Abstract
Studies of substance abuse treatment outcomes that give priority to cessation of all drug use may obscure other tangible benefits of treatment that are important to patients. The aim of this study was to examine the association between changes in quality of life (QoL) and: (a) retention in treatment and (b) opioid use as measured by self-report and urine testing. Participants were 300 African American men and women starting outpatient buprenorphine treatment. Participants completed assessments at baseline, 3- and 6-months consisting of the World Health Organization’s Quality of Life brief scale, Addiction Severity Index, and urine testing for opioids. There were statistically significant increases over time across all four QoL domains: physical, psychological, environmental, and social. Self-reported frequency of opioid use was negatively associated with psychological QoL, but opioid urine test results were not significantly associated with any QoL domains. Continued treatment enrollment was significantly associated with higher psychological QoL and environmental QoL. Patients entering buprenorphine treatment experience improvements in QoL, which are amplified for patients who remain in treatment. Point-prevalence opiate urine test results obtained at each assessment were not associated with any of the QoL domains and may not accurately reflect improvements perceived by patients receiving buprenorphine treatment.
Keywords: Quality of Life, Buprenorphine, Urine Testing, Treatment Retention, Minority Health
Introduction
Traditionally, metrics by which the substance abuse treatment field gauges “success” of illicit opioid addiction treatment interventions emphasize reductions in drug use, either as measured by self-report or by biological testing (most commonly urine specimens). This focus on drug use as the gold standard of improvement assumes that when patients test positive for illicit opioids they are probably “doing worse”, and conversely that they are doing well when they report no drug use. Although measuring drug use is an important outcome for programs or practitioners delivering treatment, a singular focus on drug use may obscure other benefits of addiction treatment, since frequency and quantity of drug use may not be directly proportional to consequences of such use and patients’ problems are often more pervasive than drug use alone (Tiffany, Friedman, Greenfield, Hasin, & Jackson, 2012). The history of considering multidimensional instruments (Darke, Hall, Wodak, Heather, & Ward, 1992; Ling, Farabee, Liepa, & Wu, 2012; McLellan, Alterman, Cacciola, Metzger, & O’Brien, 1992) to measure drug abuse treatment outcomes indicates a recognition that outcomes go beyond the presence or absence of drug use, although many such instruments incorporate drug use as a domain of functioning or encompass domains that may be directly impacted by drug use.
Over the past decade, there has been additional attention to measuring improvement as perceived by the patients. One approach has taken the form of measuring quality of life (QoL) in opioid dependent populations following treatment (Tracy et al., 2012; Zubaran & Foresti, 2009), much of it conducted outside the US in methadone treatment (Baharom, Hassan, Ali, & Shah, 2012; Chou et al., 2013; Dhawan & Chopra, 2013; Giri, Srivastava, & Shankar, 2013; Ha, 2010; Karow et al., 2011; Padaiga, Subata, & Vanagas, 2007; Wang et al., 2012; Xiao, Wu, Luo, & Wei, 2010). Many instruments used to measure QoL have the advantage of being relatively independent of changes in quantity and frequency of drug use.
Buprenorphine, like methadone, has been shown to suppress opioid use (Fudala et al., 2003; Johnson et al., 1995; Johnson, Jaffe, & Fudala, 1992; Mattick, Kimber, Breen, & Davoli, 2008) in the treatment of opioid-dependence. Although buprenorphine has been shown effective in reducing opioid use, its impact on QoL over the course of treatment has been less intensively explored, and the use of QoL as a primary outcome measure has received only limited attention. Research on changes in QoL following entry into buprenorphine treatment (Dhawan & Chopra, 2013; Giacomuzzi, Ertl, Kemmler, Riemer, & Vigl, 2005; Maremmani, Pani, Pacini, & Perugi, 2007; Ponizovsky & Grinshpoon, 2007; Raisch et al., 2012) have generally found improvement over time using a variety of QoL measures (Zubaran & Foresti, 2009). In our recent randomized trial with buprenorphine comparing standard outpatient to intensive outpatient for African American patients, we found significant improvement in physical, psychological, social, and environmental QoL domain scores on the World Health Organization’s abbreviated Quality of Life scale (WHOQOL-BREF) from treatment entry to 6-month follow-up (Mitchell et al., 2013).
Less studied has been the relationship between QoL and retention in buprenorphine treatment, or the relationship between changes in QoL and reduction in drug use, with QoL as the primary outcome measure. The focus of the present study is to examine the association between changes in quality of life (QoL) scores and: (a) retention in buprenorphine treatment and (b) opioid use, as measured by self-report and urine test results. We hypothesized that retention in treatment would be positively associated with improvements in QoL across all domains and that there would be a negative association between opioid use and QoL.
Methods
Parent Study
The current study examined data collected as part of a randomized controlled trial comparing standard outpatient and intensive outpatient levels of counseling for opioid-dependent African Americans newly admitted to buprenorphine treatment. The main trial found no significant differences between the intensive outpatient condition, in which participants received a mean of 5.2 (SD=1.7) hours of counseling per active week in treatment, vs. the standard outpatient condition, in which participants received an average of 3.7 (SD=1.30) hours of counseling per active week in treatment on a range of outcomes, including QoL. On average, participants in either counseling level experienced marked improvements in drug use and functioning (Mitchell et al., 2013). Participants in buprenorphine treatment were provided with individualized doses of buprenorphine, with a modal maintenance dose of 16 mg. Medication was initially administered under supervision at the program five days a week, with participants eventually able to receive up to monthly prescriptions. There were no differences in buprenorphine dose by study counseling condition, although prior research with this sample found that higher maintenance dose was significantly associated with lower risk of treatment dropout through 6 months (Gryczynski et al., 2014). The methodology and results of the parent study have been described in detail elsewhere (Mitchell et al., 2013). The research was approved by the Friends Research Institute’s Institutional Review Board, as well as the IRB of one of the clinic sites’ umbrella organization.
Participants
Participants were 300 opioid-dependent African Americans who were starting outpatient buprenorphine treatment. The sample was 37.7% female, with a mean age of 46.1 (SD=6.5). About half of the sample had experience with buprenorphine treatment prior to the current treatment episode (51%). All participants used heroin, with an average age of first heroin use of 22.8 years (SD=7.4). Use by injection was reported by 23.3% of the sample, with the remainder reporting use via the nasal route. On average, in the 30 days prior to entry participants reporting using opioids 22.9 days (SD=9.1) and cocaine 7.2 days (SD= 10.6). The majority of the sample (61.3%) had pre-treatment cocaine use (either by past 30-day self-report or urine test).
Assessments
Participants completed a structured interview and provided a urine sample for drug testing on three occasions: baseline, 3-, and 6-month follow-up. Participants received $30 for the baseline interview and $40 for each of the follow-up interviews. There were 300 completed interviews at treatment entry (baseline), 287 at 3 month follow-up (95.7% follow-up rate), and 279 at 6 month follow-up (93.0% follow-up rate).
Measures
Quality of Life
Quality of Life was assessed at each time point using the WHOQOL-BREF, a brief 32-item instrument developed by the World Health Organization that has been used in a wide variety of populations internationally (Chand, Mattoo, & Sharan, 2004; Hawthorne, Herrman, & Murphy, 2006; Noerholm et al., 2004; WHOQOL GROUP, 1998; World Health Organization, 2004) and has been found to have strong psychometric properties (O’Carroll, Smith, Couston, Cossar, & Hayes, 2000; Skevington, Lotfy, O’Connell, & Group, 2004). The WHOQOL-BREF produces scores ranging from 0 (low QoL) to 100 (high QoL) in four QoL domains. The physical domain addresses: activities of daily living, dependence on medicines and medical aids, energy/fatigue levels, mobility issues, pain/discomfort, sleep/rest, and work capacity. The psychological domain includes: body image and appearance, negative feelings, self-esteem, spirituality, and memory and concentration. The social domain addresses: personal relationships, social support, and sexual activity. And the environmental domain covers: financial resources, freedom and physical safety, health and social care, the home environment, opportunities for gaining new skills and information, leisure activities, the physical environment, and transportation. The WHOQOL-BREF also contains a single item, which is not incorporated into any of the four scale scores, asking participants to rate their overall QoL on a 5-point scale from very poor to very good.
Addiction Severity Index
The 5th edition of the Addiction Severity Index (ASI; (McLellan, Kushner, et al., 1992) was used to gather data on participant background and substance use characteristics. For the present analysis, we used items from the ASI corresponding to demographic characteristics of age (in years) and gender (male vs. female), self-reported number of days of opioid use in the past 30 days, self-reported number of days of cocaine use in the past 30 days, and injection drug use status (injector vs. non-injector).
Urine Test
A urine sample for research purposes was collected at each assessment point (baseline, 3-, and 6-month follow-up). These samples were sent to a certified laboratory for analysis of drug metabolites by the Enzyme-Linked Immunoassay Test (EMIT) method. For the current analysis, we examined urine test results for opiates (positive vs. negative), not including buprenorphine. A separate test for buprenorphine is reported descriptively.
Statistical Analysis
Each of the QoL scales corresponding to the physical, psychological, social, and environmental QoL domains was analyzed separately as dependent variables. Data were analyzed using mixed-effects regression equations to account for repeated measurement at baseline, 3-, and 6-month follow-up (Cohen, Cohen, West, & Aiken, 2003). First, we examined mean change in each quality of life domain over the course of the study for the full sample. Each model was then extended to include the following fixed, time-constant predictor variables: gender, age (in years), and injection drug use status as reported at baseline (non-injector vs. injector). The models also included the following time-varying predictor variables: self-reported number of days of heroin use during the last 30 days, urine opioid positive test results at the follow-up assessment points (negative vs. positive), treatment status at the assessment point (in vs. out of treatment), and number of self-reported days of cocaine use in the past 30 days. While opioid dependence is the focus of the study and of buprenorphine treatment, frequency of cocaine use is included as a control variable due to its potential independent association with QoL. In order to determine the impact of treatment status on the relationship between opioid use and QoL, these main models were extended to include multiplicative interactions between (a) treatment status and urine opioid test results, and (b) treatment status and self-reported opioid use.
The estimates in the mixed-effects model can be interpreted as they would in a linear regression analysis, with unstandardized partial regression coefficient estimates representing the amount of change in the outcome variable for a 1-unit change in a predictor variable, holding constant the other predictors in the model. The interpretation of time-varying predictors (e.g., drug test results measured at each assessment occasion) follows the same logic, with the additional nuance that estimates for such variables represent an average of within-subjects effects whereas time-constant variables (e.g., gender) represent solely between-subject effects. Consistent with the modeling framework, missing data on outcome variables was handled using maximum likelihood estimation, whereas listwise deletion was used when data were missing on predictor variables. All model equations were generated using the statistical software environment, R (R Core Team, 2012), and the add-on packages lme4 (Bates, Maechler, & Bolker, 2012), and multcomp (Hothorn, Bretz, Westfal, Heiberger, & Schuetzenmeister, 2013). In addition to examining the four WHOQOL-BREF scale scores, we also compared participants’ ratings of their overall quality of life at 6 months by treatment enrollment status using the Mann-Whitney U test.
Results
Changes in Mean Quality of Life Over Time
Table 1 shows results from simple repeated measures regression models examining mean changes in physical, psychological, environmental, and social QoL domain scores from the WHOQOL-BREF. There was a statistically significant increase over time in mean QoL scores for all four QoL domains (all ps< .001). By 6-month follow-up, on average, physical QoL scores increased by 10.8% from pre-treatment levels, psychological QoL scores increased by 7.6% from pre-treatment levels, environmental QoL increased by 8.5% from pre-treatment levels, and social QoL increased by 11.2% from pre-treatment levels.
Table 1.
Quality of Life scores at baseline, 3, and 6 months (N=300)
| Assessment Time Point | ||||
|---|---|---|---|---|
|
|
||||
| Baseline Mean (SE) |
3 Months Mean (SE) |
6 Months Mean (SE) |
Time Effect | |
|
|
||||
| Physical QoL | 60.00 (1.15) | 67.85 (1.08) | 66.50 (1.10) | χ2 (2)= 60.04; p < .001 |
| Psychological QoL | 67.5 (1.05) | 74.07 (1.01) | 72.64 (1.02) | χ2 (2)= 46.48; p < .001 |
| Environmental QoL | 60.33 (1.00) | 64.80 (0.95) | 65.46 (0.96) | χ2 (2)= 34.01; p < .001 |
| Social QoL | 63.42 (1.31) | 67.76 (1.41) | 70.54 (1.42) | χ2 (2)= 25.73; p < .001 |
Note: Values are model-predicted means (standard errors) derived from a general linear mixed model with a fixed effect for Time.
3.2. Relationship between Treatment Retention Status and Quality of Life
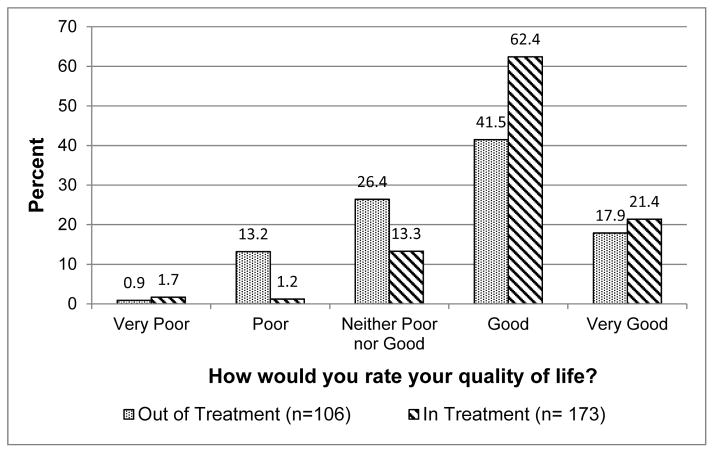
Figure 1 shows participants’ ratings of their overall QoL at 6 months by their treatment enrollment status. The Mann-Whitney U test indicated that participants in treatment at 6 months had significantly higher QoL values than their counterparts who had left treatment by 6 months (p< .001). Among those enrolled in treatment, 83.8% rated their QoL as good or very good at 6 month follow-up, compared to 59.4% of those who had discontinued treatment. Among participants who discontinued treatment by 6 months, 14.2% rated their QoL as poor or very poor, compared to 2.9% of those who remained enrolled in treatment. Importantly, those who were in vs. out of treatment at 6 months did not differ in general QoL ratings at baseline (p=.43).
Figure 1.
General quality of life at 6 month follow-up by treatment enrollment status.
Table 2 shows the results of the full mixed effects regression models predicting QoL scores for each functional domain. Compared to participants who left buprenorphine treatment, active enrollment in treatment was associated with significantly higher QoL scores in two of the four models. Treatment enrollment was associated with nearly 5-point higher mean scores in the psychological (b= 4.89; SE =1.62; p< .01) and environmental (b= 4.94; SE =1.55; p< .01) QoL domains. Treatment enrollment was not significantly associated with higher QoL scores, above and beyond the effects of the other variables in the models, in either the physical or social QoL domains.
Table 2.
Results from mixed effects regression models predicting changes in Quality of Life scores.
| Physical b (SE) |
Psychological b (SE) |
Social b (SE) |
Environmental b (SE) |
|
|---|---|---|---|---|
|
|
||||
| Treatment Enrollment Status | ||||
| Treatment status (ref= not in treatment) | 3.09 (1.81) | 4.89 (1.62)** | −0.03 (2.25) | 4.94 (1.55)** |
| Opiate Use | ||||
| Days of opiate use, past 30 days | −0.12 (0.07) | −0.22 (0.07)** | −0.16 (0.09) | −0.10 (0.06) |
| Opiate-positive urine test (ref= negative) | 1.54 (1.32) | −0.14 (1.18) | 0.83 (1.64) | −0.63 (1.13) |
| Control Variables | ||||
| Age (in years) | −0.37 (0.15)* | −0.04 (0.14) | −0.32 (0.16)* | −0.08 (0.13) |
| Female Gender (ref= male) | −4.55 (1.98)* | 0.03 (1.80) | −0.95 (2.17) | 3.32 (1.76) |
| Injection Drug User (ref= non-IDU) | −2.44 (1.95) | −1.14 (1.75) | −0.22 (2.39) | −0.23 (1.68) |
| Days of Cocaine Use, Past 30 days | −0.02 (0.08) | −0.22 (0.07)** | −0.13 (0.10) | −0.09 (0.07) |
| 3 months (ref= baseline) | 3.05 (1.86) | −2.13 (1.67) | 1.27 (2.35) | −1.53 (1.60) |
| 6 months (ref= baseline) | 2.19 (1.72) | −2.95 (1.54) | 3.71 (2.18) | −0.23 (1.47) |
| (Intercept) | 81.47 (7.46) | 76.11 (6.76) | 82.99 (8.27) | 66.21 (6.58) |
< 0.05
< 0.01.
Notes: ref=reference group. b = unstandardized partial regression coefficient. SE = standard error. Treatment enrollment status, days of opiate and cocaine use, and urine tests are time-varying predictors.
Relationship between Opioid Use and Quality of Life
As shown in Table 2, the model indicates that opioid-positive urine tests obtained at each interview point were not significantly associated with QoL in any of the four QoL domains. There was a statistically significant, negative relationship between self-reported days of opioid use in the past 30 days and psychological QoL, such that each additional day of opioid use was associated with a decrease in the psychological QoL score of 0.22 points (b= −.22; SE= .07; p< .01). Thus, a participant reporting opioid use every day in the past 30 days would be expected to have a 6.6 point-lower psychological QoL score than a participant reporting no use. However, self-reported number of days of opioid use in the past 30 days was not significantly associated with QoL scores in the physical, social, or environmental domains (all ps>.05).
Differential Relationship between Quality of Life and Opiate Use by Treatment Enrollment Status
To examine the possibility that the relationship between opioid use and QoL differs based on whether or not participants remain enrolled in treatment, the models were extended to include interactions between opioid positive urine tests and treatment enrollment status, and self-reported past 30 day opioid use and treatment enrollment status. These models revealed no significant interactions between opioid urine test results and treatment status (p= .81, .33, .52, and .42 for physical, psychological, social, and environmental QoL, respectively). Thus, treatment enrollment status does not appear to factor into the lack of a relationship between urine test results and QoL. There was no significant interaction between self-reported days of opioid use and treatment enrollment status for physical (p= .44), psychological (p= .23), or environmental (p= .44) domains. There was a significant interaction between self-reported days of opioid use and treatment enrollment status (b= −.61; SE= .29; p< .05), such that days of opioid use had a stronger, negative relationship with social QoL if the participant was enrolled in treatment than if they had discontinued treatment. For all domains, a sizeable minority (~25%) of those who remained in treatment through 6 months reported a net deterioration in quality of life from baseline.
It is important to note that in this study, 28% of participants who were no longer in treatment at 6 month follow-up nevertheless had a positive urine test for buprenorphine. Thus, being out of treatment does not necessarily equate to being off of buprenorphine entirely, although the frequency or regularity of buprenorphine use in a non-treatment context (i.e., street buprenorphine) is unknown. Among those who were out of treatment at 6 months, the presence of buprenorphine in urine was not associated with other opiate-positive urine test results, as rates of opiate-positive urine tests at 6-months were 79% and 75% for out-of-treatment participants who tested buprenorphine-positive vs. buprenorphine-negative at 6 months, respectively.
Other Predictors of Quality of Life
The models identified several significant predictors of QoL that were not an explicit focus of the inquiry (and were therefore included as control variables), but may nevertheless lend some insights about what factors shape QoL for opioid-dependent patients starting buprenorphine treatment. Higher age was associated with lower QoL in the physical (p< .05) and social (p< .05) domains, with each additional year of age associated with a decrease of approximately 1/3 of a point. On average, women had physical QoL scores approximately 4.5 points lower than men (p< .05). Injection drug use was not associated with QoL in any of the four domains. Finally, although days of cocaine use in the past 30 days was not associated with QoL in three domains (physical, social, or environmental), each additional day of cocaine use was associated with a .22-point decrease in the psychological QoL score (p< .01).
Discussion
The present study with 300 opioid-dependent participants entering buprenorphine treatment found that participants who remained in treatment had significantly higher scores at 3 and 6 month follow-ups on two of the four QoL scales on the WHOQOL-BREF (psychological and environmental), compared to participants who left treatment. In addition, participants in treatment at 6-month follow-up rated their QoL higher on the single overall QoL item than those who were no longer enrolled in treatment, even though these groups had comparable ratings on this item at baseline. Thus, although the sample as a whole reported significant improvements in QoL scores over time, remaining in treatment was associated with higher QoL on some measures, including the overall rating as well as scores for psychological and environmental scales.
Our findings are consistent with previous studies with methadone patients that examined changes in QoL scores on the WHOQOL-BREF, although such studies were typically limited to those who were retained in treatment. One study in Taiwan found improvements in QoL in the psychological and environmental domains among methadone patients retained through 6 months (Chou et al., 2013). A study in Lithuania found that patients retained in methadone treatment through 6 months had significant improvements in physical, psychological, and environmental domains (Padaiga et al., 2007), while a study in Malaysia found significant improvements in all QoL domains among methadone patients enrolled in treatment through 6 months. The only paper we found that examined differences in QoL between retained and not retained patients treated with buprenorphine failed to find a significant difference at 6-month follow-up using the German version of the Lancashire QoL Profile (Giacomuzzi et al., 2005). It is possible that the lack of significance was related to that study’s sample size (n=24).
Miller and Miller (Miller & Miller, 2009) noted that the extent to which drug abuse treatment improves the lives of patients rather than simply reducing their drug use may improve treatment retention and patient outcomes. It may be that the patients’ subjective feeling of improvement from treatment admission in their psychological and environmental functioning may have contributed to their retention in treatment. Alternatively, staying in treatment may have led to improvements in perceived QoL. While our study indicates that QoL differed on some dimensions between those who remained in treatment and those who discontinued treatment, the causal direction of this relationship is difficult to determine. Our hypothesis that participants retained in treatment would have, on average, better QoL outcomes than those not retained in treatment was confirmed for the psychological and environmental scales, but not for the physical and the social scales. It is possible that patients with medical problems did not receive relevant medical services or were not compliant with medical recommendations.
The social scale consists of three items inquiring about satisfaction with: 1. personal relationships; 2. sex life; and, 3. support from friends. In our study, the inverse relationship between self-reported days of opioid use and the score on the social QoL scale was more pronounced for participants who remained enrolled in treatment than for those who left treatment. This finding might be attributable to the negative sexual side effects of buprenorphine coupled with additional opiate use or possibly the difficulty that drug-dependent patients have in changing the people with whom they associate. Promoting the change in social networks from drug using to non-drug using individuals has been an important feature of 12-step groups such as Alcoholics Anonymous and Narcotics Anonymous, and has been the focus of specific interventions to encourage such change (Latkin, Sherman, & Knowlton, 2003).
Our study found that opiate urine test results obtained at each interview point were not associated with scores on any of the QoL scales. The number of days in which participants reported using opioids was inversely related to the score on the psychological scale only. Thus, our hypothesis that drug use would be negatively associated with QoL was largely not supported. In a study among Malaysian methadone patients, opioid positive drug tests during treatment were not significantly associated with scores on the WHOQOL-BREF social, environmental, or psychological scales and were only weakly correlated with the scores on the physical scale (Baharom et al., 2012).
The discrepancy found between self-reported drug use and drug testing results is not entirely surprising, as they measure drug use over different periods of time. Self-reported drug use has been shown to be relatively reliable under research conditions (Darke et al., 1992) such as those in the present study, in which responses were not shared with treatment providers or public safety officers and a federal certificate of confidentiality had been obtained. It is possible that changes in drug use over the 30 days prior to the interview were reliably reported and that urine test results were valid over too narrow a window to correlate with changes in the QoL scale. Given the great importance granted to urine testing results in clinical care and clinical trials, it is noteworthy that sharp decreases in self-reported drug use over the past 30-days was associated with improvement in QoL measures but negative urine tests at follow-up points were not. However, in the field of treatment for illicit drug use disorders, it has been difficult to reach consensus on what constitutes clinically significant improvement as contrasted with statistically significant. For example, it is possible to show that, as a group, patients taking methadone, when compared to their status at admission or to placebo treatment controls, show statistically significant reductions in percentage urine tests positive for illicit opioids (Mattick, Breen, Kimber, & Davoli, 2009). It can be argued that a reduction in heroin use from several times a day to just a few days a week should be associated with decreased risk of viral or bacterial infections, overdose, and other heroin-related hazards, but many professionals and people in recovery from dependence argue that such reduction does not represent improvement unless there is evidence of some improvement in quality of life (and sometimes even if there are accompanying quality of life improvements). The current study illustrates that drug use per se, whether measured by self-report or biological testing, may not align well with patients’ own perceptions of their QoL.
A minority of participants had a decrement in QoL from baseline to 6 month follow-up, despite being enrolled in treatment, including participants with confirmed opioid abstinence at follow-up. Hence, even some of those who would traditionally be considered treatment successes, when measured in terms of abstinence, may perceive that their life has gotten worse since starting treatment. This decrement in QoL could be due to exogenous factors and one might expect a subset of people to have misfortune and difficulties simply due to chance. This paradox of a significant minority of participants showing improvement on the most commonly used metrics of treatment improvement (cessation of illicit drug use) but simultaneously reporting decreases in QoL is worthy of additional exploration.
The study had some limitations. Since the patient population was restricted to African American patients in one city in the US being treated in two publicly-funded treatment centers, findings may not generalize to other ethnic groups, patients of other socio-economic status, other countries, or physician office-based treatments. Another limitation is that the clinical significance of statistically significant changes in QoL scores is currently not clear. The concept of a minimal clinical important difference (MCID) was developed by Jaeschke et al. (1989) to help interpret changes in patient reported outcomes. There have been attempts to define MCID in other chronic illnesses (Den Oudsten, Zijlstra, & De Vries, 2013; Kosinski, Zhao, Dedhiya, Osterhaus, & Ware, 2000; Lauridsen, Hartvigsen, Manniche, Korsholm, & Grunnet-Nilsson, 2006; Ringash, O’Sullivan, Bezjak, & Redelmeier, 2007) however, neither norms of QoL nor an MCID have been developed for illicit drug use disorders. Thus, how best to integrate measures of changes in drug use and QoL to capture the elusive notion of clinically significant improvement requires further research.
Acknowledgments
Funding for this study was provided by Grant No. 5RC1DA028407 (PI Mitchell) from the National Institute on Drug Abuse, which did not play a role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. We thank the National Institute on Drug Abuse for funding the study. We thank Partners in Recovery and Total Health Care, the two participating treatment programs.
References
- Baharom N, Hassan MR, Ali N, Shah SA. Improvement of quality of life following 6 months of methadone maintenance therapy in Malaysia. Substance Abuse Treatment, Prevention, and Policy. 2012;7:32. doi: 10.1186/1747-597X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999–0. 2012 Available at http://CRAN.R-project.org/package=lme4.
- Bizzarri J, Rucci P, Vallotta A, Girelli M, Scandolari A, Zerbetto E, Sbrana A, Lagher C, Dellantonio E. Dual diagnosis and quality of life in patients in treatment for opioid dependence. Substance Use & Misuse. 2005;40(12):1765–1776. doi: 10.1080/10826080500260800. [DOI] [PubMed] [Google Scholar]
- Chand PK, Mattoo SK, Sharan P. Quality of life and its correlates in patients with bipolar disorder stabilized on lithium prophylaxis. Psychiatry and Clinical Neurosciences. 2004;58(3):311–318. doi: 10.1111/j.1440-1819.2004.01237.x. [DOI] [PubMed] [Google Scholar]
- Chou YC, Shih SF, Tsai WD, Li CS, Xu K, Lee TS. Improvement of quality of life in methadone treatment patients in northern Taiwan: a follow-up study. BMC Psychiatry. 2013;13:190. doi: 10.1186/1471-244X-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler RA, Kivlahan DR, Donovan D, Mattson ME. Assessing nondrinking outcomes in combined pharmacotherapy and psychotherapy clinical trials for the treatment of alcohol dependence. Journal of Studies on Alcohol. 2005;(Suppl 15):110–118. doi: 10.15288/jsas.2005.s15.110. discussion 192–113. [DOI] [PubMed] [Google Scholar]
- Clinical Trials Network. Treatment effect & assessment measures task force, executive summary. 2010 Apr 6; Available at http://ctndisseminationlibrary.org/PDF/522.pdf.
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. British Journal of Addiction. 1992;87(5):733–742. doi: 10.1111/j.1360-0443.1992.tb02719.x. [DOI] [PubMed] [Google Scholar]
- Den Oudsten BL, Zijlstra WP, De Vries J. The minimal clinical important difference in the World Health Organization Quality of Life instrument--100. Supportive Care in Cancer. 2013;21(5):1295–1301. doi: 10.1007/s00520-012-1664-8. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Chopra A. Does buprenorphine maintenance improve the quality of life of opioid users? Indian Journal of Medical Research. 2013;137(1):130–135. [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, Marlatt GA, Preston KL, Selzer JA, Somoza EC, Wakim PG, Wells EA. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107(4):694–708. doi: 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D the Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New England Journal of Medicine. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Giacomuzzi SM, Ertl M, Kemmler G, Riemer Y, Vigl A. Sublingual buprenorphine and methadone maintenance treatment: a three-year follow-up of quality of life assessment. The Scientific World Journal. 2005;5:452–468. doi: 10.1100/tsw.2005.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri OP, Srivastava M, Shankar R. Quality of life and its correlates among substance dependent subjects: A study from a tertiary care centre in northeastern part of India. International Journal of Medicine and Medical Sciences. 2013;3(6):464–469. [Google Scholar]
- Gryczynski J, Mitchell SG, Jaffe JH, O’Grady KE, Olsen YK, Schwartz RP. Leaving buprenorphine treatment: Patients’ reasons for cessation of care. Journal of Substance Abuse Treatment. 2014;46(3):356–361. doi: 10.1016/j.jsat.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha N. The effect of methadone maintenance treatment in improvement of quality of life for heroin users in Hai Phong, Vietnam. Paper presented at the The 4th International Conference on Reproductive Health and Social Sciences Research; Bangkok, Thailand. 2010. [Google Scholar]
- Hanestad BR, Rustoen T, Knudsen O, Jr, Lerdal A, Wahl AK. Psychometric properties of the WHOQOL-BREF questionnaire for the Norwegian general population. Journal of Nursing Measurement. 2004;12(2):147–159. doi: 10.1891/jnum.2004.12.2.147. [DOI] [PubMed] [Google Scholar]
- Hawthorne G, Herrman H, Murphy B. Interpreting the WHOQOL-BREF: Preliminary population norms and effect sizes. Social Indicators Research. 2006;77:37–59. [Google Scholar]
- Hothorn T, Bretz F, Westfal P, Heiberger R, Schuetzenmeister A. Multcomp: Simultaneous Inference in General Parametric Models (Version 1.2–15) 2013. Computer software. [Google Scholar]
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug and Alcohol Dependence. 1995;40(1):17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267(20):2750–2755. [PubMed] [Google Scholar]
- Karow A, Verthein U, Pukrop R, Reimer J, Haasen C, Krausz M, Schafer I. Quality of life profiles and changes in the course of maintenance treatment among 1,015 patients with severe opioid dependence. Substance Use & Misuse. 2011;46(6):705–715. doi: 10.3109/10826084.2010.509854. [DOI] [PubMed] [Google Scholar]
- Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis & Rheumatology. 2000;43(7):1478–1487. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Sherman S, Knowlton A. HIV Prevention Among Drug Users: Outcome of a Network-Oriented Peer Outreach Intervention. Health Psychology. 2003;22(4):332–339. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskeletal Disorders. 2006;25(7):82. doi: 10.1186/1471-2474-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Farabee D, Liepa D, Wu LT. The Treatment Effectiveness Assessment (TEA): an efficient, patient-centered instrument for evaluating progress in recovery from addiction. Substance Abuse and Rehabilitation. 2012;3(1):129–136. doi: 10.2147/SAR.S38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Pacini M, Perugi G. Substance use and quality of life over 12 months among buprenorphine maintenance-treated and methadone maintenance-treated heroin-addicted patients. Journal of Substance Abuse Treatment. 2007;33(1):91–98. doi: 10.1016/j.jsat.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2008;(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. The Journal of Nervous and Mental Disease. 1992;180(2):101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller PG, Miller WR. What should we be aiming for in the treatment of addiction? Addiction. 2009;104(5):685–686. doi: 10.1111/j.1360-0443.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- Mitchell SG, Gryczynski J, Schwartz RP, O’Grady KE, Olsen YK, Jaffe JH. A randomized trial of intensive outpatient (IOP) vs. standard outpatient (OP) buprenorphine treatment for African Americans. Drug and Alcohol Depend. 2013;128(3):222–229. doi: 10.1016/j.drugalcdep.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noerholm V, Groenvold M, Watt T, Bjorner JB, Rasmussen NA, Bech P. Quality of life in the Danish general population--normative data and validity of WHOQOL-BREF using Rasch and item response theory models. Quality of Life Research. 2004;13(2):531–540. doi: 10.1023/B:QURE.0000018485.05372.d6. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Smith K, Couston M, Cossar JA, Hayes PC. A comparison of the WHOQOL-100 and the WHOQOL-BREF in detecting change in quality of life following liver transplantation. Quality of Life Research. 2000;9(1):121–124. doi: 10.1023/a:1008901320492. [DOI] [PubMed] [Google Scholar]
- Padaiga Z, Subata E, Vanagas G. Outpatient methadone maintenance treatment program. Quality of life and health of opioid-dependent persons in Lithuania. Medicina (Kaunas) 2007;43(3):235–241. [PubMed] [Google Scholar]
- Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. The American Journal of Drug and Alcohol Abuse. 2007;33(5):631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Raisch DW, Campbell HM, Garnand DA, Jones MA, Sather MR, Naik R, Ling W. Health-related quality of life changes associated with buprenorphine treatment for opioid dependence. Quality of Life Research. 2012;21(7):1177–1183. doi: 10.1007/s11136-011-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110(1):196–202. doi: 10.1002/cncr.22799. [DOI] [PubMed] [Google Scholar]
- Skevington SM, Lotfy M, O’Connell KA, Group W. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research. 2004;13(2):299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R. Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction. 2012;107(4):709–718. doi: 10.1111/j.1360-0443.2011.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy EM, Laudet AB, Min MO, Kim H, Brown S, Jun MK, Singer L. Prospective patterns and correlates of quality of life among women in substance abuse treatment. Drug and Alcohol Depend. 2012;124(3):242–249. doi: 10.1016/j.drugalcdep.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PW, Wu HC, Yen CN, Yeh YC, Chung KS, Chang HC, Yen CF. Change in quality of life and its predictors in heroin users receiving methadone maintenance treatment in Taiwan: an 18-month follow-up study. The American Journal of Drug and Alcohol Abuse. 2012;38(3):213–219. doi: 10.3109/00952990.2011.649222. [DOI] [PubMed] [Google Scholar]
- WHOQOL GROUP. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychological Medicine. 1998;28(3):551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The World Health Organization Quality of Life (WHOQOL) - Bref. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- Xiao L, Wu Z, Luo W, Wei X. Quality of life of outpatients in methadone maintenance treatment clinics. Journal of Acquired Immune Deficiency Syndromes. 2010;53(Suppl 1):S116–120. doi: 10.1097/QAI.0b013e3181c7dfb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubaran C, Foresti K. Quality of life and substance use: concepts and recent tendencies. Current Opinion in Psychiatry. 2009;22(3):281–286. doi: 10.1097/yco.0b013e328328d154. [DOI] [PubMed] [Google Scholar]