–DNA adducts in cells. Free Radic. Biol. Med. 2010;49:392–400. doi: 10.1016/j.freeradbiomed.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saeed M, Zahid M, Rogan EG, Cavalieri EL. Synthesis of the catechols of natural and synthetic estrogens by using 2-iodoxybenzoic acid (IBX) as the oxidizing agent. Steroids. 2005;70:173–178. doi: 10.1016/j.steroids.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine N-acetylcysteine and glutathione. Chem. Res. Toxicol. 1998;11:909–916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- 44.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Meth. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 45.Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in the randomized block and split-plot designs. J. Educ. Behav. Stats. 1976;1:69–82. [Google Scholar]
- 46.Bliss CI. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939;26:585–615. [Google Scholar]
- 47.Samuni AM, Chuang EY, Krishna MC, et al. Semiquinone radical intermediate in catecholic estrogen-mediated cytotoxicity and mutagenesis: chemoprevention strategies with antioxidants. Proc. Natl. Acad. Sci. USA. 2003;100:5390–5395. doi: 10.1073/pnas.0930078100. [DOI] [PMC free article] [PubMed] [Google Scholar]