Abstract
Brown spot of pear is a fungal disease producing high economical losses in several pear-growing areas in Europe. Fungicide applications during the growing period either at fixed schedule or delivered according to the BSPcast forecasting system are not enough to control the disease under favorable conditions. New strategies have been introduced to control the inoculum production using sanitation methods. These methods are based on combinations of leaf litter removal during winter and biological control agent applications during late winter, spring and summer. These practices reduce both the inoculum pressure and disease levels. Therefore, the resulting optimized disease management consists of a combination of sanitation methods applied during the whole year with chemical fungicides scheduled according to the BSPcast forecasting model during the vegetative period. It is expected that the control of brown spot could be further refined upon availability of rapid methods for inoculum potential analysis. However, this analysis is difficult due to the variability in pathogenicity within the pathogen population.
Keywords: Stemphylium vesicarium, Pleospora allii, Sanitation measures, Inoculum production, Integrated disease management
Introduction
Brown spot of pear (Pyrus communis L.) is a disease caused by the fungus Stemphylium vesicarium (Wallr.) E. Simmons, that was first reported in 1975 in Italy in the Emilia-Romagna region, thereafter in 1984, it was observed in Girona (Spain), and in 1987, it was reported in Bouches du Rhône (France) (Blancard et al. 1989; Ponti et al. 1982; Vilardell 1988). In the recent years, new outbreaks have been reported in La Rioja (Spain), the Netherlands and Portugal, indicating that the spread of the disease has taken place within the main pear-growing areas in Europe (Heijne and Mourik 2001; Llorente and Montesinos 2006; Rossi et al. 2005b).
Brown spot of pear (BSP) symptoms consist of necrotic lesions on pear fruits, leaves and twigs. Symptoms on young fruits are usually located on the calyx, whereas on mature fruits, necrotic spots develop in the equatorial zone. Fruit lesions expand on the fruit surface and the secondary colonization by saprophytic fungi as Alternaria sp. may produce fruit rotting. Severe attacks on leaves can produce a premature defoliation. First, disease symptoms are observed in late spring and progressively increase until harvest. Losses of production have high economic impact in several Mediterranean pear-growing areas of Europe and the intensity of the disease depends on different factors as the inoculum level or the weather conditions, but global disease incidence may be estimated between 1 and 10%, with an impact comparable to apple scab in some areas (Montesinos and Vilardell 1992).
The causal agent is the Deuteromycete S. vesicarium that produces erect conidiophores with a single terminal conidium (Simmons 1969). S. vesicarium has been described as saprophyte (Ellis 1971; Simmons 1969) and as pathogenic on different plant species as well as pear, like garlic (Aveling and Naude 1992; Basallote Ureba et al. 1999), onion (Shishkoff and Lorbeer 1989), asparagus (Blancard et al. 1984; Falloon et al. 1987), and alfalfa (Lamprecht et al. 1984). The teleomorph corresponds to the Ascomycete Pleospora allii (Rabenh.) Ces. & De Not which produces pseudothecia with asci carrying eight yellow-brown ascospores (Simmons 1985).
Differences in susceptibility to the disease have been observed among pear cultivars. Cultivars Passe Crassane, Abate Fetel, Alexandrine and Conference are very susceptible, whereas cultivars Williams, Blanquilla, Beurre Hardy, Louis Bonne, Grand Champion and Highland are moderate or low susceptible (Blancard et al. 1989; Cavanni and Ponti 1994; Montesinos et al. 1995a). These differences in the susceptibility can be explained by the production of two host-specific toxins (SV-toxins I and II) (Singh et al. 1999, 2000). Most frequently cultivated and economically important pear cultivars are very susceptible to the disease in BSP affected areas. A difference in the susceptibility by aging has been reported, where young leaves and immature fruits are more susceptible than mature one (Montesinos et al. 1995a).
Epidemiology and inoculum production
The biological cycle is characterized by two kinds of inoculum: the sexual inoculum corresponds to ascospores of Pleospora allii, whereas the asexual inoculum consists of conidia of S. vesicarium (Fig. 1). It has been described that the sexual inoculum production occurs mainly during autumn and winter when pseudothecia of P. allii are formed mainly on pear leaf litter, but in the recent years, it has been demonstrated that the ascosporic season is more extense from late summer to early autumn. The most important ascospore production period occurs between February and June, and the second period between August and October, but ascospores are also trapped during July in pear orchards (Llorente and Montesinos 2006; Rossi et al. 2008). Although these ascospores are pathogenic to pear and are capable to produce infections on pear fruit and leaves (Llorente et al. 2006), their most important role probably consists of initiating the saprophytic colonization of pear debris on the orchard ground. The resulting mycelium produces conidia that become airborne and infect pear trees during the growing period (Llorente and Montesinos 2006a; Llorente et al. 2010a; Rossi et al. 2005b, 2008). Maturation of P. allii during winter and spring mainly depends on the temperature and relative humidity. During winter, pseudothecia developed only at high RH (>96%) and the optimum temperature for maturation was between 10 and 15°C (Llorente et al. 2006). Information is lacking on conditions that favor maturation of pseudothecia during the summer. As in other fungi, the key factor related to the release of mature ascospores is the rain (Llorente and Montesinos 2006; Llorente et al. 2008).
Fig. 1.
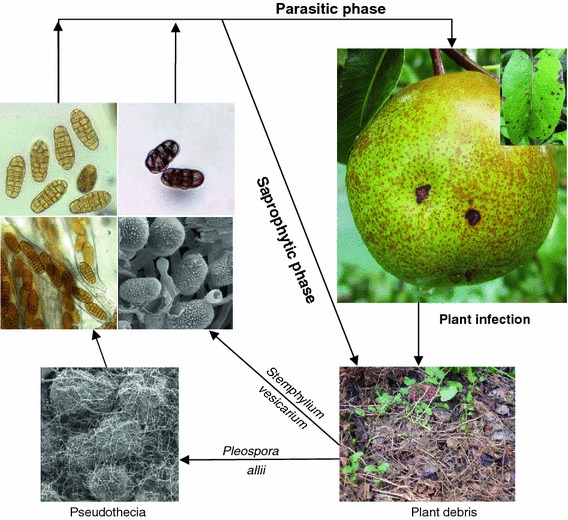
Parasitic and saprophytic phases in the life cycle of Stemphylium vesicarium and Pleospora allii on pear orchards
The conidial season takes place from April to November and the release of S. vesicarium conidia starts in April or May achieving a maximal production in summer, with more than 90% of conidia caught between July and September (Llorente and Montesinos 2006; Llorente et al. 2008; Rossi et al. 2005a). However, the production of S. vesicarium conidia on pear trees does not match with the airborne inoculum levels detected in this period. This fact can be explained on the basis of the existence of two phases, a pathogenic phase on the aerial pear organs during the pear-growing period and a saprophytic phase on the plant debris (pear and herbaceous) at the orchard ground, also in spring and summer. The existence of these two phases leads to a permanent colonization of the plant debris on the orchard over the year. In favor to this hypothesis, it has been demonstrated that under controlled environmental conditions, S. vesicarium colonizes different plant debris material and is able to produce high amounts of conidia at 20–25°C under high relative humidity conditions, which maintain their pathogenicity (Giosuè et al. 2006; Köhl et al. 2009a; Llorente and Montesinos 2006; Rossi et al. 2005b, 2008).
The optimal temperature for conidial germination ranges from 20 to 30°C with a very fast rate of germination (50% germinated conidia in 60 min) (Cugier and Humbert 1991; Montesinos and Vilardell 1992). The optimal conditions for disease establishment in susceptible cultivars are 20–25°C and leaf or fruit wetness, and under these conditions, a 6-h wetness period is enough to start infections (Montesinos et al. 1995b).
On the basis of the existing information, it is assumed that the inoculum is produced on plant debris at the orchard ground or in the neighboring orchards. Recent studies have demonstrated that the exclusion of soil inoculum reduces significantly the disease level, and dispersal patterns indicate that S. vesicarium inoculum moves only short distances (de Jong and Heijne 2008; Llorente et al. 2008; Rossi et al. 2008). In addition, the characterization of the airborne inoculum population has demonstrated that not all conidia trapped are pathogenic on pear and this may be explained by the saprophytic ability of S. vesicarium (Llorente et al. 2010a).
The detection and identification of S. vesicarium conidia and P. allii ascospores and the assessment of inoculum levels are usually achieved through spore trap devices and optical microscope observations. Currently, species differentiation is performed according to the morphological traits (Simmons 1969, 1985). Interestingly, qualitative and quantitative molecular tools have been developed for specific analysis of S. vesicarium and P. allii and molecular markers have been identified and used for differentiation of pathogenic and non-pathogenic S. vesicarium isolates in natural populations (Köhl et al. 2009a, b; Llorente et al. 2010a).
Forecasting models
Different forecasting models have been developed to determine the effect of environmental parameters on different stages of the biological cycle of the pathogen or the disease. The PAMcast model consists of a monomolecular mathematical function and was developed from controlled environmental and field observations to predict the percentage of mature pseudothecia on the basis of temperature and relative humidity during the winter (Llorente and Montesinos 2004). This model quantifies the effect of cumulative degree days (CDD) in maturation process of pseudothecia assuming that under high relative humidity, the development of P. allii is dependent on temperature and uses 0°C as the base temperature. The first mature pseudothecia usually are observed between December and February in Europe, depending on orchard conditions, but once pseudothecia began to mature, the development continued at a similar rate in response to degree day accumulation. Most pseudothecia are fully mature after 750 CDD and the release of mature ascospores is related to the rain or heavy dew. PAMcast may be used to determine the initiation of measures to prevent primary infections from debris colonization, and has been evaluated and validated under field conditions in pear orchards in several years (Llorente and Montesinos 2004).
The BSPcast model was developed to predict infection risk and has been evaluated and validated under very wide conditions. This model quantifies the effect of daily wetness duration and temperature during wetness periods on BSP disease (Llorente et al. 2000a, b; Montesinos et al. 1995a). Optimal conditions for infections are >24 h of continuous wetness at 22.5°C. Daily wetness duration and mean air temperature during wetness periods are used to compute a daily disease severity. Every day a relative daily infection risk (R) is calculated and then a cumulative daily infection risk (CR) is obtained by totaling R values for the past 3 days. Indexes R and CR are calculated every 24 h. The CR is used as an action threshold for spraying fungicides. In addition, the effect of interrupted wetness periods and relative humidity during the interruption was determined and incorporated into the BSPcast model. This effect concerns to wetness periods that should be considered interrupted if the length of interruption is ≥3 h at low relative humidity (Llorente and Montesinos 2002). BSPcast model has been evaluated and validated during several years in Spain and Italy (Llorente et al. 2000a; Montesinos et al. 1995a), and is currently used to schedule fungicide sprays.
Control of infections during the growing season
Chemical control
Chemical control is the most efficient method to control brown spot of pear. Disease control is based on the preventative sprays with fungicides applied at 7–14 day intervals. Most effective fungicides are dithiocarbamates (thiram and mancozeb), and strobilurins (kresoxim-metil, trifloxystrobin or pyraclostrobin), and other products as captan or tebuconazole (Brunelli et al. 1984, 1986, 1997; Llorente 1997; Ponti et al. 1993, 1996; Vilardell 1988). However, other fungicides are also suitable. The fungicide sprays start after petal fall and finish a few weeks before harvest. In orchards moderately to highly affected by the disease, 15–25 fungicide sprays are required to keep low levels of disease incidence in fruits (under 1–2%). This high number of fungicide applications may produce non-target effects in affected areas, and is not suitable for integrated production systems. In addition, isolates of S. vesicarium resistant to strobilurines have been reported (Alberoni et al. 2010) that reinforce the need for a rational use of fungicides against BSP. In addition, some applications of fungicides may be unnecessary because environmental conditions are not always suitable for infections by S. vesicarium. BSPcast is used as a tool to schedule fungicides and is currently used or tested in pear production areas of Spain, Portugal, Italy, Belgium and the Netherlands. Values of the cumulative daily infection risk CR = 0.4 or 0.5 are used as threshold to schedule fungicide sprays in Spain and Italy in orchards with moderate disease pressure. The BSPcast gives 30–40% fungicide savings in commercial orchards with similar efficacy as standard fungicide fixed schedules at moderate to low disease levels (Llorente et al. 2000a) (Fig. 2). However, under high disease pressure, fungicides are not sufficiently effective and the disease is not reduced to economically acceptable levels. Unfortunately, no curative fungicides are available to control BSP because once conidia germinate they begin to produce the SV-toxins and the inhibition of germination or germ tube elongation by the curative fungicides occurs too late, when the toxin has already been released and the necrosis will appear (Llorente 1997; Llorente and Montesinos 2006; Singh et al. 1999). Therefore, the timing of sprays before infection is critical for optimal efficacy.
Fig. 2.
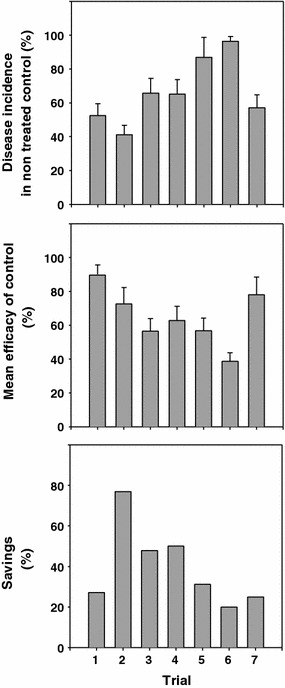
Savings of fungicide treatments according to BSPcast schedule in comparison to fixed spray timing in seven trials performed in Spain and Italy. The efficacy of control was calculated as reduction in the disease incidence (fruits with lesions %) at harvest relative to a non-treated control. The level of disease incidence on fruits in non-treated controls is also presented. Data correspond to field tests where no significant differences were observed between BSPcast and fixed spray schedule for disease control (modified from Llorente et al. 2000a)
To increase the efficacy of disease control using the BSPcast for scheduling fungicides, modifications were introduced into the model, mainly the use of a daily infection risk (R) instead of the 3-day cumulative infection risk (CR) to guide the fungicide sprays. However, modifications introduced did not result in increased disease control efficacy, as compared to the original BSPcast system (Llorente et al. 2011). Hence, new and complementary disease control strategies and methods need to be developed to further reduce the disease pressure in epidemics areas (Llorente et al. 2006, 2008).
Biological control
Several biological control agents have been evaluated for disease control on leaves and fruits. Trichoderma koningii and T. viride has been applied on trees, but the efficacy was very low (Ponti et al. 1993). A Pseudomonas fluorescens strain was selected among 400 potential biological control candidates for its efficacy in disease control under greenhouse conditions, but the biological control activity decreased when it was used under field conditions, probably due to the low survival on the pear leaf and fruit surface (Montesinos and Bonaterra 1996a; Montesinos et al. 1996). Therefore, much effort has to be done in the search of biocontrol agents able to control BSP in the aerial plant part.
Control of inoculum production
Complementary methods focused to decrease the inoculum are recommended, and mainly sanitation methods have been tested. Because two kinds of inoculum are produced, ascospores of P. allii and conidia of S. vesicarium, these strategies should be focused to the reduction of both. With this purpose, methods to assess the inoculum potential are critical. The inoculum potential is function of the pathogenicity and the amount of conidia or ascospores. The characterization of natural S. vesicarium/P. allii populations in terms of their pathogenic activity has been conducted. In a study performed in Spain during 2008 and 2009 (unpublished data), different isolates were obtained from several sources, mainly in pear orchards, and characterized onto their virulence (Fig. 3). The isolates showed different patterns of disease progression (Llorente et al. 2010a), but 40% of isolates were non-pathogenic, that is in agreement with the saprophytic ability of S. vesicarium. Among the pathogenic isolates, 26% showed a slow disease progress and in 19% of isolates the disease progress was slow at the beginning, but fast at the end. Most isolates showing these disease progression patterns were obtained from pear leaf debris, indicating that they probably need a period of time before infection. In addition, in 55% of isolates, corresponding to those obtained from infected organs, the disease progress was fast and final disease levels were high. The non-pathogenic group included 78% of air isolates, 50% of non-host isolates, 17% of leaf debris isolates and 2% of isolates from pear lesions. Therefore, the direct measurements of airborne inoculum using volumetric spore traps may overestimate the actual pathogen population.
Fig. 3.
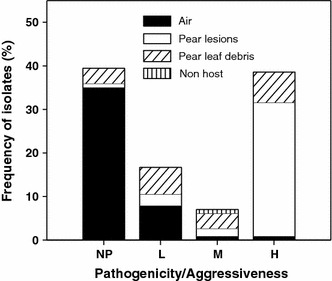
Pathogenicity and aggressiveness of S. vesicarium isolates recovered from different sources in pear orchards (NP non-pathogenic, L low, M moderate and H high aggressiveness). A total of 114 isolates were collected from 27 pear orchards in Northereastern Spain
To decrease the development of P. allii during the winter in the orchard ground and disrupt the biological cycle of the pathogen, several methods have been evaluated. Application of copper compounds and urea at different doses and timing during the autumn or winter does not decrease consistently the number of ascospores trapped (Llorente et al. 2006). Biocontrol methods using Trichoderma sp.-based products applied during the winter and spring have some efficacy in reducing the number of ascospores released. Methods of leaf shredding and leaf removal during winter are effective in reducing ascospores release. So, a strategy based on the control of P. allii during winter and spring was designed. This strategy is focused on sanitation practices consisting of removal leaf and fruit litter from the orchard floor in autumn in combination to applications of effective Trichoderma strains.
To reduce the S. vesicarium inoculum throughout the growing season in summer, applications of different Trichoderma sp. products are very promising as reported by Rossi and Pattori (2009). Laboratory and field trials using microplots showed that some Trichoderma-based products reduced in more than 90% conidia production by S. vesicarium.
Integrated control
Spraying fungicides during the pear-growing season is the main strategy to control BSP, but the efficacy of the fungicides, either for fixed or BSPcast-guided schedules, is low under high disease pressure, due to inoculum presence, favorable environmental conditions, or to the high susceptibility of pear cultivars. Sanitation methods on orchard ground consisting of combinations of leaf litter removal during winter and biological control agent applications during late winter and spring have been tested to increase the efficacy of disease control. An integrated disease management program (IDM) was evaluated in nine trials in Girona (Spain) and Ferrara (Italy) over a 4-year period. The IDM program consisted of sanitation methods and fungicide treatments. The sanitation methods were leaf litter removal from December to February and application of biological control agents (commercial formulates of Trichoderma spp.) to the orchard ground cover from February to May. Fungicides were also applied to the trees during the pear-growing season, scheduled according to the BSP-cast model. The different methods were tested as stand-alone applications or in combination. All methods consistently reduced disease incidence at harvest on fruit with an efficacy between 30 and 60% for leaf litter removal and more than 60% for combination of leaf litter removal and biological control. Efficacy of sanitation alone (leaf litter removal and biological control) in reducing the brown spot level on fruit was similar in most of the trials to the efficacy obtained when fungicides were applied alone (Fig. 4; Llorente et al. 2010b). Interestingly, these results open the possibility to use a disease control strategy in organic pear production because sanitation methods may be an alternative to the application of chemical fungicides. However, integration of sanitation methods and fungicides did not improve the efficacy of disease control over the level provided by fungicides alone.
Fig. 4.
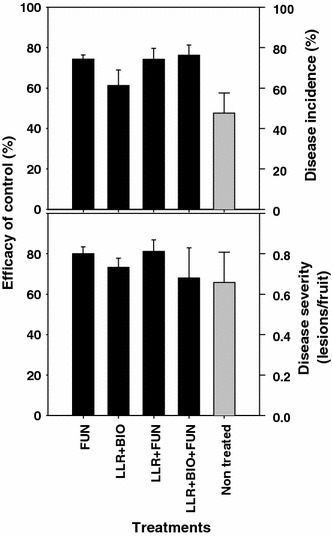
Efficacy of different treatments aimed at controlling brown spot of pear by sanitation. The efficacy (black bars) is expressed as reduction in the disease incidence (fruits with lesions %) or disease severity (number lesions/fruit) at harvest relative to a non-treated control. The level of disease in non-treated control is presented in gray bars. Treatments were applied alone or combined. FUN fungicide applications during the growing season, LLR pear leaf litter removal during the winter, BIO biological control using Trichoderma sp.-based products applied during winter or spring. Bars correspond to the mean standard error (modified from Llorente et al. 2010b)
Because pathogenic S. vesicarium isolates also have the potential to develop saprophytically on debris of plant species different from pear, the epidemiological situation is more complex than such as apple scab. The leaf debris on the ground are also sources of inoculum, which are present during the entire year and may also play a role during summer epidemics as a continuous source of conidia. In this situation, sanitation measures focusing on fallen pear leaves during autumn can lead only to partial success (Köhl et al. 2009a).
Acknowledgments
This research was supported in part by grants from Ministerio de Educación y Ciencia (AGL2006-04987/AGR and AGL2009-09829/AGR) of Spain; Comissió Interdepartamental de Recerca i Tecnologia of the Generalitat de Catalunya (2009SGR00812); COST-Action 864: ‘Pome Fruit Health’ from the European Cooperation Science and Technology.
References
- Alberoni G, Cavallini D, Collina M, Brunelli A. Characterisation of the first Stemphylium vesicarium isolates resistant to strobilurins in Italian pear orchards. Eur J Plant Pathol. 2010;126:453–457. doi: 10.1007/s10658-009-9559-3. [DOI] [Google Scholar]
- Aveling TAS, Naude SP. First report of Stemphylium vesicarium on garlic in South Africa. Plant Dis. 1992;76:426. doi: 10.1094/PD-76-0426E. [DOI] [Google Scholar]
- Basallote Ureba MJ, Prados-Ligero AM, Melero-Vara JM. Aetiology of leaf spot of garlic and onion caused by Stemphylium vesicarium in Spain. Plant Pathol. 1999;48:139–145. doi: 10.1046/j.1365-3059.1999.00313.x. [DOI] [Google Scholar]
- Blancard D, Piquemal JP, Gindrat D. La stemphyliose de l’asperge. Rev Hortic. 1984;248:27–30. [Google Scholar]
- Blancard D, Allard E, Brest P. La stemphyliose du poirier ou “macules brunes”. Phytoma. 1989;406:35–37. [Google Scholar]
- Brunelli A, Di Marco G, Contarelli G, Ponti I (1984) Prove di lotta contro la maculatura bruna delle pere. ATTI Gior Fitopat I:203–212
- Brunelli R, Rovesti R, Di Marco S, Ponti I. Attivita di diversi fungicide contro la maculatura bruna del pero. Riv Frutticolt e Ortofloricolt. 1986;1:51–54. [Google Scholar]
- Brunelli A, Gherardi I, Adani N. Ridotta sensibilità di Stemphylium vesicarium, agente della maculatura bruna del pero, ai fungicidi dicarbossimidici. Inform Fitopat. 1997;9:44–48. [Google Scholar]
- Cavanni P, Ponti I. Maculatura bruna del pero: una micopatia sempre d’attualità. Riv di Fruticult. 1994;12:37–42. [Google Scholar]
- Cugier JP, Humbert W. Stemphyliose du poirier. Etude de la biologie du parasite et recherches des fongicides actifs. Phytoma-La Défense des végétaux. 1991;431:47–50. [Google Scholar]
- de Jong P, Heijne B. Exclusion of the inoculum source of bron spot (Stemphylium vesicarium) Acta Hortic. 2008;800:833–838. [Google Scholar]
- Ellis MB. Dematiaceus hyphomycetes. Kew: Commonwealth Mycological Institute; 1971. [Google Scholar]
- Falloon PG, Falloon LM, Grogan RG. Aetiology and epidemiology of Stemphylium leaf spot and purple spot of asparagus in California. Phytopathology. 1987;77:407–413. doi: 10.1094/Phyto-77-407. [DOI] [Google Scholar]
- Giosuè S, Rossi V, Buginai R, Mazzoni C. Modelling dynamics of airborne conidia of Stemphylium vesicarium, the causal agent of brown spot of pear. IOBC/WPRS Bull. 2006;29:169–176. [Google Scholar]
- Heijne B, Van Mourik J. Zwartvruchtrot op peer neemt toe. Fruitteelt Den Haag. 2001;91(8):18–19. [Google Scholar]
- Köhl J, Groenenboom-de Haas B, Goossen-van de Geijn H, Speksnijder A, Kastelein P, de Hoog S, Gerrits van den Ende B. Pathogenicity of Stemphylium vesicarium from different hosts causing brown spot in pear. Eur J Plant Pathol. 2009;124:151–162. doi: 10.1007/s10658-008-9402-2. [DOI] [Google Scholar]
- Köhl J, Groenenboom-de Haas B, Kastelein P, Rossi V, Waalwijck C. Quantitative detection of pear-pathogenic Stemphylium vesicarium in orchards. Phytopathology. 2009;99:1377–1386. doi: 10.1094/PHYTO-99-12-1377. [DOI] [PubMed] [Google Scholar]
- Lamprecht SC, Baxter A, Thompson AH. Stemphylium vesicarium on Medicago spp. in South Africa. Phytophylactica. 1984;16:73–75. [Google Scholar]
- Llorente I (1997) Development of an infection forecasting model for Stemphylium vesicarium. Evaluation, validation and implementation on experimental plots in pear commercial orchards. Ph D thesis. University of Girona, Girona, pp 327
- Llorente I, Montesinos E. Effect of relative humidity and interrupted wetness periods on brown spot severity of pear caused by Stemphylium vesicarium. Phytopathology. 2002;92:99–104. doi: 10.1094/PHYTO.2002.92.1.99. [DOI] [PubMed] [Google Scholar]
- Llorente I, Montesinos E. Development and field evaluation of a model to estimate the maturity of pseudothecia of Pleospora allii on pear. Plant Dis. 2004;88:215–219. doi: 10.1094/PDIS.2004.88.2.215. [DOI] [PubMed] [Google Scholar]
- Llorente I, Montesinos E. Brown spot of pear: an emerging disease of economic importance in Europe. Plant Dis. 2006;92:99–104. doi: 10.1094/PD-90-1368. [DOI] [PubMed] [Google Scholar]
- Llorente I, Vilardell P, Bugiani R, Gherardi I, Montesinos E. Evaluation of BSPcast disease warning system in reduced fungicide use programs for management of brown spot of pear. Plant Dis. 2000;84:631–637. doi: 10.1094/PDIS.2000.84.6.631. [DOI] [PubMed] [Google Scholar]
- Llorente I, Vilardell P, Moragrega C, Montesinos E. Development and evaluation of a forecasting system for scheduling fungicide sprays for control of brown spot (Stemphylium vesicarium) of pear. IOBC/WPRS Bull. 2000;23(12):81–87. [Google Scholar]
- Llorente I, Vilardell A, Montesinos E. Infection potential of Pleospora allii and evaluation of methods for reduction of the overwintering inoculum of brown spot of pear. Plant Dis. 2006;90:1511–1516. doi: 10.1094/PD-90-1511. [DOI] [PubMed] [Google Scholar]
- Llorente I, Vilardell A, Vilardell P, Montesinos E. Evaluation of new methods in integrated control of brown spot of pear (Stemphylium vesicarium, teleomorph Pleospora allii) Acta Hortic. 2008;800:825–831. [Google Scholar]
- Llorente I, Moragrega C, Ruz L, Santamaría G, Vilardell A, Vilardell P, Montesinos E. Basis for new strategies in integratred control of brown spot of pear (Stemphylium vesicarium, telemorpoh Pleospora allii) IOBC/WPRS Bull. 2010;54:35–39. [Google Scholar]
- Llorente I, Vilardell A, Vilardell P, Pattori E, Bugiani R, Rossi V, Montesinos E. Control of brown spot of pear by reducing the overwintering inoculum trough sanitation. Eur J Plant Pathol. 2010;128:127–141. doi: 10.1007/s10658-010-9637-6. [DOI] [Google Scholar]
- Llorente I, Vilardell P, Montesinos E. Evaluation of a revision of the BSPcast decision support system for control of brown spot of pear. Phytopathol Mediterr. 2011;50:3–13. [Google Scholar]
- Montesinos E, Bonaterra A. Dose-response models in biological control of plant pathogens: an empirical verification. Phytopathology. 1996;86:464–472. doi: 10.1094/Phyto-86-464. [DOI] [Google Scholar]
- Montesinos E, Vilardell P. Evaluation of FAST as a forecasting system for scheduling fungicide sprays for control of Stemphylium vesicarium on pear. Plant Dis. 1992;76:1221–1226. doi: 10.1094/PD-76-1221. [DOI] [Google Scholar]
- Montesinos E, Moragrega C, Llorente I, Vilardell P. Susceptibility of selected European pear cultivars to infection by Stemphylium vesicarium and influence of leaf and fruit age. Plant Dis. 1995;79:471–473. doi: 10.1094/PD-79-0471. [DOI] [Google Scholar]
- Montesinos E, Moragrega C, Llorente I, Vilardell P, Bonaterra A, Ponti I, Bugiani R, Cavanni P, Brunelli A. Development and evaluation of an infection model for Stemphylium vesicarium on pear based on temperature and wetness duration. Phytopathology. 1995;85:586–592. doi: 10.1094/Phyto-85-586. [DOI] [Google Scholar]
- Montesinos E, Bonaterra A, Ophir Y, Beer SV. Antagonism of selected bacterial strains to Stemphylium vesicarium and biological control of brown spot of pear under controlled environment conditions. Phytopathology. 1996;86:856–863. doi: 10.1094/Phyto-86-856. [DOI] [Google Scholar]
- Ponti I, Cavani P, Brunelli A. “Maculatura bruna” delle pere: eziologia e difesa. Inform Fitopat. 1982;32:35–40. [Google Scholar]
- Ponti I, Brunelli A, Tosi C, Basaglia M, Bevilacqua T, Emiliani G, Cont C, Viccinelli R. Verifica dell’attivita di diversi preparati contro la maculatura bruna del pero. Inform Fitopat. 1993;43:45–52. [Google Scholar]
- Ponti I, Brunelli A, Tosi C, Cavallini G, Mazzini F. Aggiornamenti sull ‘attivita’ dei fungicide contro la maculatura bruna del pero. ATTI Gior Fitopat. 1996;2:165–172. [Google Scholar]
- Rossi V, Pattori E. Inoculum reduction of Stemphylium vesicarium, the causal agent of brown spot of pear, through application of Trichoderma-based products. Biol Control. 2009;49:52–57. doi: 10.1016/j.biocontrol.2008.12.012. [DOI] [Google Scholar]
- Rossi V, Bugiani R, Giosué S, Natali P. Patterns of airborne conidia of Stemphylium vesicarium, the causal agent of brown spot disease of pears, in relation to weather conditions. Aerobiologia. 2005;21:203–216. doi: 10.1007/s10453-005-9002-y. [DOI] [Google Scholar]
- Rossi V, Pattori E, Giosuè S, Bugiani R. Growth and sporulation of Stemphylium vesicarium, the causal agent of brown spot of pear, on herb plants of orchard lawns. Eur J Plant Pathol. 2005;111:361–370. doi: 10.1007/s10658-004-5273-3. [DOI] [Google Scholar]
- Rossi V, Pattori E, Bugiani R. Sources and seasonal dynamics of inoculum for brown spot disease of pear. Eur J Plant Pathol. 2008;121:147–159. doi: 10.1007/s10658-007-9258-x. [DOI] [Google Scholar]
- Shishkoff N, Lorbeer JW. Etiology of Stemphylium leaf blight of onion. Phytopathology. 1989;79:301–304. doi: 10.1094/Phyto-79-301. [DOI] [Google Scholar]
- Simmons EG. Perfect stages of Stemphylium. Mycologia. 1969;61:1–26. doi: 10.2307/3757341. [DOI] [PubMed] [Google Scholar]
- Simmons EG. Perfect states of Stemphylium II. Sydowia. 1985;38:284–293. [Google Scholar]
- Singh P, Bugiani R, Cavanni P, Nakajima H, Kodama M, Otani H, Kohmoto K. Purification and biological characterization of host-specific SV-toxins from Stemphylium vesicarium causing brown spot of European pear. Phytopathology. 1999;89:947–953. doi: 10.1094/PHYTO.1999.89.10.947. [DOI] [PubMed] [Google Scholar]
- Singh P, Park P, Bugiani R, Cavanni P, Nakajima H, Kodama M, Otani H, Kohmoto K. Effects of host-selective SV-toxin from Stemphylium vesicarium, the cause of brown spot of European pear plants, on ultrastructure of leaf cells. J Phytopathol. 2000;148:87–93. doi: 10.1046/j.1439-0434.2000.00474.x. [DOI] [Google Scholar]
- Vilardell P. Stemphylium vesicarium en plantaciones de peral. Fruticult Profes. 1988;18:51–55. [Google Scholar]


