Abstract
Hypoxia is known to play important role in cancer biology. In sarcomas, hypoxia-induced protein biomarkers such as Hypoxia Inducible Factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), and Erythropoietin (Epo) have been previously reported in only a few studies. Moreover, the biologic significance and relationship to tumorigenesis of these hypoxia-induced biomarkers is not well understood in the context of sarcoma. The HIF negative regulator, Prolyl Hydroxylase Domain protein 2 (PHD2) has not been evaluated in sarcomas. We examined the expression of PHD2, HIF-1α, and several other hypoxia induced biomarkers in a series of clinically characterized, retroperitoneal sarcomas with immunohistochemical methods. Expression of these proteins was analyzed and correlated with clinical outcome. Increased HIF-1α expression was associated with shorter overall and disease free survival. PHD2 expression was detected in the majority of sarcoma cases, with increased expression correlating with high tumor grade but not with survival. Though changes in PHD2 expression alone did not correlate with overall and disease free survival, reduced/absent PHD2 expression in the presence of HIF-1α expression was associated with shorter overall and disease-free survival than that of other HIF-1α/PHD2 expression profiles. These observations suggest that regulation and expression of both PHD2 and HIF-1α are important to the biology of sarcomas, and that loss of PHD2 function has an additional adverse effect in the prognosis of sarcomas in tumors expressing HIF-1α. The biologic and therapeutic implications of HIF-1α and PHD2 expression in retroperitoneal sarcomas warrant further investigation.
Keywords: hypoxia, HIF, PHD2, retroperitoneal sarcoma, pathology, survival
Introduction
Hypoxia is a recognized risk factor for poor prognosis in various types of cancers, including carcinoma, melanoma, glioblastoma, and sarcoma.1,2 Hypoxia has been directly and indirectly measured in sarcomas 3,4 and has been shown to be associated with an increased risk of metastasis 5 and poor outcome in sarcoma.5 The availability of immunohistochemical and molecular tools to study hypoxia-induced gene expression has led to an understanding of the role and function of individual hypoxia-induced genes in tumor biology, and this may-ultimately prove useful prognostically or therapeutically in sarcomas.6
There has been substantial progress in understanding the control of cellular hypoxic responses in recent years.7,8 Transcriptional control of hypoxia-inducible responses is largely mediated through hypoxia inducible factors (HIFs). HIF is comprised of a constitutively expressed β polypeptide, the aryl hydrocarbon nuclear translocator, and an α polypeptide, the two main isoforms of which are HIF-1α and HIF-2α.9 HIF can be induced by numerous stimuli, including reduced oxygen tension, growth factors, oncogene activation, and loss of tumor suppressor gene function. Post-translational regulation critically regulates HIF-α in an oxygen-dependent manner. Several oxygen-sensing enzymes covalently hydroxylate HIF-α proteins on specific prolyl residues, thereby targeting the hydroxylated HIF proteins for recognition by the von Hippel-Lindau (VHL) tumor suppressor protein, leading to ubiquitination and subsequent proteolytic degradation of HIF via the cytosolic proteasome.10 The HIF prolyl hydroxylases include PHD1 (also known as Egln-2/HPH3), PHD2/Egln-1/HPH2, and PHD3/Egln-3/HPH1.11 In many cell types, PHD2 plays a particularly important role in downregulating HIF activity.12,13 There is also the asparagine hydroxylase, Factor Inhibiting HIF (FIH); modification of HIF-α catalyzed by FIH interferes with the interaction of HIF with the transcriptional coactivator CBP/p300.14,15,16
Expression of HIF-1α has been detected in sarcomas of diverse types, and in some cases, the expression correlated with poor clinical outcome.17 In addition, the angiogenic factor, vascular endothelial growth factor (VEGF), has been shown to be up-regulated in high grade sarcomas,18,19 and Erythropoietin (Epo) and the Erythropoietin receptor (EpoR) have also been detected in a series of pediatric sarcomas13 and other adult sarcomas, including angiosarcoma.20
The expression of the HIF negative regulator, PHD2, has not been previously analyzed in sarcomas. This is all the more relevant, given the recent observation of loss of heterozygosity of the PHD2 gene in a paraganglioma from a patient with a heterozygous germline PHD2 mutation,21 and other studies that point to a role for PHD2 in regulating tumor angiogenesis and tumor-forming potential.22,23 We hypothesized that the expression of hypoxia-induced proteins and the HIF regulatory protein PHD2 might be interrelated. In this study, we examined whether expression of hypoxia-induced proteins or PHD2 alone, or the combined patterns of expression of these factors were associated with any pathologic parameters and clinical outcome in sarcomas. To eliminate factors related to differences in sarcomas from differing sites, the study was limited to retroperitoneal sarcomas.
Materials and Methods
Patients
Fifty-six patients with retroperitoneal sarcomas were identified from case files of the Hospital of the University of Pennsylvania from between 1987 and 2006. Paraffin blocks were available in 46 cases. The original histologic slides in 46 cases with available paraffin blocks were reviewed to confirm the diagnosis and representative blocks were selected for the study. In the majority of dedifferentiated liposarcoma, two blocks containing either well-differentiated or dedifferentiated areas were evaluated when possible. Clinical follow-up data was available from clinical records for 39 of the cases.
Antibodies
Rabbit polyclonal antibodies specific for Epo (H-162; 1:200 dilution) and EpoR (C-20; 1:500 dilution) were purchased from Santa Cruz Biotechnologies Inc. (Santa Cruz, CA), and that for VEGF (1:25 dilution) was purchased from Labvision (Fremont, CA). Mouse monoclonal antibody specific for HIF-1α (clone H1α67) was purchased from Labvision. Mouse monoclonal antibody to PHD2 (clone 6.9) was obtained by immunizing mice with recombinant full-length PHD2. The protein was purified from baculovirus-infected insect cells.24 Splenic B cells were fused to myeloma cells, and supernatants from hybridomas were tested by ELISA for reactivity to PHD2 at the Hybridoma Facility of The Wistar Institute. Of 192 hybridomas tested, one clone 6.9, showed reactivity. This clone was expanded, and additional testing demonstrated that it could specifically immunoprecipitate either endogenous or overexpressed PHD2, but not overexpressed PHD1 or PHD3, from mammalian cell lysates (data not shown).
Immunohistochemistry
Paraffin-embedded tissue sections were analyzed for expression of HIF-1α, PHD2, Epo, EpoR, and VEGF by routine immunohistochemistry (IHC). Except for HIF-1α, immunohistochemical detection of Epo- and Epo-R-specific antibodies was performed manually, as previously described,25 with minor modifications. Slides were deparaffinized in xylene and rehydrated in graded alcohols. Antigen retrieval for VEGF, HIF-1α, Epo and EpoR antibodies was performed in citrate buffer, pH 6.0 for 20 minutes at 95-100°C, followed by rinsing in water. No antigen retrieval was performed for PHD2. Endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide for 10 minutes at room temperature, followed by rinsing in water and then Tris-buffered saline containing Tween-20 (TBST). Primary antibody was diluted in Dako diluent, applied to slides, and incubated at 4°C overnight (12-16 hours). Slides were washed twice for 3 minutes each in TBST. Except for HIF-1α primary antibody, detection was performed using EnVision Plus/HRP, Rabbit (Dako Cytomation) reagent for 30 minutes at room temperature, followed by rinsing twice for 3 minutes with TBST, and colorimetric development with 3, 3-diaminobenzidine. HIF-1α primary antibody detection was performed using the Catalyzed Signal Amplification kit (Dako Cytomation) according to manufacturer’s specifications. Slides were counterstained with hematoxylin. Slides were reviewed by two pathologists (PJZ and JHH) and immunostaining was semiquantitated and scored for the percentage of positive tumor cells when unequivocal immunoreactivity was detected. Immunoreactivity was also classified as negative or positive when the percentage was scored <10% or ≥10%, respectively.
Statistical analysis
One-way analysis of variance (ANOVA) and Mann-Whitney U tests were used to compare expression among the diagnostic groups. Kaplan-Meier plots and log-rank tests were used to assess associations between overall and disease-free survival and expression as defined as a continuous percentage of cells, and using a cut-off of ≥10%. A multivariable analysis was performed using Cox proportional hazards regression to adjust observed associations for important clinical confounders such as patient age, sex, and tumor grade. For all analyses, two-sided tests of significance were used with P <0.05 considered significant. Statistical analyses were performed using STATA version 8.0 (College Station, TX) and GraphPad 4 Prism.
Results
Clinical data
The diagnosis, grade and the corresponding main patient demographics are summarized in Table 1. The age at diagnosis ranged from 21 to 85 years with a mean of 64 years. The male to female ratio was 1:1. Twenty-one cases were classified as dedifferentiated liposarcoma (D-LPS), 9 well-differentiated liposarcoma (WD-LPS), 12 leiomyosarcoma (LMS), and 4 others (2 myxoid liposarcoma, 1 osteosarcoma, and 1 undifferentiated sarcoma). Sarcomas ranged from 4.6 - 60.0 cm in size with a mean of 22.5 cm. For tumor grade, 27 and 11 cases were classified as high and low grade, respectively, as per NCI grading criteria.16 Eight dedifferentiated liposarcomas with low grade dedifferentiation were classified as intermediate grade. Clinical follow-up was available in 39 primary sarcomas with a range of 0.7 to 229 months with a mean of 27.5 months. During follow-up of patients with primary retroperitoneal sarcoma, recurrence occurred in 15 of the 39 cases (38%). Among the recurrences, 6 cases (40% of recurrent cases) metastasized. Thirteen (87%) of the 15 patients with recurrent disease expired and the remaining 2 patients (13%) were alive with disease at the end of the follow-up.
Table 1.
Summary of 46 retroperitoneal sarcomas
| Diagnosis | n | Grade | Ave. Age | Ave. Size (cm) | Survival (months) |
|---|---|---|---|---|---|
|
| |||||
| D-LPS | 13 | High | 67.6 | 24.0 | 29.1 |
| D-LPS | 8 | Int | 63.1 | 28.6 | 23.5 |
| WD-LPS | 9 | Low | 68.1 | 32.7 | 15.7 |
| LMS | 12 | High | 61.3 | 10.1 | 29.4 |
| Other | 2 | High | 46.0 | 18.0 | 32.5 |
| Other | 2 | Low | 65.0 | 8.8 | 59.7 |
| Overall | 46 | 64.3 | 22.5 | 27.5 | |
LMS: leiomyosarcoma, WD-LPS: well -differentiated liposarcoma, D-LPS: Dedifferentiated liposarcoma, Int: intermediate.
Hypoxia-induced protein expression in retroperitoneal sarcomas
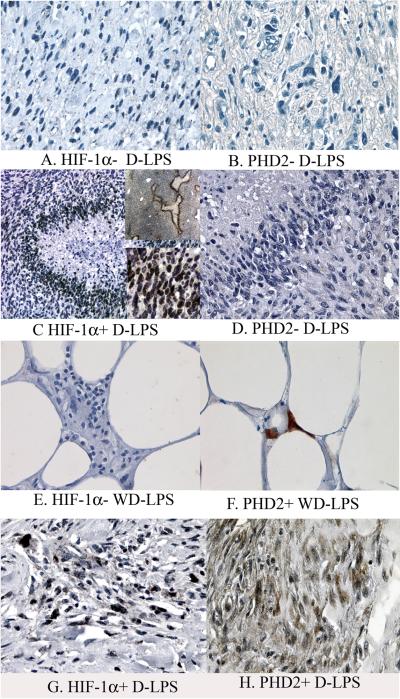
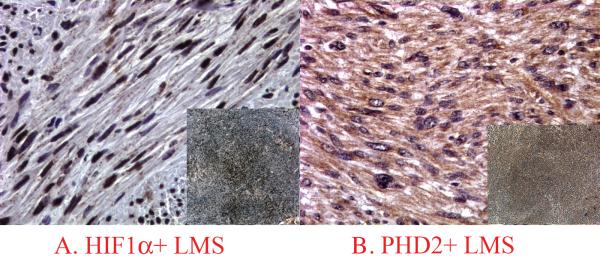
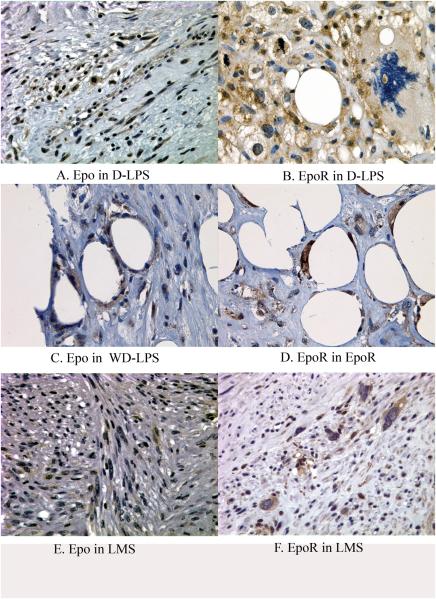
Expression of HIF-1α demonstrated a nuclear pattern that varied from weak to strong intensity (Figures 1 and 2) and was detected in 29 cases (63% of all cases), and 17 such cases (37%) had ≥10% tumor cells being positive. PHD2 Epo and EpoR demonstrated a predominantly cytoplasmic staining pattern with weak to moderate intensity (Figures 1 to 3). Faint nuclear reactivity was seen occasionally in cells with moderate cytoplasmic PHD2 and EpoR staining. Because this faint nuclear reactivity could not be reliably distinguished from nonspecific staining from stronger background cytoplasmic staining, it was considered equivocal and not further analyzed in this study. For VEGF expression, a membrane/cytoplasmic staining pattern was seen with weak to moderate intensity (Figure 4). When dedifferentiated and well differentiated areas from the same tumor were compared in D-LPS, reactivity of these hypoxia induced proteins was primarily detected in the dedifferentiated area (Figure 5). In some high grade tumors with necrosis, HIF-1α expression was more concentrated around the areas of necrosis (Figure 1C). The results of positive expression (as determined as ≥10%), individual percentage score, and mean value of the percentages of each protein analyzed in the study groups are presented in Table 2.
Figure 1.
HIF-1α and PHD2 expression in liposarcomas. A and B: HIF-1α−/PHD2− D-LPS; C and D: HIF-1α+/PHD2− D-LPS; E and F: HIF-1α−/PHD2+ WD-LPS; G and H: HIF-1α+/PHD2+ D-LPS. Note the increased HIF-1α nuclear reactivity in perinecrotic area in case 2 D-LPS (image C contains one low magnified and one high magnified inserts).
Figure 2.
HIF-1α and PHD2 expression profiles in a HIF-1α+/PHD2+ a leiomyosarcoma, (A and B contain inserts showing low magnification of the same staining in each image).
Figure 3.
Epo (A,C,E) and EpoR (B,D,F) immunoreactivity in dedifferentiated liposarcoma (A and B), well differentiated liposarcoma (C and D) and leiomyosarcoma (E and F).
Figure 4.
VEGF immunoreactivity in dedifferentiated liposarcoma (A), well-differentiated liposarcoma (B) and leiomyosarcoma (C).
Figure 5.
HIF-1α expression in dedifferentiated area (A) and well-differentiated area (B) of a dedifferentiated liposarcoma
Table 2.
Immunoreactivity (%) of HRPs in retroperitoneal sarcomas
| Case | Diagnosis | Grade | HIF-1a (%) | PHD2 (%) | EPO (%) | EPO-R (%) | VEGF (%) |
|---|---|---|---|---|---|---|---|
| 1 2 3 4 5 6 7 8 9 10 11 12 13 |
DLPS | High High High High High High High High High High High High High |
2 0 20 40 30 10 5 20 0 30 60 5 10 |
40 50 100 80 20 80 80 5 0 5 90 50 100 |
1 5 1 5 5 0 0 50 0 0 0 30 2 |
40 90 30 70 70 80 90 80 0 90 40 50 80 |
2 60 90 0 80 10 30 10 100 80 5 20 50 |
|
Mean
Positive |
(≥ 10%) |
17.8
8 (62%) |
53.8
10 (77%) |
7.6
2 (15%) |
62.3
12 (92%) |
41.3
10 (77%) |
|
| 1 2 3 4 5 6 7 8 |
DLPS | Int Int Int Int Int Int Int Int |
20 0 3 0 0 15 0 1 |
1 10 5 10 20 5 0 70 |
80 0 0 5 0 0 0 10 |
80 60 30 60 10 30 3 100 |
5 10 10 30 0 5 0 60 |
|
Mean
Positive |
(≥ 10%) |
4.8
2 (25%) |
15.1
4 (50%) |
11.8
2 (25%) |
46.6
7 (89%) |
15
4 (50%) |
|
| 1 2 3 4 5 6 7 8 9 |
WD LPS | Low Low Low Low Low Low Low Low Low |
0 1 0 0 5 1 0 0 5 |
0 40 0 1 0 10 0 0 5 |
0 40 0 0 0 0 0 30 0 |
10 80 100 60 50 90 80 60 80 |
0 40 0 0 0 10 0 45 10 |
|
Mean
Positive |
(≥ 10%) |
1.3
0 |
6.2
2 (22%) |
7.8
2 (22%) |
67.8
9 (100%) |
11.7
4 (44%) |
|
| 1 2 3 4 5 6 7 8 9 10 11 12 |
LMS | High High High High High High High High High High High High |
30 80 80 1 60 5 30 30 5 20 0 0 |
90 20 20 70 80 100 90 90 70 100 100 100 |
0 0 10 10 5 60 70 10 0 40 10 70 |
60 20 70 90 40 100 90 60 20 80 80 80 |
50 0 5 80 0 100 30 10 40 20 5 70 |
|
Mean
Positive |
(≥ 10%) |
28.0
7 (58%) |
77.5
12 (100%) |
27.6
8 (66%) |
64.1
12 (100%) |
31.6
8 (66%) |
|
| 1 2 3 4 |
Osteosarcoma UD sarcoma Myxoid LPS Myxoid LPS |
High High Low Low |
0 0 0 0 |
0 20 10 20 |
1 0 0 0 |
30 80 <5 90 |
1 30 5 10 |
| P (ANOVA) | <0.0001 | 0.008 | 0.24 | 0.544 | 0.157 |
LMS: leiomyosarcoma, WD-LPS: well-differentiated liposarcoma, D-LPS: Dedifferentiated liposarcoma, Int: intermediate, UD: undifferentiated
Univariable analyses
As determined by analysis of variance, only the means of HIF-1α and PHD2 expression among the groups were statistically different (Table 2). Pairwise comparisons in the expression percentages of the various proteins were analyzed between sarcoma types and grades (Table 3). Both HIF-1α and PHD2 expression was significantly higher in LMS and D-LPS (as a group including both high and intermediate grades) when compared to WD-LPS (P-value = 0.009 and 0.037, respectively, for HIF-1α; and 0.0002 and 0.005, respectively, for PHD2) (Table 3). In addition, the difference in expression of PHD2 was significant when comparing LMS to D-LPS (P-value = 0.005). Epo expression was significantly higher in LMS than WD-LPS (P-value = 0.0488), and VEGF expression was significantly higher in high grade D-LPS than WD-LPS (P-value 0.0384). Except for these two comparisons, there were no significant differences in mean expression percentages of Epo, EpoR, and VEGF between groups or by grade (P-value >0.05). No significant relationships were identified when comparing tumor size to percentage of expression of all proteins examined.
Table 3.
P values for comparison of the expression percentages of HRFs between sarcoma groups (Two-tailed, Mann-Whitney U-test)
| Sarcoma groups | HIF-1a | Epo | EpoR | PHD2 | VEGF |
|---|---|---|---|---|---|
| LMS vs. D-LPS (H + I) | 0.139 | 0.0539 | 0.421 | 0.005 | 0.694 |
| LMS vs. WD-LPS | 0.0094 | 0.0488 | 0.943 | 0.0002 | 0.0812 |
| D-LPS vs. WD-LPS | 0.0373 | 0.248 | 0.353 | 0.0058 | 0.0855 |
| D-LPS (H) vs. WD-LPS | 0.0068 | 0.161 | 0.64 | 0.003 | 0.0384 |
| D-LPS (I) vs. WD-LPS | 0.596 | 0.665 | 0.194 | 0.112 | 0.501 |
LMS: leiomyosarcoma, WD-LPS: well differentiated liposarcoma, D-LPS: Dedifferentiated liposarcoma, (H): high grade, (I): intermediate grade.
Survival in relation to HIF-1α and PHD2 expression
The relationships of HIF-1α and PHD2, individually, and as co-expressed proteins to overall survival (OS) or disease-free survival (DFS) were evaluated using log-rank tests. Shorter OS was associated with positive expression of HIF-1α (HIF-1α+, median survival 14.2 months) compared to negative HIF-1α (HIF-1α−) expression (median survival 56.1 months, P-value = 0.014, log-rank test) (Figure 6B left). No association of OS with positive PHD2 expression (median survival 51 months) or negative PHD2 expression (median survival 46 months) was observed (P-value = 0.365) (Figure 6B right). For all cases, HIF-1α+ (P-value = 0.032) but not PHD2+ (P-value = 0.399) expression was significantly associated with shorter DFS (data not shown). Similarly in high grade tumors, shorter DFS were associated with HIF-1α+ (median survival 14.2 months) compared to HIF-1α− (median survival 50.7 months, P-value = 0.031) (Figure 6C left).
Figure 6.
Kaplan-Meier plots and Log-rank tests:
(A) Overall survival (left) and disease-free survival (right) as a function of HIF-1α / PHD2 expression profiles. HIF-1α+/PHD2− expression (solid squares) was compared to HIF-1α+/PHD2+ (OS P value = 0.018, DFS P value = 0.16), HIF-1α−/PHD2+ (OS P value = 0.11, DFS P value = 0.0059), and HIF-1α−/PHD2− (OS P value = 0.039, DFS P value = 0.13) expression.
(B) Overall survival in relation to HIF-1α (left) or PHD-2 (right) expression. HIF-1α positive expression (solid squares) was associated with shorter OS when compared to HIF-1α negative expression (open squares) (P value = 0.014). There was no difference in OS between PHD2 positive (solid circles) versus PHD2 negative (open circles) expression (P value = 0.365).
(C) Disease-free survival for high grade retroperitoneal sarcomas with follow-up data (n = 22) in relation to HIF-1α expression alone (left) or HIF-1α/ PHD-2 expression profile (right). There was significant difference in DFS between HIF-1α positive (solid squares) versus HIF-1α negative (open squares) high grade tumors (P value = 0.032). Two cases with HIF-1α+/PHD2− profile (solid squares, right) were associated with the worst DFS.
The relationships of HIF-1α and PHD2 expression profiles to OS and DFS in all cases and high grade sarcomas were examined further. Tumors with HIF-1α+/PHD2− expression pattern demonstrated shorter OS and DFS (8.3 months and 9.8 months) compared to tumors with HIF-1α+/ PHD2+ (OS =13.5 months, DFS = 14.2 months, P value = 0.018 and 0.16, respectively), HIF-1α−/ PHD2− (OS =46.0 months, DFS = 27.4 months, P value=0.039, and 0.13, respectively), and HIF-1α− /PHD2+ (OS =35.9 months, DFS = 47.6 months; P value = 0.11 and 0.0059, respectively) expression (Figure 6A). A similar trend was observed in patients with high grade HIF-1α+/ PHD2− sarcomas (median survival 8.3 months) compared to HIF-1α+/PHD2 + (median survival 18.7 months,) and HIF-1α−/PHD2+ (median survival 50.7 months,) (Figure 6C right). Disease-free survival was longer in HIF-1α− /PHD2− (median survival 46 months) in the high grade tumors, but the difference was not statistically significant due to the small number of cases evaluated (P value = 0.22). In contrast to high grade sarcomas, the outcomes of low grade sarcomas showed no association with any of the hypoxia-related proteins in (P-values >0.05, data not shown).
No significant associations with DFS or OS were identified for positive versus negative expression of Epo (P value = 0.801 (OS), 0.813 (DFS)), EpoR (P value = 0.977 (OS), 0.448 (DFS)), or VEGF (P value = 0.275 (OS), 0.223 (DFS)) using the log-rank test.
Multivariate analyses
Age- and grade-adjusted multivariable analyses were performed using a Cox proportional hazards model for OS and DFS (analyses for OS shown in Table 4). Negative expression of Epo was associated with worse OS (hazard ratio (HR) = 10.7, P value = 0.010) and DFS (HR = 3.65, P value = 0.031). Negative expression of PHD2 was associated with a worse OS (HR = 3.75, P value = 0.043), and approached statistical significance for DFS (HR = 2.77, P value = 0.064). Positive expression of HIF-1α approached statistical significance for worse DFS (HR = 3.13, P value = 0.061), but was not significant for OS (HR = 0.393, P value = 0.193). The HIF-1α+/ PHD2− expression profile was significant for worse DFS (HR = 7.10, P value = 0.034), but not for OS (HR = 3.06, P value = 0.345) (data not shown). No significant associations for EpoR or VEGF with either OS or DFS were identified.
Table 4.
Multivariate Cox model for overall survival
| Unadjusted HR |
95% CI | Adjusted HR* | 95% CI | p-value | |
|---|---|---|---|---|---|
| Epo (<10%) |
3.508 | 0.790- 15.581 |
10.73 | 1.764- 65.316 |
0.01 |
| EpoR (≥10%) |
1.73 | 0.224- 13.342 |
0.739 | 0.080- 6.821 |
0.79 |
| VEGF (>10%) |
5.967 | 1.316- 27.048 |
4.186 | 0.717- 24.450 |
0.112 |
| PHD2 (<10%) |
2.061 | 0.727- 5.844 |
3.748 | 1.042- 13.472 |
0.043 |
| HIF-1α (>10%) | 1.864 | 0.587- 5.927 |
2.452 | 0.625- 10.335 |
0.193 |
HR: Hazard ratio. *Adjusted for tumor grade and age.
Discussion
In this study, expression of PHD2 and a panel of hypoxia-induced biomarkers was evaluated in retroperitoneal sarcomas. We found that 1) high grade LMS and D-LPS express HIF-1α and PHD2 more frequently and at a higher level than do low grade tumors or WD-LPS, 2) HIF-1α positive expression alone or HIF-1α positive with PHD2 negative expression profile is associated with shorter survival, 3) positive Epo expression is present in a small number of cases with no association with tumor grade and type, but negative expression is associated with worse DFS and OS, 4) VEGF expression is detected in a small subset of retroperitoneal sarcomas without significant clinical correlation, 5) most retroperitoneal sarcomas express a high level of EpoR without significant clinical correlation.
Expression of hypoxia-related proteins has been evaluated in sarcomas in only a few reports. Positive HIF-1α expression has been associated with shorter overall survival in a series of sarcomas which was mainly composed of malignant fibrous histiocytoma and synovial sarcoma from various sites.9 Our finding of VEGF expression in retroperitoneal sarcomas with no prognostic association is also concordant with the observations of a prior study.18
Epo expression has been previously reported in pediatric sarcomas but little is known about its role in sarcoma biology.17 Compared to EpoR, Epo expression was low and infrequent in sarcomas. Although high grade leiomyosarcomas were found to express Epo more frequently when compared to low grade well-differentiated liposarcoma (P=0.049), the lack of Epo reactivity in tumor was actually associated with shorter survival in multivariate analysis. The significance of this apparently paradoxical Epo effect in sarcoma is unclear, and it might be a result of a small series used in the study. Further investigation is needed to validate this finding.
Recently, two papers demonstrated that the detection of EpoR using polyclonal C-20 antibody cross-react with non-EpoR proteins in paraffin-embedded tissues26,27 and therefore, the finding of EpoR expression in sarcomas in this study and in another series of pediatric sarcomas17 should be interpreted with caution. Further evaluation using additional EpoR antibodies is currently underway.
PHD2 expression has been previously observed in renal cell, bronchogenic, breast, and thyroid carcinomas28 and in head and neck squamous cell carcinoma;29 and in the latter case is associated with higher tumor grade. This report is now the first to demonstrate that PHD2 expression is associated with high tumor grade in retroperitoneal sarcomas, and that negative PHD2 expression in the presence of HIF-1α expression in these tumors is associated with shorter survival. This association between prognosis and HIF-1α positive/ PHD2 negative expression pattern is compelling and warrants further investigation. However, there were only four such cases in the present study, so identifying and evaluating additional cases will be important to further validate the importance of this initial observation. In additional studies, the HIF-1α positive and PHD2 negative expression pattern has been found in malignant, but not benign, uterine smooth muscle tumors (JHH, FSL, PJZ unpublished observations).
PHD2 is typically constitutively expressed in many non-neoplastic tissues and PHD2 expression can be induced by HIF-1α.28 This may provide an explanation for the coexpression of PHD2 and HIF-1α in some of the tumors examined in this study. In addition, the down-regulation of HIF-1α by PHD2, which is the normal biologic function of the latter, provides explanation for some tumors in which PHD2 but not HIF-1α was detected. The observation of HIF-1α positive, PHD2 negative phenotype in sarcomas raises interesting parallels with the VHL syndrome, in which loss of heterozygosity at the VHL tumor suppressor gene locus predisposes to tumors that include renal cell carcinoma, pheochromocytoma, and hemangioblastoma.30 Indeed, loss of heterozygosity at the PHD2 locus has recently been reported in a paraganglioma from a patient with a heterozygous PHD2 missense mutation and erythrocytosis.21 However, it must also be recognized that the role of PHD2 loss of function in tumorigenesis may be unrelated to HIF. Studies examining PHD2 knockdown in transformed human colonic and pancreatic adenocarcinoma cell lines identify an important role for PHD2 in regulating tumor angiogenesis in a manner that does not depend on HIF.22 It should also be recognized that loss of PHD2 function has important functions in the tumor microenvironment. For example, impaired metastasis of tumors is observed in Phd2 +/− mice due to normalization of endothelial cells31. Future studies to evaluate PHD2 gene sequences in sarcomas and their relationship to HIF-1α expression and clinical outcome are warranted. In addition, it will be of interest to investigate other members of the HIF-α and PHD families, in addition to the evaluation of HIF-1α and PHD2.32
Retroperitoneal sarcomas are often high clinical stage at diagnosis and are typically treated by surgical excision with difficulty in securing complete wide surgical margin resulting in poor local disease control. In addition to radiotherapy, other options for adjuvant therapy are very limited currently in these patients. The finding of HIF-1α up-regulation in sarcomas reported in this study raises the possibility of therapeutic targeting of HIF-1α. One strategy would be a direct inhibition of HIF-1α, as has been proposed for other types of cancers. Mammalian target of rapamycin (mTOR) activates S6 kinase which is known to stimulate HIF-1α translation. Pharmacologic inhibition of mTOR in an in vivo xenograft model of LMS has been reported to reduce tumor burden.33 Recently, partial responses using an inhibitor have been reported in early data from a human clinical trial and an anecdotal study.34,35 These promising findings suggest a reduction in HIF-1α levels may be responsible for the apparent clinical response.
In summary, this study identifies the expression of hypoxia induced proteins and the HIF regulatory protein PHD2 in a series of retroperitoneal sarcomas and correlates the expression of these proteins to clinical outcome. To our knowledge, this is the first report of the analysis of the expression of PHD2 in sarcomas. We found that the pattern of positive HIF-1α expression in the presence of negative PHD2 expression was associated with poor overall and disease-free survival. Additional studies aimed at determining the generality and the mechanisms of PHD2 expression and its role in regulating HIF in tumors are indicated.
Acknowledgements
The authors would like to thank Kristen M. Huang for her critical reading of this manuscript. Work in Dr. Lee’s laboratory was supported by R01-CA090261.
Abbreviations
- HIF-1α
Hypoxia Inducible Factor-1α
- PHD2
Prolyl Hydroxylase Domain protein 2
- VEGF
Vascular endothelial growth factor
- Epo
Erythropoietin
- HIFs
Hypoxia-inducible factors
- FIH
Factor Inhibiting HIF
- EpoR
Erythropoietin receptor
- IHC
Immunohistochemistry
- VHL
von Hippel-Lindau
- D-LPS
Dedifferentiated liposarcoma
- WD-LPS
Well differentiated liposarcoma
- LMS
Leiomyosarcoma
- OS
Overall Survival
- DFS
Disease-Free Survival
- HRF
Hypoxia-related factor
References
- 1.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Brizel DM, Rosner GL, Prosnitz LR, Dewhirst MW. Patterns and variability of tumor oxygenation in human soft tissue sarcomas, cervical carcinomas, and lymph node metastases. Int J Radiat Oncol Biol Phys. 1995;32:1121–1125. doi: 10.1016/0360-3016(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 4.Koch CJ, Evans SM, Lord EM. Identification of hypoxia in cells and tissues of epigastric 9L rat glioma using EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide] Br J Cancer. 1995;72:869–874. doi: 10.1038/bjc.1995.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 6.Nordsmark M, Alsner J, Keller J, Nielsen OS, Jensen OM, Horsman MR, et al. Hypoxia in human soft tissue sarcomas: adverse impact on survival and no association with p53 mutations. Br J Cancer. 2001;84:1070–1075. doi: 10.1054/bjoc.2001.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–34. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. Involvement of hypoxia-inducible factor 1 in human cancer. Intern Med. 2002;41:79–83. doi: 10.2169/internalmedicine.41.79. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 11.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 12.Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–90. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–65. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 14.Hewitson KS, Schofield CJ. The HIF pathway as a therapeutic target. Drug Discov Today. 2004;9:704–711. doi: 10.1016/S1359-6446(04)03202-7. [DOI] [PubMed] [Google Scholar]
- 15.del Peso L, Castellanos MC, Temes E, Martin-Puig S, Cuevas Y, Olmos G, et al. The von Hippel Lindau/hypoxia-inducible factor (HIF) pathway regulates the transcription of the HIF-proline hydroxylase genes in response to low oxygen. J Biol Chem. 2003;278:48690–48695. doi: 10.1074/jbc.M308862200. [DOI] [PubMed] [Google Scholar]
- 16.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batra S, Perelman N, Luck LR, Shimada H, Malik P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab Invest. 2003;83:1477–1487. doi: 10.1097/01.lab.0000090156.94795.48. [DOI] [PubMed] [Google Scholar]
- 18.Chao C, Al-Saleem T, Brooks JJ, Rogatko A, Kraybill WG, Eisenberg B. Vascular endothelial growth factor and soft tissue sarcomas: tumor expression correlates with grade. Ann Surg Oncol. 2001;8:260–267. doi: 10.1007/s10434-001-0260-9. [DOI] [PubMed] [Google Scholar]
- 19.Pakos EE, Goussia AC, Tsekeris PG, Papachristou DJ, Stefanou D, Agnantis NJ. Expression of vascular endothelial growth factor and its receptor, KDR/Flk-1, in soft tissue sarcomas. Anticancer Res. 2005;25:3591–3596. [PubMed] [Google Scholar]
- 20.Rathmell WK, Acs G, Simon MC, Vaughn DJ. HIF transcription factor expression and induction of hypoxic response genes in a retroperitoneal angiosarcoma. Anticancer Res. 2004;24:167–169. [PubMed] [Google Scholar]
- 21.Ladroue C, Carcenac R, Leporrier M, Gad S, Hello CL, Galateau-Salle F, et al. Mutation and Congenital Erythrocytosis with Paraganglioma. N Engl J Med. 2008;359(25):2685–2692. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- 22.Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT, Giaccia AJ. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell. 2009;15:527–38. doi: 10.1016/j.ccr.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KA, Lynd JD, O'Reilly S, Kiupel M, McCormick JJ, LaPres JJ. The biphasic role of the hypoxia-inducible factor prolyl-4-hydroxylase, PHD2, in modulating tumor-forming potential. Mol Cancer Res. 2008;6:829–42. doi: 10.1158/1541-7786.MCR-07-2113. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–39800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53:530–541. doi: 10.1002/1097-0142(19840201)53:3<530::aid-cncr2820530327>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM, et al. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:1892–1895. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- 27.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soilleux EJ, Turley H, Tian YM, Pugh CW, Gatter KC, Harris AL. Use of novel monoclonal antibodies to determine the expression and distribution of the hypoxia regulatory factors PHD-1, PHD-2, PHD-3 and FIH in normal and neoplastic human tissues. Histopathology. 2005;47:602–610. doi: 10.1111/j.1365-2559.2005.02280.x. [DOI] [PubMed] [Google Scholar]
- 29.Jokilehto T, Rantanen K, Luukkaa M, Heikkinen P, Grenman R, Minn H, et al. Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase PHD2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness. Clin Cancer Res. 2006;12:1080–1087. doi: 10.1158/1078-0432.CCR-05-2022. [DOI] [PubMed] [Google Scholar]
- 30.Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 31.Mazzone M, Dettori D, Oliveira RL, Loges S, Schmidt R, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–51. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emanuel F, Petricoin I, Espina V, Araujo RP, Midura B, Yeung C, et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res. 2007;67:3431–3440. doi: 10.1158/0008-5472.CAN-06-1344. [DOI] [PubMed] [Google Scholar]
- 34.Iwenofu O, Goodwin D, Staddon A, Haupt H, Brooks J. Phospho-S6 ribosomal protein: a potential new predictive sarcoma marker for targeted mTOR therapy. Mod Pathol. 2008;3:231–237. doi: 10.1038/modpathol.3800995. [DOI] [PubMed] [Google Scholar]
- 35.Merimsky O. Targeting metastatic leiomyosarcoma by rapamycin plus gemcitabine: an intriguing clinical observation. Int J Mol Med. 2004;14:931–935. [PubMed] [Google Scholar]