Abstract
Purpose
It is thought that following a stroke the contralesional motor region exerts an undue inhibitory influence on the lesional motor region which might limit recovery. Pilot studies have shown that suppressing the contralesional motor region with cathodal transcranial Direct Current Stimulation (tDCS) can induce a short lasting functional benefit; greater and longer lasting effects might be achieved with combining tDCS with simultaneous occupational therapy (OT) and applying this intervention for multiple sessions.
Methods
We carried out a randomized, double blind, sham controlled study of chronic stroke patients receiving either 5 consecutive days of cathodal tDCS (for 30 minutes) applied to the contralesional motor region and simultaneous OT, or sham tDCS+OT.
Results
We showed that cathodal tDCS+OT resulted in significantly more improvement in Range-Of-Motion in multiple joints of the paretic upper extremity and in the Upper-Extremity Fugl-Meyer scores than sham tDCS+OT, and that the effects lasted at least one week post-stimulation. Improvement in motor outcome scores was correlated with decrease in fMRI activation in the contralesional motor region exposed to cathodal stimulation.
Conclusions
This suggests that cathodal tDCS combined with OT leads to significant motor improvement after stroke due to a decrease in the inhibitory effect that the contralesional hemisphere exerts onto the lesional hemisphere.
Keywords: Rehabilitation, stroke recovery, non-invasive brain-stimulation, fMRI, tDCS
1. Introduction
Transcranial Direct Current Stimulation (tDCS) – a non-invasive brain stimulation technique (Nitsche & Paulus, 2000; Priori et al., 1998; Schlaug & Renga, 2008; Schlaug et al., 2008) - has recently gained a lot of attention as a tool to modulate cortical activity in two principal ways, and several groups of researchers have hence explored its rehabilitative potential (Schlaug & Renga, 2008; Schlaug et al., 2008). Anodal stimulation (which increases excitability of the brain tissue underlying the electrode) to the intact parts of the lesional hemisphere or cathodal stimulation (which decreases excitability of the brain tissue underlying the electrode) to the contralesional hemisphere have shown some efficacy in affecting motor outcome measures, although most studies tested the efficacy in single session trials of pilot or proof-of-principle studies (Celnik et al., 2009; Fregni et al., 2005; Hummel et al., 2005; Hummel & Cohen, 2005; Hummel et al., 2006). The use of non-invasive brain stimulation to facilitate recovery from a stroke is based on neurophysiological and imaging findings. Neurophysiological studies in chronic stroke patients have demonstrated that disinhibition of contralesional motor regions coexists with increased inhibition of ipsilesional motor regions that results in an imbalance of interhemispheric interactions (Duque et al., 2005b; Liepert et al., 2000b; Murase et al., 2004; Shimizu et al., 2002). The indirect effect of this imbalance on the lesioned hemisphere combined with the stroke’s direct effect on the unimpaired/intact parts of the lesioned motor region and its efferent motor system may interfere with the recovery process. Similarly, imaging studies in well-recovered patients have shown that brain reorganization during the recovery phase is associated with re-activation or over-activation of unimpaired sensorimotor and premotor networks in the lesional hemisphere (Calautti & Baron, 2003; Cramer et al., 2002; Loubinoux et al., 2003; Nair et al., 2007). The significance of activation in the contralesional motor regions when the affected arm/hand performs a motor task remains under study (Johansen-Berg et al., 2002; Lotze et al., 2006). One explanation is that this contralesional activation when the affected hand is doing a motor task is a sign of disinhibition (lack of lesional hemisphere’s inhibitory effect on the contralesional hemisphere’s motor region) that could potentially impede recovery. Although this model of interhemispheric imbalance (Fig. 1A) may appear to be a simplified representation of the many underlying pathophysiological processes involved in recovery from stroke, it provides a framework for generating hypotheses focused on three approaches: 1) downregulating activity in the contralesional motor region to check its unbalanced influence on the lesional motor region (Fig. 1C), 2) facilitating activity in the intact portions of the ipsilesional motor region (Fig. 1B) (Fregni et al., 2005; Hesse et al., 2007; Hummel et al., 2005; Hummel & Cohen, 2005; Lindenberg et al., 2010b; Mansur et al., 2005; Schlaug et al., 2008; Vines et al., 2008a; Ward & Cohen, 2004), or 3) a combination of both with dual hemispheric stimulation (Lindenberg et al., 2010b; Vines et al., 2008a).
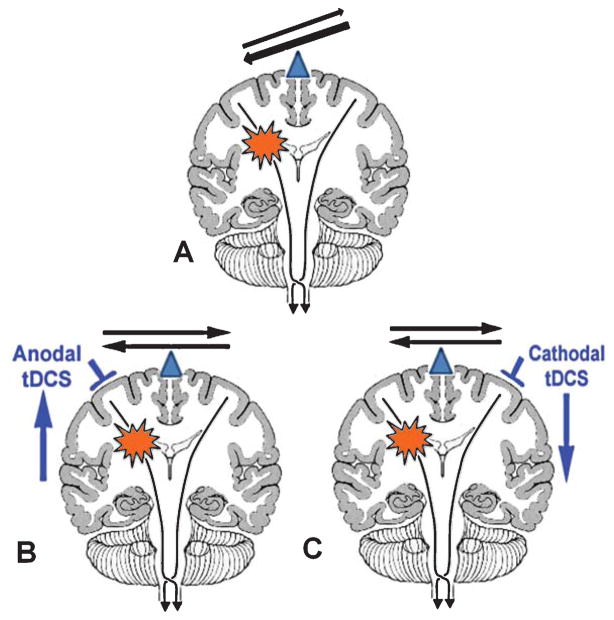
Fig. 1.
Brain model of altered interhemispheric inhibition in patients with a unihemispheric stroke and the therapeutic options to ameliorate this imbalance. The balance of interhemispheric inhibition becomes disrupted after a stroke (A), such that the healthy hemisphere exerts an unopposed inhibitory influence onto the lesional hemisphere and possibly interferes in the recovery process. There are three possible ways to ameliorate this process by non-invasive brain-stimulation with tDCS: the excitability in the affected (lesional) hemisphere is upregulated through anodal tDCS (B), the excitability in the unaffected (normal) hemisphere is down regulated through cathodal tDCS (C) or a combination of B and C is applied. In the current study, we tested the efficacy of option C.
One way to improve motor function due to a unihemispheric stroke is to suppress the activity of the contralesional hemisphere and release the lesional hemisphere from the unbalanced transcallosal inhibitory influence of the contralesional hemisphere (see Fig. 1C). So far no study has used functional imaging to validate this theory or understand how tDCS affects neural reorganization. Furthermore, it has not been tested whether the effects of central stimulation with tDCS can be enhanced by combining tDCS with other peripheral stimulation techniques to optimally modulate neural plasticity, similar to paired associative stimulation protocols that have been successfully used in the past to enhance/facilitate brain plasticity (Stefan et al., 2000). It is also not known whether a multisession protocol leads to longer lasting and stronger effects compared to a single session of brain-stimulation that have mainly shown short lasting effects. Thus, in the current study, we tested the effects of cathodal stimulation to the contralesional hemisphere possibly validating or refuting the theory of disinhibition in motor recovery after stroke (Duque et al., 2005a; Ebisu et al., 1991; Liepert et al., 2000a; Liepert et al., 2000b; Murase et al., 2004; Shimizu et al., 2002). We chose to first test cathodal stimulation to the contralesional hemisphere in our multipronged approach, since models of the current density distribution have shown that anodal stimulation to the lesional hemisphere is somewhat less predictable since a cortical stroke can alter the current distribution and current density maxima (Wagner et al., 2007). Nevertheless, anodal stimulation over the affected hemisphere and dual hemispheric stimulation studies are currently underway in several laboratories. We hypothesized that cathodal tDCS to the contralesional hemisphere simultaneously with OT is more beneficial than sham tDCS+OT. In addition, we hoped to see changes in fMRI parameters that correlate with the intervention and behavioral improvement, thus serving as predictors of the therapeutic response.
2. Subjects and methods
2.1. Subjects
Fourteen right-handed patients, aged 40–76 (mean 55.8) years who had suffered their first ever unihemispheric stroke, participated in this study. Six patients had lesions in the right hemisphere and eight patients in the left. Patients were randomized to either the cathodal group or the sham group. The cathodal group had 5 patients with predominantly cortical lesions including the immediate underlying subcortical region and 2 patients with deep white matter/striato-capsular lesions. The sham group consisted of 4 patients with predominantly cortical lesions including the immediate underlying subcortical region and 3 patients with deep white matter/striato-capsular regions. Patient details (e.g., age, gender, time elapsed after the stroke, baseline UE-FM, lesion volume, and the lesion load of the corticospinal tract) separated by groups are given in Table 1. Although there are slight differences to the disadvantage of the cathodal group with regard to patient age, time elapsed after their stroke and enrollment in our study, lesion size, and CST lesion load, none of these differences were statistically significant. All patients gave their written informed consent to the study, which had been approved by the Institutional Review Board. Patients with a previous history of stroke, bilateral infarcts, hemorrhage, arthritis, chronic pain and other neurological diseases were excluded from participating in the study. None of the patients were taking anti-depressant medications or stimulants. Patients had moderate to severe upper extremity impairment as revealed by a mean Upper-Extremity Fugl-Meyer (UE-FM) score of 30.1 (±10.4) (Fugl-Meyer et al., 1975) out of a maximum possible score of 66. A mean score of 30 makes it very likely that these patients had either a partially intact pyramidal tract or intact alternate descending corticospinal fibers (see Lindenberg et al., 2010a for more details). All patients underwent MRI using a 3T GE scanner, which included a set of highly T1-weighted images (0.93 × 0.93 × 1.5 mm3), a FLAIR sequence (0.5 × 0.5 × 5 mm3) and a gradient echo T2*-weighted EPI sequence for functional imaging. Head motion was minimized using foam pads and forehead restraining straps. The T1-weighted images were spatially normalized using established routines for brains with large lesions and then each patient’s stroke lesion was manually mapped on the T1-weighted images using the co-registered FLAIR images as an additional guide to confirm location and extent of the chronic lesion (Brett et al., 2001; Karnath et al., 2004; Rorden & Karnath, 2004). Furthermore, we calculated an overlap of each lesion with a canonical corticospinal tract (CST) derived from a group of ten age-matched healthy control subjects (55.8 ± 12.7 years) using diffusion tensor imaging. The detailed methods for this analysis were published recently (Zhu et al., 2010). We used lesion volume as well as CST-lesion load, a combined measure of lesion site and size that has been shown to be an excellent predictor of motor impairment in chronic stroke patients, to test for group differences at baseline.
Table 1.
Mean baseline measures of patients in the cathodal and sham groups
| Cathodal | Sham | |
|---|---|---|
| Age | 61 ± 12 | 56 ± 15 |
| Sex | S male, 2 female | 4 male, 3 female |
| Months post-stroke | 33 ± 20 | 28 ± 28 |
| Affected hemisphere | 3 left, 4 right | 5 left, 2 right |
| Baseline UE-FM | 30 ± 11 | 31 ± 10 |
| Lesion volume (cc) | 90 ± 78 | 59 ± 79 |
| CST Lesion load (cc) | 1.6 ± 1.5 | 1.2 ± 0.8 |
None of the measures showed a significant between-group difference (all p-values > 0.05).
2.2. tDCS and occupational therapy (OT)
After baseline motor evaluations patients were randomly assigned to start with either real (cathodal) tDCS+OT or sham tDCS+OT. TDCS was delivered using a Phoresor® II Auto (Model No. PM850, IOMED®, Salt Lake City, Utah, USA) through two sponge electrodes (for more technical details see Schlaug & Renga, 2008). The real tDCS+OT sessions consisted of 30 minutes of 1 mA direct current with the cathodal electrode over the non-affected (contralesional) motor region (either C3 or C4 of the 10–20 EEG system) and a reference electrode over the contralateral supraorbital region. Typically the occupational therapy and the tDCS treatment were started at the same time; each patient received 60 min of occupational therapy and simultaneously 30 minutes of tDCS (during the first 30 minutes of OT) each day for 5 days in a row. Repeat assessments using Range-Of-Motion (ROM) and UE-FM were done 7 days after the end of the 5-day treatment. fMRI was done prior to the treatment phase and in the week after the treatment. The sham tDCS intervention was done by turning the stimulator on, letting it ramp up to the desired current strength and then turning it off without the patient or therapist noticing it (see also Gandiga et al., 2006; Schlaug & Renga, 2008; Schlaug et al., 2008; Vines et al., 2008a; Vines et al., 2008b for details on the sham treatment approach in tDCS). As patients received tDCS, an experienced occupational therapist performed OT focusing on proprioceptive neuromuscular facilitation (PNF) techniques. The OT was blinded as to whether patients received real or sham tDCS. Typically patients would be trained to combine shoulder abduction, external rotation, elbow extension and forearm pronation into one single movement across the midline. As far as possible (within the extent of individual disabilities), the therapist made sure that all patients received similar exercises for the same amount of time. The movements used in the fMRI experiment were not specifically trained in the therapy sessions, although the individual movements tested may have been part of a multi-joint movement program.
2.3. Primary outcome measures
Range-Of-Motion (ROM)
ROM for shoulder abduction, elbow extension & wrist extension, (calculated as active ROM* 100/passive ROM for each joint) was one of our two primary outcome measures. A mean of the ROM scores from these three joints (hence called 3J-ROM) was calculated after the 5-day intervention and then again 7 days later (day 12) in addition to two baseline assessments.
Upper-Extremity Fugl-Meyer Assessment (UE-FM)
The UE-FM score was our second primary outcome measure for motor improvement. UE-FM was done for each patient at baseline and 7 days after the end of the 5-day treatment period. The change in UE-FM score compared to the baseline UE-FM was then calculated as a percentage ((post-pre* 100)/pre) for each patient. The 3J-ROM and the FM assessments were done by an investigator who was blind with regard to whether real tDCS or sham tDCS was applied.
2.4. Secondary outcome measure
FMRI was mainly used as a predictor of therapeutic response and also to understand the neural correlates of improvements in the primary outcome measures of the investigation. All patients performed one of the two motor tasks while in the scanner: 1) full wrist extension and flexion, or if they could not perform reliably the wrist extension and flexion movements, they performed 2) full elbow flexion and extension up to the maximum excursion they could perform while still synchronizing with an auditory pacer. The same paced movements were repeated after the intervention. Subjects performed these movements unimanually, first using the non-affected hand and then the affected hand. Movements were paced by a metronome which they heard through MRI compatible headphones. Subjects were asked to have their eyes closed, listen to the metronome and make a full excursion (flexion and extension) at a rate of 1 Hz. One of the investigators stood beside the subject in the scanner to observe whether the tasks were performed as instructed and at the required pace and also watched for possible mirror movements.
2.5. Image acquisition and data analysis
Subjects were scanned using a 3T GE scanner. A gradient echo T2* weighted echo planar (EPI) sequence was used to acquire 32 contiguous axial slices, parallel to the anterior-posterior commissure plane and covering the entire brain. A block design of 35 s ON (task) and 35 s OFF (rest) epochs was used. There were five acquisitions per epoch, with a clustered volume acquisition time of 2.6 s and a delay of 4.4 s between acquisitions (effective TR = 7 s). A long TR was used so that patients were able to hear the metronome well during the interscan intervals and to exclude the possibility that the scanner noise itself could serve as a metronome for movements. A set of axial Diffusion Weighted and FLAIR images were also acquired to rule out the possibility of any new infarct (for more details of the fMRI acquisition see Nair et al., 2007).
FMRI data from one patient was discarded due to extensive head motion while performing the motor tasks (more than 5 mm movements in inferior-superior direction), resulting in thirteen good data sets. Off-line data pre-processing including image realignment, normalization and spatial smoothing (FWHM 8 mm) was done using SPM. For patients with extensive lesions we used MRIcro (Rorden & Brett, 2000) to mask the lesion in order to perform an accurate spatial normalization (Brett et al., 2001). Voxels with task-related activity were identified by using the General Linear Model (GLM) and a boxcar reference vector was convolved with the canonical hemodynamic response function to model the expected blood oxygenation level dependent (BOLD) response. A template of the primary motor cortex (corresponding roughly to the posterior bank of the precentral gyrus) (see www.fmri.wfubmc.edu/downloads/WFUPickAtlas for details) of each hemisphere was used to determine regional mean beta-parameters (that is, the coefficient of the task effect in the GLM) before and after the experimental intervention. Regional beta-values were determined to examine whether a relationship existed between a possible decrease in contralesional beta (due to the cathodal stimulation) and an improvement in Fugl-Meyer score in the affected limb.
3. Results
Although there were slight between-group differences in age at treatment, time-post stroke, lesion size, and CST lesion load (see Table 1 for baseline measures), none of these small differences were significant suggesting that our two groups were relatively well matched. The mean baseline fluctuation in 3J-ROM in our patients was 2.26% (±0.8%). An average of the two baseline assessments was used for further analysis. The mean proportional improvement in 3J-ROM in the affected upper extremity was 19.2% (SD 11.3) at the end of the 5-day intervention and 17.5% (SD 12.8) one week later (day 12) for the patients receiving cathodal tDCS+OT. The mean proportional improvement in 3J-ROM for the sham tDCS+OT group was 3.6% (SD 1.7) at the end of the 5-day intervention and 4.2% (SD3.1) one week later (see Table 2). A repeated measures analysis of variance with factors TIME and GROUP showed a significant effect of TIME (F(2,11) = 13.89, p = 0.001) and a significant interaction between TIME and GROUP (F(2,11) = 12.0, p = 0.002) suggesting that the effect of TIME was different between the cathodal tDCS and sham tDCS groups for ROM. Pairwise posthoc comparisons (Bonferroni adjusted for multiple comparisons) showed significant differences in ROM changes between day 5 and baseline as well as between day 12 and baseline (both p-values < 0.04) but not between day 5 and day 12 (p > 0.05).
Table 2.
Outcome measures and their changes over time
| Cathodal | Sham | |
|---|---|---|
| Baseline UE-FM | 29.6 ± 11.4 | 30.6 ± 10.2 |
| UE-FM 1 week later (day 12) | 33.7 ± 12.9 | 32.3 ± 9.8 |
| Absolute change in UE-FM (day 12)* | 4.14 ± 2.7 | 1.61 ± 1.5 |
| Proportional change in UE-FM (day 12)* | 13.9 ± 7.7 | 6.4 ± 7.2 |
| Baseline 3J-ROM | 0.67 ± 0.19 | 0.72 ± 0.11 |
| 3J-ROM after end of 5-day intervention (day 5) | 0.78 ± 0.19 | 0.73 ± 0.13 |
| 3J-ROM 1 week later (day 12) | 0.78 ± 0.20 | 0.75 ± 0.13 |
| Absolute change in 3J-ROM (day 12)* | 0.10 ± 0.05 | 0.03 ± 0.02 |
| Proportional change in 3J-ROM (day 12)* | 17.5 ± 12.8 | 4.2 ± 3.1 |
3J-ROM values at baseline, at end of the intervention (day 5) and one week later (day 12). UE-FM scores were done at baseline and one week after the end of the 5-day intervention (day 12). Proportional change scores are in percentage. Between group differences marked by * were statistically significant. For details of statistical tests see Results section.
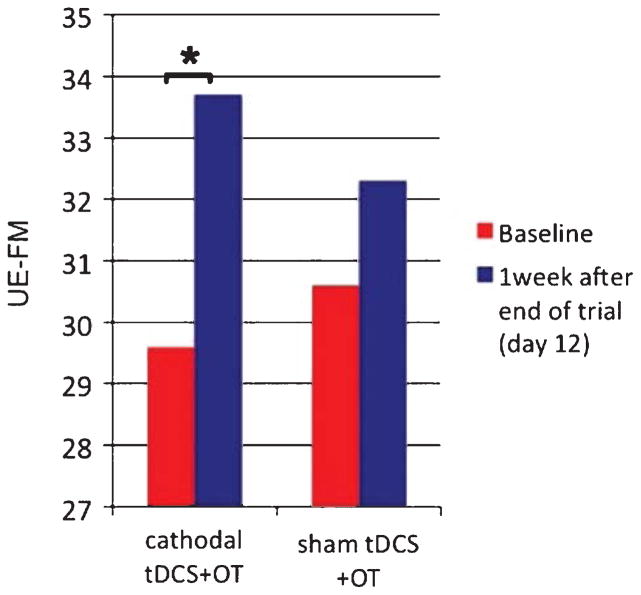
UE-FM assessments were only done twice (at baseline and then again one week after the end of the 5-day intervention, on day 12). The mean proportional change in UE-FM scores, tested 1 week after the end of the 5-day intervention, was 13.9% (7.7) with an absolute mean improvement of 4.14 points (SD 2.7) in the cathodal tDCS+OT group. The mean proportional change in UE-FM scores in the sham tDCS+OT group was 6.4% (SD 7.2) with an absolute mean improvement of 1.6 points (SD 1.5) (see Table 2 and Fig. 2). Again, an analysis of variance with factors TIME and GROUP showed a significant effect of TIME (F(1,12) = 24.9, p < 0.001) and a significant interaction between TIME and GROUP (F(1,12) = 4.8, p = 0.048) suggesting that the effect of TIME was different between the cathodal tDCS and sham tDCS groups for UE-FM scores.
Fig. 2.
Absolute UE-FM scores at baseline and 1 week after the 5-day intervention. The bar graph shows the significantly (*) higher changes in the cathodal tDCS+OT group compared to the sham tDCS+OT group.
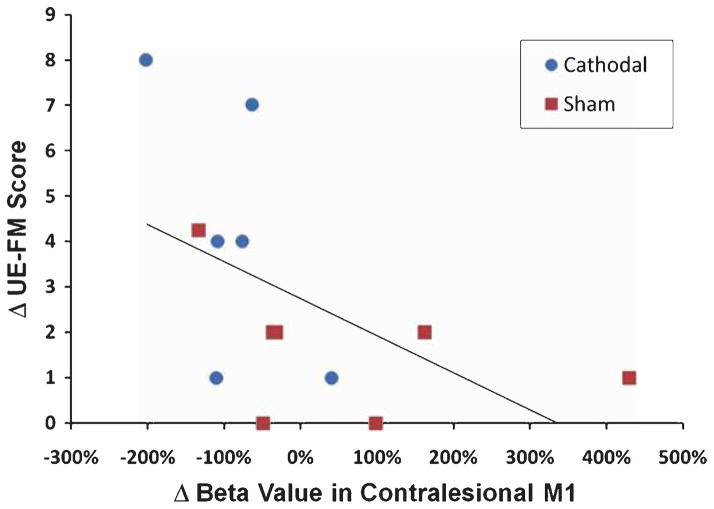
The magnitude of the activation (beta values) in the contralesional motor region decreased in the majority of patients (5 out of 7) treated with cathodal tDCS to the contralesional motor region, while only 3 out of 6 patients in the sham group showed slight decreases. Figure 3 shows an example of a decrease in motor activation in the contralesional hemisphere in a patient undergoing cathodal tDCS. Across the entire group of patients we found an inverse correlation between the change in contralesional activation and a positive change in the UE-FM score (R2 = 0.275; p = 0.033; one–tailed). Thus, the more the activation decreased (between pre- and post-therapy imaging time points) in the contralesional motor region when the affected hand performed a motor task, the greater the improvement in the UE-FM score (Fig. 4).
Fig. 3.
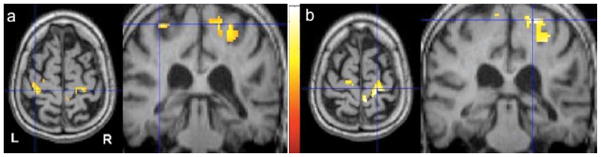
FMRI activation pattern of a motor task in a stroke patient before and after cathodal tDCS applied to the contralesional hemisphere. FMRI study of paced wrist flexion and extension movements before (Fig. 2a) and after (Fig. 2b) 5 days of cathodal tDCS+OT. It has been shown that the ipsilateral (to the moving hand) sensorimotor cortex can become active when a patient performs a flexion and extension movement with the recovering wrist. Applying cathodal stimulation to the nonlesional motor cortex – the left hemisphere in this patient - significantly decreased this activation and was associated with an improvement in this patient’s functional motor status. There was also an increase in activation in the motor region of the lesional hemisphere (right hemisphere) in this patient. However, this effect was seen in some patients, but was not significant across the entire group. Functional images are thresholded at p < 0.05 (FWE corrected).
Fig. 4.
Correlation of improvement in UE-FM and change in activation in the contralesional motor cortex. There was a strong trend for an inverse correlation between a greater decrease in contralesional motor cortex activation and a larger improvement in UE-FM scores after the 5-day intervention. Patients in the cathodal tDCS+OT group are marked in blue and patients in the sham tDCS+OT group are marked in red.
4. Discussion
This study shows that a combined central and peripheral stimulation protocol using cathodal tDCS applied to the contralesional hemisphere (central stimulation) and occupational therapy (peripheral sensorimotor stimulation) enhances motor function in chronic stroke patients with moderate to severe motor impairment, more than a similarly intense protocol of sham tDCS with OT. Results of this single center, randomized, double blind, and sham controlled trial suggest that there is efficacy to the combined peripheral and central stimulation approach in chronic stroke patients. The use of cathodal stimulation applied to the contralesional hemisphere is based on a model of presumed interhemispheric imbalance after a unihemispheric stroke. This hypothetical model of impairment provides a framework for three facets of interventions using non-invasive brain-stimulation: (1) up-regulating excitability of intact portions of the ipsilesional motor cortex, (2) down-regulating excitability of the contralesional motor cortex to modulate its unrestrained inhibitory influence on ipsilesional regions, or (3) a combination of both approaches in a dual hemispheric stimulation paradigm. Pilot and proof-of-principle studies, using either rTMS (Mansur et al., 2005; Nowak et al., 2008; Yozbatiran et al., 2009) or tDCS (Celnik et al., 2009; Fregni et al., 2005; Hesse et al., 2007; Hummel & Cohen, 2005; Hummel et al., 2006; Lindenberg, 2009; Schlaug et al., 2008) have shown all three approaches to have beneficial effects on motor functions after a stroke and motor skill acquisition in normal subjects. Cathodal tDCS decreases excitability in the brain tissue under the electrode (Nitsche & Paulus, 2000), in our case, the contralesional M1. A few previous studies, mostly single session studies, have also shown that decreasing excitability of the contralesional hemisphere by TMS (Boggio et al., 2006; Mansur et al., 2005; Nowak et al., 2008) or tDCS (Fregni et al., 2005), results in improvement in motor function in stroke patients, most likely by decreasing the transcallosal inhibition from the contralesional to the lesional hemisphere. Considering the size and location of the electrode, it is possible that cathodal stimulation applied at C3 or C4 of the contralesional hemisphere not only affected the primary motor cortex, but also the adjacent premotor cortex. Imaging studies in well-recovered patients have shown that brain reorganization during the recovery phase is associated with re-activation or over-activation of unimpaired sensorimotor and premotor networks in the lesional hemisphere (Calautti & Baron, 2003; Cramer et al., 2002; Loubinoux et al., 2003; Nair et al., 2007) in addition to activation in the contralesional motor regions. The significance of this activation in the contralesional motor regions when the affected arm/hand performs a motor task is a matter of active research in several laboratories (Johansen-Berg et al., 2002; Lotze et al., 2006). Down-regulating excitability of contralesional motor regions has consistently resulted in transient improvements of motor function in chronic stroke patients (Mansur et al., 2005; Nowak et al., 2008) which has been attributed to a modulation of a potentially unbalanced inhibitory effect of the contralesional hemisphere (Lang et al., 2004).
Our primary outcome variables (3J-ROM and UE-FM scores) were sensitive enough to detect a meaningful (for the patient) change in motor measures as a function of the combined central and peripheral intervention. An increase in ROM over the baseline fluctuation was observed, not only during the cathodal but also in the sham group. But the improvement in ROM in the sham group was only marginal, probably because traditional OT by itself may not change motor function significantly in our patients, who being several months out of their strokes, had relatively stable deficits. A similar small change in motor function was seen in a recent study in which patients in one arm of the study received traditional intense physical/occupational therapy, although the patients in this multicenter study were more severely affected than the patients in our study (Lo et al., 2010). The improvement in ROM and UE-FM was significantly greater even in our chronic stroke population when central and peripheral stimulations were combined. This result encourages us to surmise that tDCS-induced changes in excitability and OT may have served as converging inputs to the lesional M1 (via cortico-cortical and intracortical pathways) and induced a mechanism similar to Hebbian or associative LTP. Paired stimulation (in vivo and in vitro) has been successfully used in the past to induce associative LTP in M1 (Baranyi & Feher, 1981; Hess et al., 1996; Hess & Donoghue, 1994) and as suggested by our data, has facilitating effects on motor recovery in chronic stroke patients. There is some evidence already that a combination of tDCS and peripheral stimulation, either peripheral sensory nerve stimulation or peripheral sensorimotor activities as part of occupational/robotic therapy, seems to enhance the effects of either intervention by itself (Celnik et al., 2009; Hesse et al., 2007; Lindenberg et al., 2010b).
Previous studies (mainly single session studies) have reported electrophysiological effects lasting up to 90 minutes and behavioral effects lasting up to 30 minutes after a 20–30 min session of direct current stimulation (Nitsche et al., 2003; Nitsche & Paulus, 2001). A more recent study in healthy subjects, indicated that daily applications of anodal tDCS over the scalp location of the primary motor cortex for 5 days in association with motor training led to a substantial improvement in skill acquisition through facilitation of off-line learning that remained present for 3 months after the end of the training-stimulation period relative to controls (Reis et al., 2009). The present study in chronic stroke patients undergoing 5 days of central and peripheral stimulation suggests that the improvement lasted at least one week post-stimulation and possibly longer as seen in some patients coming back for follow-up appointments 4 weeks after the end of the study, although no formal longer-term outcome assessment was done in our patients beyond one week. The optimal number and duration of experimental neurorehabilitation sessions in chronic stroke patients with stable neurological deficits remains to be determined. Current data suggests that changes of more than 4 points in the UE-FM are possible after multiple combined central and peripheral stimulation sessions in chronic stroke patients with moderate impairment (see also Lindenberg et al., 2010b) which is more than the approximately 1 point change in UE-FM observed with an intense peripheral stimulation protocol only (Lo et al., 2010), although patients were much more severely impaired in this multicenter study.
It seems that combining central and peripheral stimulation jumpstarts the recovery network providing greater recovery potential than when peripheral stimulation is given alone. A possible explanation of the enhanced effect is that the combination of peripheral sensorimotor activities (which provides increased sensory feedback to the cortex) and the simultaneous modulation of intrinsic cortical excitability might enhance skill acquisition/consolidation through synaptic plasticity and long-term potentiation-like mechanism (Fritsch et al., 2010; Stefan et al., 2002). This is supported by a recent publication combining peripheral sensory stimulation with noninvasive brain stimulation (Celnik et al., 2009). Cortical stimulation studies in experimental stroke models have shown stronger effects when peripheral sensorimotor activities were combined with central stimulation (Adkins-Muir & Jones, 2003).
Functional MRI was specifically used in this study to examine changes in activation in the precentral gyrus region. Finding a correlation between changes in activation on the contralesional hemisphere and motor improvement suggests that cathodal tDCS when applied to the contralesional hemisphere resulted in a reduced fMRI activation level, presumably due to a decrease in the abnormal (post-stroke) excitability levels in that hemisphere.
In summary, our results confirm that tDCS is a safe, non-invasive technique with minimal adverse effects that can be routinely used in the clinical setting for motor rehabilitation in chronic stroke patients. Cathodal tDCS combined with OT might have synergistic effect on neural plasticity and motor recovery, compared to OT with sham stimulation which is essentially OT only, since sham stimulation would have no biological effect (although a placebo effect cannot be completely ruled out). Obviously, there are still several open questions such as the optimal timing of this combined intervention in the recovery process (all of our patients were chronic stroke patients), the optimal number and duration of treatment sessions, and effects of various stimulation parameters (e.g., current strength, electrode montage) that could be altered to optimize the stimulation conditions.
Acknowledgments
This research work was supported by grants from the National Institute of Health (RO1 NS045049, RO1 DC008796), CIMIT, Mary Crown and William Ellis Family Fund.
References
- Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: Enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Feher O. Synaptic facilitation requires paired activation of convergent pathways in the neocortex. Nature. 1981;290:413–415. doi: 10.1038/290413a0. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Alonso-Alonso M, Mansur CG, Rigonatti SP, Schlaug G, et al. Hand function improvement with low-frequency repetitive transcranial magnetic stimulation of the unaffected hemisphere in a severe case of stroke. Am J Phys Med Rehabil. 2006;85:927–930. doi: 10.1097/01.phm.0000242635.88129.38. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40:1764–1771. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Mark A, Barquist K, Nhan H, Stegbauer KC, et al. Motor cortex activation is preserved in patients with chronic hemiplegic stroke. Ann Neurol. 2002;52:607–616. doi: 10.1002/ana.10351. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. NeuroImage. 2005a;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005b;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Ebisu T, Naruse S, Horikawa Y, Ueda S, Tanaka C, et al. The application of in vivo diffusion weighted magnetic resonance imaging to intracranial disorders. No To Shinkei. 1991;43:677–684. [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophys. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophys. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: A pilot study. Restor Neurol Neurosci. 2007;25:9–15. [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Voller B, Celnik P, Floel A, Giraux P, et al. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Fruhmann Berger M, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cereb Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hallett M, Samii A, Oddo D, Celnik P, et al. Motor cortex excitability in patients with cerebellar degeneration. Clin Neurophysiol. 2000a;111:1157–1164. doi: 10.1016/s1388-2457(00)00308-4. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000b;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010a;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke. Neurology. 2010b;75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen L. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. NeuroImage. 2007;34:253–263. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation–technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Renga V. Transcranial direct current stimulation: A noninvasive tool to facilitate stroke recovery. Expert Rev Med Devices. 2008;5:759–768. doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008a;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Nair D, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci. 2008b;28:1667–1673. doi: 10.1111/j.1460-9568.2008.06459.x. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: A computer-based human model study. NeuroImage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozbatiran N, Alonso-Alonso M, See J, Demirtas-Tatlidede A, Luu D, et al. Safety and behavioral effects of high-frequency repetitive transcranial magnetic stimulation in stroke. Stroke. 2009;40:309–312. doi: 10.1161/STROKEAHA.108.522144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]