Abstract
Hippocampal atrophy is associated with memory impairment and dementia and serves as a key biomarker in the preclinical stages of Alzheimer's disease. Physical activity, one of the most promising behavioral interventions to prevent or delay cognitive decline, has been shown to be associated with hippocampal volume; specifically increased aerobic activity and fitness may have a positive effect on the size of the hippocampus. The majority of older adults, however, are sedentary and have difficulty initiating and maintaining exercise programs. A modestly more active lifestyle may nonetheless be beneficial. This study explored whether greater objectively measured daily walking activity was associated with larger hippocampal volume. We additionally explored whether greater low-intensity walking activity, which may be related to leisure-time physical, functional, and social activities, was associated with larger hippocampal volume independent of exercise and higher-intensity walking activity. Segmentation of hippocampal volumes was performed using FMRIB's Software Library (FSL) and daily walking activity was assessed using a step activity monitor (SAM) on 92, non-demented, older adult participants. After controlling for age, education, body mass index (BMI), cardiovascular disease risk factors, and the Mini Mental State Exam (MMSE), we found that a greater amount, duration, and frequency of total daily walking activity were each associated with larger hippocampal volume among older women, but not men. These relationships were specific to hippocampal volume, compared to the thalamus, used as a control brain region, and remained significant for low-intensity walking activity, independent of moderate- to vigorous-intensity activity and self-reported exercise. This is the first study, to our knowledge, to explore the relationship between objectively measured daily walking activity and hippocampal volume in an older adult sample. Findings suggest the importance of better understanding whether increasing non-exercise, lifestyle physical activities may produce measurable cognitive benefits and effect hippocampal volume through molecular pathways unique to those related to moderate-intensity exercise.
Keywords: aging, physical activity, African Americans, cognition, brain
Introduction
Given the disappointing results of numerous dietary and pharmaceutical studies and primary prevention trials to delay or halt Alzheimer's Disease (AD) (Daviglus et al., 2010), focus has shifted towards more promising behavioral and activity-based approaches to prevent or delay cognitive decline. The relationship between increased physical activity and reduced risk of AD (Abbott et al., 2004; Buchman et al., 2012; Larson et al., 2006; Podewils et al., 2005) as well as increased physical activity and cognitive health (Angevaren et al., 2008; Lautenschlager et al., 2008; Rockwood and Middleton, 2007; Weuve et al., 2004; Yaffe et al., 2001) have been shown in a number of large, epidemiological studies.
Animal models have provided much of the neurobiological evidence linking physical activity to enhanced brain function, implicating the hippocampus as particularly structurally and functionally sensitive to exercise (Cotman and Berchtold, 2007), a subcategory of physical activity. In mice models, voluntary exercise increases cell proliferation in the adult hippocampus (van Praag et al., 1999a; van Praag et al., 1999b) and reduces age-dependent decline in hippocampal neurogenesis (Kronenberg et al., 2006); this relationship may be partially mediated through trophic factors, including insulin-like growth factor 1 (IGF-1) and brain-derived neurotrophic factor (BDNF) (Ferris et al., 2007; Vaynman et al., 2004) and increased cerebral blood flow and angiogenesis (Pereira et al., 2007; van Praag et al., 2005). Mice with access to a running wheel have additionally shown better spatial learning and memory on water maze tests (Adlard et al., 2004; van Praag et al., 1999b).
Recent neurobiological evidence in human models have began to link physical activity to key neurocognitive pathways vulnerable to dementia. Research has shown that exercise training may reduce brain atrophy in non-demented individuals (Colcombe et al., 2006; Colcombe et al., 2004; Erickson et al., 2010). Exercise and fitness may have a positive effect specifically on the size of the hippocampus (Erickson et al., 2011; Erickson et al., 2010; Erickson et al., 2009). Hippocampal atrophy is associated with memory impairment and dementia (Driscoll et al., 2003; Jack et al., 2009; Mueller et al., 2010) and may serve as a key biomarker in early and presymptomatic diagnosis of AD (Cummings, 2009; Jack et al., 2013). Thus, identifying modifiable factors which can have a positive effect on the hippocampus is critical for preventive and therapeutic strategies to preserve cognitive health and delay the onset of cognitive impairment (Fotuhi et al., 2012).
The majority of neurobiological evidence showing the benefits of physical activity on cognition has indicated that exercise and improvements in cardiovascular fitness are associated with structural and functional benefits. Physical activity, however, includes a broad range of activities, in addition to exercise that increase energy expenditure above a resting level (Howley, 2001; “What is Physical Activity?,” 2011). These activities can include non-exercise leisure-time and life-style activities (e.g. walking, gardening, etc.) and instrumental activities of daily living (IADLs) (e.g. shopping, housework, etc.), which are typically in the low-intensity range. Research using self-report measures of walking activity indicates that these non-exercise physical activities may be associated with cognitive health benefits (Scarmeas et al., 2001; Yaffe et al., 2001). More recently, studies using objective measures of physical activity found that total physical activity and daytime movement, including both exercise and non-exercise physical activity, as well as total energy expenditure, were associated with better cognitive function, lower odds of cognitive impairment, and reduced risk of AD (Barnes et al., 2008; Buchman et al., 2012; Buchman et al., 2008; Middleton et al., 2011).
Research into the cognitive benefits of low-intensity physical activity is extremely important considering that the majority of older adults are sedentary (Harvey et al., 2013; “One in five adults meet physical activity guidelines,” 2013) and have difficulty initiating and adhering to exercise programs (Resnick and Spellbring, 2000; Schutzer and Graves, 2004). This is of particular concern for older adults of low socio-economic status (SES) who have low baseline levels of physical activity, and fewer physical activity-related facilities due to restrictive environmental and neighborhood characteristics (Day, 2006; Parra-Medina et al., 2010; Physical Activity Guidelines Advisory Committee Report, 2008; Powell et al., 2006). For older adults who may not participate in or have access to formal exercise programs, a more active lifestyle may be beneficial.
To our knowledge, no study has yet explored the relationship between objectively measured daily physical activity and brain structure, as well as the independent relationship between low-intensity physical activity and brain structure, in an older adult cohort. We therefore explored the cross-sectional association between objectively measured daily walking activity and hippocampal volume in a non-demented, older, mostly sedentary cohort at elevated socio-demographic risk for cognitive and functional decline.
Materials and Methods
Participants
Participants were from the Brain Health Study (BHS), a sub-study within the larger Baltimore Experience Corps Trial (BECT), a sex-stratified randomized, controlled effectiveness trial to evaluate the health benefits for older adults participating in Experience Corps Baltimore, a high-intensity volunteer service program, vs. a control group offered other low-service volunteer opportunities. Details on sex-stratification, randomization, study design, sampling methodology, and recruitment have been described previously (Fried et al., 2013; Fried et al., 2004). Enrollment criteria included : aged ≥ 60 years; ≥24 on the Mini-Mental State Exam (MMSE) (Folstein et al., 1975); and ability to read at a minimum 6th grade level (Wilkinson, 1993). BHS enrollment criteria have been described previously (Agbedia et al., 2011; Carlson et al., 2014; Chuang et al., 2013), and included right-hand dominance; free of a pacemaker or other ferrous metals in the body; and no history of brain cancer or brain aneurism/ stroke in the past year.
Of 123 participants enrolled in the BHS, 10 participants did not complete the MRI evaluation due to excessive head movement or claustrophobia, and 21 did not complete or provide usable data for the objective walking activity assessment (see below for exclusion criteria). The final usable sample included 92 participants. The baseline evaluation occurred prior to randomization to BECT intervention or control groups. Participants in the final study sample did not vary significantly (p<0.05) from the remaining BECT participants on any socio-demographic or health characteristic at baseline other than sex. The study protocol was approved by the Johns Hopkins School of Medicine Institutional Review Board and each participant provided written informed consent.
Walking Activity Measure
Walking activity was measured using a step activity monitor (SAM; Orthocare Innovations, Mt. Terrace, WA), an accelerometer that is worn on the dominant ankle and measures step activity in daily life over continuous periods of time. The device measures the number of steps at one-minute intervals using acceleration, position, and timing information, and can therefore characterize the amount, duration and frequency of daily walking activity. The SAM has been validated across a range of community-dwelling older adult populations with varying levels of function using self-report and objective measures (e.g. hand-tallied step counts and accelerometers) (Cavanaugh et al., 2007; Resnick et al., 2001; Storti et al., 2008). The SAM is particularly sensitive in measuring activity at decreased gait speeds (Storti et al., 2008), and is well tolerated by older adults because it is placed on the ankle vs. the hip (Algase et al., 2003).
Participants were instructed to wear the SAM for three to seven days while keeping a wear time/ activity diary at approximately one-hour intervals. Participants were instructed to remove the SAM only when bathing, showering or swimming, and replace the device immediately after. The majority of participants wore the SAM during the late summer and fall which reduced the influence of seasonal effects. Additionally, the majority of participants were not employed at baseline (79%), which reduced the influence of weekday/weekend effects. The data cleaning protocol included exclusion of days that represented noncompliance based on SAM inactivity and by participants' self-reported noncompliance in their activity diaries. Detailed cleaning protocol for a larger sample, of which participants in this study are a subsample, has been described previously (Varma et al., 2013).
In order to characterize the proportion of participants meeting physical activity guidelines, we used the 10,000 steps/day threshold developed in previous studies as a reasonable equivalent of U.S. physical activity guidelines (Tudor-Locke et al., 2008; Tudor-Locke and Bassett, 2004) (Table 1). We classified participants who met the 10,000 steps/day threshold across all days surveyed as active. Based on previous studies translating physical activity recommendations (30 minutes of moderate-intensity activity/day that can be split into three, 10 minute bouts) into a pedometer based step goal (3 bouts/day of 1,000 steps in 10 min (Marshall et al., 2009; Tudor-Locke et al., 2005), we additionally classified participants who met the 3 bouts/day threshold across all days surveyed as active.
Table 1. Baseline characteristics of Brain Health Study Subjects (N=92).
| Characteristic | N (%) or Mean ± SD | |
|---|---|---|
| Age (years) | 67.3 ± 6.1 | |
| Sex (women) | 64 (69.6) | |
| Race (African American) | 82 (89.1) | |
| Education (≤ high school) | 34 (37.0) | |
| Income (< $15,000) | 27 (29.4) | |
| MMSE | 28.4 (1.5) | |
| Chronic Disease | ||
| Obesity (BMI ≥ 30) | 52 (56.5) | |
| Hypertension | 66 (72.5) | |
| Diabetes | 29 (31.9) | |
| Brain volume a | ||
| Hippocampus (cm3) | 6.9 ± 0.8 | |
| Thalamus (cm3) | 15.1 ± 1.0 | |
| % meeting physical activity guidelines | ||
| 10,000 steps/dayb | ||
| Active (≥ 10,000 steps/day) | 11 (12.0) | |
| 30 minutes of moderate-intensity activityc | ||
| Active (≥ 30 min/day) | 0 (0.0) | |
| Daily walking activity metrics | ||
| Amount: | Steps/ day (total) | 7969.4 ± 3516.2 |
| Steps/ day at low-intensity d | 7212.1 ± 2814.3 | |
| Steps/ day at moderate- to vigorous-intensity e | 757.3 ± 1045.1 | |
| Duration: | Minutes of activity (total) | 337.8 ± 94.8 |
| Minutes of activity at low-intensity d | 330.9 ± 91.4 | |
| Minutes of activity at moderate- to vigorous-intensity e | 6.9 ± 9.6 | |
| Frequency: | Bouts of 10 min activity (total) | 11.9 ± 6.9 |
| Bouts of 10 min activity at low-intensity d | 10.5 ± 6.0 | |
| Bouts of 10 min activity at moderate- to vigorous-intensity e | 0.1 ± 0.4 | |
| Exercise | ||
| Total caloric expenditure/week f | 1209.3 ± 1616.8 | |
SD = standard deviation; MMSE = Mini Mental State Exam
Adjusted for intercranial volume (ICV)
10,000 steps/day considered an estimate of daily recommended walking activity
30 minutes/day of moderate-intensity activity (≥ 100 steps/min) considered an estimate of daily recommended walking activity
Low-intensity defined as walking activity at < 100 steps/min
Moderate- to vigorous-intensity is defined as walking activity at ≥ 100 steps/min
Assessed using the self-report of exercise-related physical activities from the Community Health Activities Model Program for Seniors (CHAMPS) questionnaire
Intensity ranges (effort associated with walking) included low-intensity (> 0 steps/min and < 100 steps/min) and moderate- to vigorous-intensity (≥ 100 steps/min) based on studies translating laboratory measurements of oxygen consumption while walking into pedometer-based metrics (Marshall et al., 2009; Tudor-Locke et al., 2005). Metrics representing components of activity, including amount, duration and frequency (Howley, 2001; Kesaniemi et al., 2001) within intensity ranges are described below and summarized in Table 1.
Activity amount was defined as the number of steps/day, and included total steps/day segmented into steps/day at low-intensity and at moderate- to vigorous-intensity. Activity duration was defined as the number of minutes/day of any activity, and included total minutes/day segmented into minutes/day at low-intensity and at moderate- to vigorous-intensity. Activity frequency was defined as the number of bouts/day of continuous 10-minute activity, and calculated by adding the number of times participants completed 10 minutes of activity. We included total bouts/day as well as bouts/day at low-intensity and at moderate- to vigorous-intensity. All metrics were averaged across all valid days surveyed.
MR Image Acquisition and Preprocessing
High resolution brain images were acquired on a 3.0T Phillips scanner (Best, the Netherlands) using a 3D T1-weighted MPRAGE sequence (Magnetization Prepared Rapid Gradient Echo Imaging) with the following parameters: repetition time (TR)= 8.037 ms; echo time (TE)= 3.7 ms; flip angle= 8°; 200 contiguous 1mm sagittal slices; FOV= 200 mm × 256 mm × 200 mm; matrix size=256mm × 256 mm; voxel size (1×1×1mm); protocol has been described previously (Carlson et al., 2009; Chuang et al., 2013). Segmentation of hippocampal and thalamus volumes, were performed using FMRIB's Integrated Registration and Segmentation Tool (FIRST) in FMRIB's Software Library (FSL) version 4.1 (Patenaude et al., 2011) and has been successfully used previously in older adult populations (e.g., (Erickson et al., 2011; Erickson et al., 2009) and validated against other automated methods and manual tracing (Eggert et al., 2012; Seixas and de Souza, 2010). FIRST is a model-based segmentation/ registration tool using a Bayesian framework from shape and appearance models obtained from manually segmented images from the Center for Morphometric Analysis, Massachusetts General Hospital, Boston. Briefly, images were first registered to MNI (Montreal Neurological Institute) 152 standard space using 2-stage affine transformations based on 12-degrees of freedom. A subcortical mask was then applied to exclude voxels outside the subcortical regions. Then the volumes were segmented with 30 modes of variation. Last, boundary correction was performed to classify the boundary voxels as belonging to the structure or not according to a statistical probability (z score > 3.00; p<0.001). Additional pre-processing steps included motion correction and non-uniform intensity normalization. All processed images were then visually inspected to identify any significant errors resulting from the segmentation process. No participants were excluded due to segmentation errors.
All brain volumes (hippocampus, thalamus) were adjusted for sex and height using a measure of intracranial volume (ICV) as a covariate in all analyses. ICV was calculated as the sum of gray, white, and cerebrospinal fluid using FMRIB's automated segmentation tool in FSL version 4.1 (Smith et al., 2004; Zhang et al., 2001), and used as a covariate in all analyses.
Covariates
In order to control for potential confounders of the relationship between daily walking activity and brain volume, all models included a number of covariates associated with both physical activity and hippocampal volume in prior studies. These included ICV, age, years of education, body mass index (BMI), cardiovascular disease (CVD) burden, exercise, and global cognitive function measured by the MMSE. All covariates were assessed at baseline. BMI was calculated using height and weight. CVD burden was calculated by summing participants' self-report of hypertension, diabetes, heart attack/ myocardial infarction, intermittent claudication, congestive heart failure, and angina/ chest pain due to heart disease. Self-reported exercise was assessed using the Community Health Activities Model Program for Seniors (CHAMPS)(Stewart et al., 2001) and included estimated caloric expenditure/week for 12 exercise-related physical activities: jogging/running, walking fast for exercise, aerobic machines, water exercises, swimming moderately/fast, swimming gently, stretching/ flexibility exercises, yoga/ tai-chi, aerobics/aerobic dancing, moderate/heavy strength training, light strength training, and general conditioning. The MMSE, a global test of cognitive function useful in quantitatively estimating the severity of cognitive impairment (Folstein et al., 1975), was administered by a trained evaluator.
Statistical Analysis
The main objective of the analyses was to explore whether components of daily walking activity, including amount, duration, and frequency, were associated with hippocampal volume independent of covariates. Multiple linear regression using Stata version 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) was used to model the relationship between the dependent variable (brain volume) and explanatory variables (e.g. daily walking activity) (Table 2, Table 3). The models were fit using the least squares approach to estimate model parameters. Standardized Beta (β) coefficients, standard errors (SE), and p-values of two-sided statistical tests are presented in Tables; F-statistics with degrees of freedom for the model and residuals, R2 values (e.g. percent of variance of brain volume explained by daily walking activity) t-statistics, and p-values, in addition to coefficient values as a percentage of mean hippocampal volume, are reported in the Results section. Pearson correlations between the explanatory variable of interest – daily walking activity – and confounders are also included in the Results section. In order to assess regional specificity of daily walking activity we explored the thalamus as a control region; this brain structure has been used previously to investigate regional specificity of physical activity (Erickson et al., 2011).
Table 2. Multiple linear regression models of daily walking activity and brain volume stratified by sex.
| Model 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||||
|
|
||||||||
| Hippocampus (cm3) | Hippocampus (cm3) | Thalamus (cm3) | Thalamus (cm3) | |||||
|
| ||||||||
| β | SE | β | SE | β | SE | β | SE | |
| Amount | ||||||||
|
| ||||||||
| Total steps/day (per 1000 steps increase) | 0.10** | 0.03 | -0.08 | 0.05 | 0.02 | 0.05 | -0.10 | 0.05 |
|
| ||||||||
| Age | -0.03* | 0.01 | 0.00 | 0.04 | -0.06** | 0.02 | -0.05 | 0.04 |
| CVD a | -0.21* | 0.09 | -0.59* | 0.24 | -0.15 | 0.12 | -0.55 | 0.26 |
| Duration | ||||||||
|
| ||||||||
| Total minutes/day (per 10 minute increase) | 0.02* | 0.01 | -0.05 | 0.02 | 0.00 | 0.01 | -0.05 | 0.03 |
|
| ||||||||
| Age | -0.03* | 0.01 | 0.01 | 0.04 | -0.06** | 0.02 | -0.05 | 0.04 |
| CVD a | -0.20* | 0.09 | -0.61* | 0.23 | -0.13 | 0.13 | -0.55 | 0.26 |
| Frequency | ||||||||
|
| ||||||||
| Total bouts/day (per 1, 10-minute bout) | 0.04* | 0.02 | -0.04 | 0.02 | 0.01 | 0.02 | -0.05 | 0.03 |
|
| ||||||||
| Age | -0.03* | 0.01 | 0.00 | 0.04 | -0.06** | 0.02 | -0.05 | 0.04 |
| CVD b | -0.17 | 0.09 | -0.59* | 0.24 | -0.14 | 0.12 | -0.54 | 0.27 |
SE = standard error; CVD = cardiovascular disease burden;
p<0.05
p<0.01
Note: all models included the following covariates; intercranial volume (ICV), age, years of education, body mass index (BMI), CVD, and the Mini Mental State Exam (MMSE)
CVD calculated by summing participants self-report of vascular disease
Table 3. Multiple linear regression models of low-intensity daily walking activity and hippocampal volume stratified by sex.
| Model 2 | ||||
|---|---|---|---|---|
| Women | Men | |||
|
|
||||
| Hippocampus (cm3) | Hippocampus (cm3) | |||
|
| ||||
| Amount | β | SE | β | SE |
| Low-intensity steps/daya (per 1000 steps increase) | 0.10** | 0.04 | -0.16 | 0.10 |
|
| ||||
| Moderate- to vigorous-intensity steps/dayb (per 1000 steps increase) | 0.04 | 0.10 | 0.09 | 0.25 |
| Age | -0.03 | 0.01 | -0.01 | 0.04 |
| CVD c | -0.22* | 0.09 | -0.50* | 0.22 |
| Exercise d | 0.08** | 0.07 | 0.12 | 0.06 |
| Duration | ||||
|
| ||||
| Low-intensity min/daya (per 1000 steps increase) | 0.02* | 0.01 | -0.04 | 0.03 |
|
| ||||
| Moderate-intensity min/dayb (per 1000 steps increase) | 0.06 | 0.11 | -0.12 | 0.20 |
| Age | -0.03* | 0.01 | -0.01 | 0.04 |
| CVD c | -0.21* | 0.09 | -0.50* | 0.23 |
| Exercise d | 0.08** | 0.03 | 0.13 | 0.07 |
| Frequency | ||||
|
| ||||
| Low-intensity bouts/daya (per 1, 10-minute bout) | 0.04* | 0.02 | -0.04 | 0.04 |
|
| ||||
| Moderate- to vigorous-intensity bouts/dayb (per 1, 10-minute bout) | 0.06 | 0.21 | -0.38 | 0.79 |
| Age | -0.03* | 0.01 | -0.01 | 0.04 |
| CVD c | -0.18* | 0.09 | -0.49 | 0.23 |
| Exercise d | 0.08** | 0.03 | 0.12 | 0.06 |
SE = standard error; CVD = cardiovascular disease burden;
p<0.05
p<0.01
Note: all models included the following covariates; intercranial volume (ICV), age, years of education, body mass index (BMI), CVD, exercise, moderate- to vigorous-intensity walking activity, and the Mini Mental State Exam (MMSE)
Low-intensity defined as walking activity at < 100 steps/min
Moderate- to vigorous-intensity is defined as walking activity at ≥ 100 steps/min
CVD calculated by summing participants self-report of vascular disease
Exercise calculated as estimated caloric expenditure/week of self-reported exercise-related physical activities using the Community Health Activities Model Program for Seniors (CHAMPS) questionnaire
Model 1 explored the relationship between components of total daily walking activity and hippocampal volume as well as the control brain region. Model 2 explored whether objectively measured, low-intensity daily walking activity was associated with hippocampal volume independent of the relationship between exercise and hippocampal volume. Low-intensity walking activity, which may include non-exercise lifestyle activities (e.g. walking for pleasure) and IADLS (e.g. walking related to housework or shopping) (Physical Activity Guidelines Advisory Committee Report, 2008) and moderate- to vigorous-intensity walking activity were included as separate variables. Self-reported exercise and covariates included in Model 1 were additionally included in Model 2.
Analyses were stratified by sex a priori. The BHS was designed to allow for sex-stratification in analyses of brain volume (Fried et al., 2013) given differences in brain morphology and differences in the association between exercise and neurocognition (Coelho et al., 2012; Colcombe and Kramer, 2003; Vaughan et al., 2012). Additionally, in exploratory analysis in the non-stratified models, we observed a significant sex × walking activity interaction (not shown). Estimated effect sizes of steps/day (amount) and minutes of activity (duration) were expressed in units of 1000 steps/day and 10 minutes/day, respectively, based on previous studies as well as ease of interpretation (Sisson et al., 2010). The CHAMPS variable was log transformed due to its skewed distribution.
Results
Baseline Characteristics of Study Subjects
Table 1 presents baseline characteristics of the study sample. A large percentage of participants had low education (37.0% reporting high school or less education) and income (29.4% reporting household income less than $15,000). Participants were additionally at risk for cognitive and physical function decline due to high rates of chronic disease: 56.52% of participants were obese (BMI ≥ 30), 72.53% reported hypertension, and 31.87% reported diabetes. Women were more obese than men (P < .05) and did not vary significantly on any other socio-demographic or health characteristic.
According to the 10,000 steps/day walking activity guideline, 12.0% of participants were considered active. According to Department of Health and Human Services guidelines of 30 minutes of moderate-intensity activity/day, no participants met guidelines by completing three or more 10-minute bouts/day of 1000 steps/ bout. Results did not vary significantly by sex.
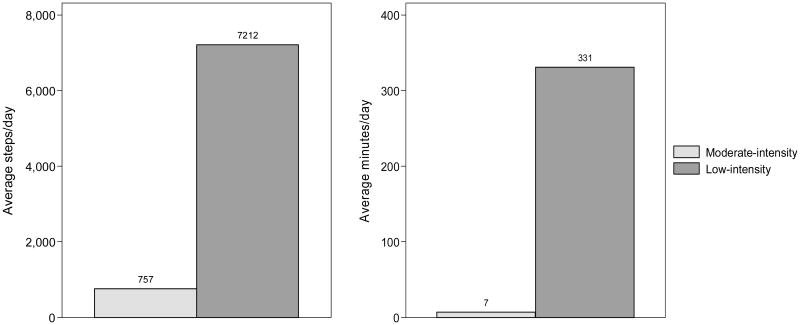
Participants completed a total of 7969.4 (SD: 3516.2) steps/day, and 337.9 (SD: 94.8) minutes of activity/day. The majority of activity was in the low-intensity range (7212.1 (SD: 2814.3); 90.5% of total steps/day and 330.9 min/day (SD: 91.4); 97.9% of total minutes of activity/day) with minimal activity in the moderate- to vigorous-intensity ranges (757.3 (SD: 1045.1); 9.5% of total steps/day and 6.9 min/day (SD: 9.6); 2.1% of total daily minutes of activity) (Figure 1). Participants averaged 11.9 (SD: 6.9) bouts/day of 10-minute activity. On average, participants expended 1209.2 (SD: 1616.8) calories/week in exercise-related physical activity. Women had a marginally greater number of low-intensity minutes of activity/day and fewer exercise-related calories/ week expended compared to men (P < .10).
Figure 1. Distribution of walking activity by intensity.
Moderate- to vigorous-intensity walking activity contributed to only 9.5% of total steps/day and 2.1% of total minutes of activity/day; Note. low-intensity activity: <100 steps/min; moderate- to vigorous-intensity activity: ≥ 100 steps/min
The mean volume of participants' hippocampus was 6.9 cm3 (SD: 0.8) and thalamus was 15.1 cm3 (SD: 1.0). Women, on average, had significantly smaller hippocampal and thalamus volumes, and ICV than men (P < .05).
Daily Walking Activity and Hippocampal Volume
Total steps/day, total minutes/day and total bouts/day were significantly correlated with BMI (r = -0.45, P < .01; r = -0.40, P < .01; r = -0.45, P < .01) and no other covariates in women. Daily walking activity metrics were not significantly correlated with any other covariates in men. As expected, low-intensity steps/day, minutes/day and bouts/day were each significantly correlated with moderate- to vigorous-intensity walking activity metrics in women (r = 0.32, P = .01; r = 0.27, P = .03; bouts/day not significantly correlated) and men (r = 0.80, P < .01; r = 0.52, P < .01; r = 0.60, P < .01).
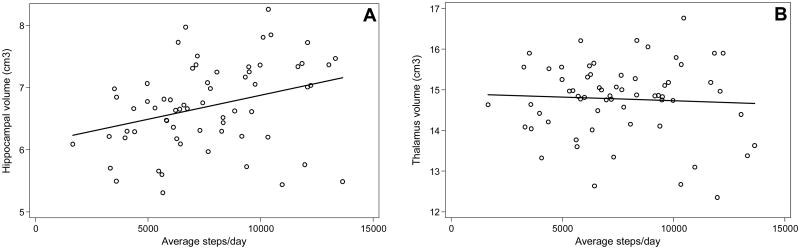
Table 2 presents the sex-stratified associations between walking activity metrics and hippocampal volume. In Model 1, after adjusting for ICV, age, years of education, BMI, cardiovascular disease burden, and MMSE, in women, an additional 1000 steps/day was significantly associated with a 0.10 cm3 larger hippocampal volume (F (7, 54) = 5.63; R2 = 0.42; t = 2.96; P < .01) (Figure 2); an additional 10 minutes/day of total walking activity was significantly associated with a 0.02 cm3 larger hippocampal volume (F (7, 54) = 4.94; R2 = 0.39; t = 2.35; P = .02); and an additional 10-minute bout of total walking activity was significantly associated with a 0.04 cm3 larger hippocampal volume (F (7, 54) = 5.11; R2 = 0.40; t = 2.52; P = .02). These associations were approximately 0.2-1.4% of the mean hippocampal volume of the sample.
Figure 2. Relationship between daily walking activity and brain volume.
A. Greater total daily walking activity was associated with larger hippocampal volume (adjusted for intracranial volume; ICV) in women. Associations remained significant for low-intensity daily walking activity, and after adding covariates exercise, moderate- to vigorous-intensity daily walking activity, age, years of education, body mass index (BMI), cardiovascular disease burden, and Mini Mental State Exam (MMSE).
B. Total daily walking activity was not significantly associated with thalamus volume in women.
Table 3 presents the sex-stratified associations between low-intensity walking activity metrics and hippocampal volume independent of moderate- to vigorous-intensity walking activity and exercise. In women, an additional 1000 steps/day in the low-intensity range was significantly associated with a 0.10 cm3 larger hippocampal volume (F (9, 52) = 6.04; R2 = 0.51; t = 2.88; P < .01); an additional 10 minutes/day of low-intensity walking activity was significantly associated with a 0.02 cm3 larger hippocampal volume (F (9, 52) = 5.57; R2 = 0.49; t = 2.44; P = .02); and an additional 10-minute bout of low-intensity walking activity was significantly associated with a 0.04 cm3 larger hippocampal volume (F (9, 52) = 5.54; R2 = 0.49; t = 2.50; P = .02).
In men across all models, metrics of daily walking activity were not significantly associated with hippocampal volume or thalamus volume.
Covariates and Hippocampal Volume
We displayed covariates of interest (i.e. age, CVD burden, and exercise) in Tables 2 and 3. As expected, in women, older age was significantly associated with smaller brain regions (hippocampus and thalamus) across all models. CVD burden was associated with smaller hippocampal volume in Model 1 and Model 2 in women, and in men associated with smaller hippocampal volumes in Model 1 and Model 2 (other than Model 2 - Frequency). Greater exercise in women was significantly associated with larger hippocampal volume in Model 2.
We explored the relationship between daily walking activity and memory function measured by the Rey Auditory Verbal Learning Test (RAVLT) and found no significant associations.
Discussion
We observed that greater daily walking activity was cross-sectionally associated with larger hippocampal volumes among older women, but not men, in a sample of non-demented, mostly sedentary older adults. As hypothesized, this relationship was specific to hippocampal volume, compared to the control brain region, thalamus volume, and remained significant for low-intensity walking activity, independent of moderate- to vigorous-intensity activity and self-reported exercise. Effect sizes in this sample ranged from 0.2-1.4% of average hippocampal volumes; considering annual hippocampal atrophy rates from 0.8-2.0% in healthy older adults (Barnes et al., 2009; Du et al., 2006; Fjell et al., 2009), these findings underscore the importance of exploring whether modest increases in non-exercise, lifestyle activities in the low-intensity range may promote cognitive health related to memory and reduced risk of dementia.
These study results add to a growing body of evidence suggesting that in humans, physical activity may be linked to key brain regions vulnerable to dementia including hippocampal volume. Prior evidence from human models suggests that moderate-intensity exercise and aerobic fitness may be associated with hippocampal volume (Erickson et al., 2011; Erickson et al., 2010; Erickson et al., 2009). Animal models have provided much of the mechanistic evidence supporting this relationship, suggesting that exercise may increase cerebral blood flow and angiogenesis, and may also promote neurogenesis through the upregulation of neurotrophic factors (Cotman and Berchtold, 2007; van Praag, 2008).
Recent research utilizing objective physical activity monitors that can sensitively measure a broad range of physical activities in-community, suggest that non-exercise physical activity may also be associated with cognitive health benefits (Buchman et al., 2012; Middleton et al., 2011). The results from this study expand this body of evidence to suggest that non-exercise walking activity within the low-intensity range may be associated with the same brain region most consistently shown to be effected by increased aerobic fitness and exercise. These findings encourage us to better understand whether increasing non-exercise, lifestyle physical activities may produce measurable cognitive benefits and effect hippocampal volume through molecular pathways unique to those related to moderate-intensity exercise (Voss et al., 2014). Additionally, these findings suggest that it may be useful to explore the extent to which environmental enrichment and associated molecular pathways may be critical to brain benefits independent of the intensity of physical activity within those contexts (van Praag et al., 2000).
Objective metrics of daily walking activity, including amount, duration, and frequency, may be important to accurately measure walking activity within a community setting, particularly among mostly sedentary older adults at elevated risk for cognitive and functional decline. Older adults within this cohort were mostly non-active by traditional standards for exercise; very few met estimated physical activity guidelines, and the majority of daily walking activity was within the low-intensity range. Low-intensity walking activity in this study sample may be related to lifestyle physical activities as well as functional activities (e.g. walking to catch a bus, shopping, housekeeping, and caretaking of grandchildren). By adjusting for moderate- to vigorous-intensity walking activity and exercise – as well as age, education, CVD burden, BMI and MMSE – all of which have been shown to be associated with hippocampal volume in older adults (e.g. (Erickson et al., 2011; Erickson et al., 2009; Gattringer et al., 2012; Jack et al., 1998; Noble et al., 2012; Szabo et al., 2011)), these findings indicate that low-intensity daily walking activity is associated with hippocampal volume independent of these covariates. Hippocampal atrophy is one of the strongest predictors of progression to AD (Henneman et al., 2009). The results of this study suggest the importance of better understanding the brain health benefits of interventions that may increase low-intensity walking activity, particularly for older adults who may be at high risk for functional decline and disability and may not be able to participate in moderate-intensity exercise.
In this study we found a clear sex difference in the relationship between daily walking activity and hippocampal volume. These findings are consistent with a meta-analysis showing that physical activity may be more cognitively beneficial for women (Colcombe and Kramer, 2003) as well as the results of a number of randomized-clinical trials of exercise (Baker et al., 2010; van Uffelen et al., 2008; Vaughan et al., 2012). Additionally, compared to men in this study, women were more obese, and expended fewer calories/ week in exercise-related activities. These risk factors may place women at elevated risk for physical and mobility difficulties, and therefore hippocampal volumes in older women may be more sensitive to modest increases in low-intensity walking activity.
This study has limitations. While the cognitive screening criteria used in the trial (MMSE≥24) as well as the inclusion of a cognitive covariate in all analytic models may account for the possibility of reverse causation, the cross-sectional design of the study precludes causal inferences. Future longitudinal analyses within a randomized-clinical trial design such as the BECT, will enable us to explore whether intervention related increases in non-exercise related physical activity may result in increased hippocampal volume. In addition, while we excluded moderate- to vigorous-intensity from the low-intensity walking activity metrics, and controlled for self-reported exercise, the SAM does not differentiate among types of activities. In future analyses, we hope to utilize daily activity diaries to better understand how various types of daily walking activities may be associated with cognitive health. Finally, while the study sample represented an understudied and at-risk segment of the older adult population, a trial of high-intensity volunteer service may select for more health conscious members of the community. Therefore generalization of findings to a larger population must be done so carefully.
To the best of our knowledge, this is the first study to explore the relationship between objectively measured total daily walking activity and hippocampal volume within a non-demented, community-dwelling cohort. The significant relationship between low-intensity daily walking activity and hippocampal volume, independent of moderate- to vigorous-intensity walking activity and self-reported exercise, indicates the importance of understanding the longitudinal benefits of modest increases in daily walking activity.
Acknowledgments
Grant information: Grant sponsor: National Institute on Aging; Grant numbers: P01 AG027735, 3P01AG027735-03S2, 3P01AG027735-02S3, K01AG031332, P30-AG021334, 5T32AG027668; Grant Sponsor: U.S. Public Health Service; Grant numbers: R01 HL077141, R01 HL089694, R21 CA127511, RC1 HL099340, and U01 AG022376; Grant Sponsor: John A. Hartford Foundation and the John D. and Catherine T. MacArthur Foundation; Johns Hopkins Neurobehavioral Research Unit
References
- Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. Jama. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Agbedia O, Varma V, Seplaki C, Seeman T, Fried L, Li L, et al. Blunted diurnal decline of cortisol among older adults with low socioeconomic status. Ann N Y Acad Sci. 2011:56–64. doi: 10.1111/j.1749-6632.2011.06151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algase DL, Beattie ER, Leitsch SA, Beel-Bates CA. Biomechanical activity devices to index wandering behavior in dementia. Am J Alzheimers Dis Other Demen. 2003;18:85–92. doi: 10.1177/153331750301800202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008;56:1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, et al. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009;30:1711–1723. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry. 2008;16:697–701. doi: 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- Carlson M, Kuo J, Chuang Y, Varma V, Harris G, Albert M, et al. Impact of the Baltimore Experience Corps Trial on Cortical and Hippocampal Volumes. Under Review. 2014 doi: 10.1016/j.jalz.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N, Mielke M, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J Gerontol A Biol Sci Med Sci. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Coleman KL, Gaines JM, Laing L, Morey MC. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. J Am Geriatr Soc. 2007;55:120–124. doi: 10.1111/j.1532-5415.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- Chuang YF, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, et al. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging. 2013;35:1396–1403. doi: 10.1016/j.neurobiolaging.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FM, Pereira DS, Lustosa LP, Silva JP, Dias JM, Dias RC, et al. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr. 2012;54:415–420. doi: 10.1016/j.archger.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement. 2007;3:S30–37. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Defining and labeling disease-modifying treatments for Alzheimer's disease. Alzheimers Dement. 2009;5:406–418. doi: 10.1016/j.jalz.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Jr, Cox NJ, et al. National Institutes of Health State-of-the-Science Conference statement: preventing alzheimer disease and cognitive decline. Ann Intern Med. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- Day K. Active living and social justice: planning for physical activity in low-income, Black, and Latino communities. Journal of the American Planning Association. 2006;72:88–99. [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, et al. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert LD, Sommer J, Jansen A, Kircher T, Konrad C. Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS One. 2012;7:e45081. doi: 10.1371/journal.pone.0045081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- Fried LP, Carlson MC, McGill S, Seeman T, Xue QL, Frick K, et al. Experience Corps: A dual trial to promote the health of older adults and children's academic success. Contemp Clin Trials. 2013 doi: 10.1016/j.cct.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Carlson MC, Freedman M, Frick KD, Glass TA, Hill J, et al. A social model for health promotion for an aging population: initial evidence on the Experience Corps model. J Urban Health. 2004;81:64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattringer T, Enzinger C, Ropele S, Gorani F, Petrovic KE, Schmidt R, et al. Vascular risk factors, white matter hyperintensities and hippocampal volume in normal elderly individuals. Dement Geriatr Cogn Disord. 2012;33:29–34. doi: 10.1159/000336052. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Chastin SF, Skelton DA. Prevalence of sedentary behavior in older adults: a systematic review. Int J Environ Res Public Health. 2013;10:6645–6661. doi: 10.3390/ijerph10126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33:S364–369. doi: 10.1097/00005768-200106001-00005. discussion S419-320. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesaniemi YK, Danforth E, Jr, Jensen MD, Kopelman PG, Lefebvre P, Reeder BA. Dose-response issues concerning physical activity and health: an evidence-based symposium. Med Sci Sports Exerc. 2001;33:S351–358. doi: 10.1097/00005768-200106001-00003. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Jama. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Marshall SJ, Levy SS, Tudor-Locke CE, Kolkhorst FW, Wooten KM, Ji M, et al. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med. 2009;36:410–415. doi: 10.1016/j.amepre.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Middleton LE, Manini TM, Simonsick EM, Harris TB, Barnes DE, Tylavsky F, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- One in five adults meet physical activity guidelines. 2013 May 2; 2013. Retrieved January 31, 2013, from http://www.cdc.gov/media/releases/2013/p0502-physical-activity.html.
- Parra-Medina D, Wilcox S, Wilson DK, Addy CL, Felton G, Poston MB. Heart Healthy and Ethnically Relevant (HHER) Lifestyle trial for improving diet and physical activity in underserved African American women. Contemp Clin Trials. 2010;31:92–104. doi: 10.1016/j.cct.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Heddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlated of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee Report. Washington D.C.: United States Department of Health and Human services; 2008. [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Powell LM, Slater S, Chaloupka FJ, Harper D. Availability of physical activity-related facilities and neighborhood demographic and socioeconomic characteristics: a national study. Am J Public Health. 2006;96:1676–1680. doi: 10.2105/AJPH.2005.065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick B, Nahm ES, Orwig D, Zimmerman SS, Magaziner J. Measurement of activity in older adults: reliability and validity of the Step Activity Monitor. J Nurs Meas. 2001;9:275–290. [PubMed] [Google Scholar]
- Resnick B, Spellbring A. The factors that influence exercise behavior in older adults. Journal of Gerontological Nursing. 2000;26:34–42. doi: 10.3928/0098-9134-20000301-08. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Middleton L. Physical activity and the maintenance of cognitive function. Alzheimers Dement. 2007;3:S38–44. doi: 10.1016/j.jalz.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39:1056–1061. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Seixas F, de Souza A. Anatomical brain MRI segmentation methods: volumetric assessment of the hippocampus. Paper presented at the 17th International Conference on Systems, Signals and Image Processing 2010 [Google Scholar]
- Sisson SB, Camhi SM, Church TS, Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps/day and metabolic syndrome. Am J Prev Med. 2010;38:575–582. doi: 10.1016/j.amepre.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Storti KL, Pettee KK, Brach JS, Talkowski JB, Richardson CR, Kriska AM. Gait speed and step-count monitor accuracy in community-dwelling older adults. Med Sci Sports Exerc. 2008;40:59–64. doi: 10.1249/mss.0b013e318158b504. [DOI] [PubMed] [Google Scholar]
- Szabo AN, McAuley E, Erickson KI, Voss M, Prakash RS, Mailey EL, et al. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology. 2011;25:545–553. doi: 10.1037/a0022733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C, Hatano Y, Pangrazi RP, Kang M. Revisiting “how many steps are enough? Med Sci Sports Exerc. 2008;40:S537–543. doi: 10.1249/MSS.0b013e31817c7133. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Sisson SB, Collova T, Lee SM, Swan PD. Pedometer-determined step count guidelines for classifying walking intensity in a young ostensibly healthy population. Can J Appl Physiol. 2005;30:666–676. doi: 10.1139/h05-147. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med. 2008;42:344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- Varma VR, Tan EJ, Wang T, Xue QL, Fried LP, Seplaki CL, et al. Low-Intensity Walking Activity is Associated with Better Health. Journal of Applied Gerontology. 2013 doi: 10.1177/0733464813512896. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan S, Morris N, Shum D, O'Dwyer S, Polit D. Study protocol: a randomised controlled trial of the effects of a multi-modal exercise program on cognition and physical functioning in older women. BMC Geriatr. 2012;12:60. doi: 10.1186/1471-2318-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Voss M, Carr L, Clark R, Weng T. Revenge of the “sit” II: Does lifestyle impact neuronal and cognitive health through distinct mechanisms associated with sedentary behavior and physical activity? Mental Health and Physical Activity. 2014 In Press. [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Jama. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- What is Physical Activity? Explore Physical Activity and Your Heart. 11 Sep 26; 2011. Retrieved 3/19/13, 2012, from http://www.nhlbi.nih.gov/health/health-topics/topics/phys/
- Wilkinson GS. WRAT3: Wide Range Achievement Test 3. Wilmington, DE: Wide Range Inc.; 1993. [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]