Abstract
Background
Elevated pediatric asthma morbidity has been observed in rural US communities, but the role of the ambient environment in exacerbating rural asthma is poorly understood.
Objectives
To investigate associations between particulate matter less than 2.5 μm in diameter (PM2.5) and pediatric asthma exacerbations in an agricultural community of Washington State.
Methods
School-aged children with asthma (n=58) were followed for up to 25 months with repeated measures of respiratory health. Asthma symptoms and quick-relief medication use were assessed biweekly through phone administered surveys (n=2023 interviews). In addition, subjects used home peak flow meters on a daily basis to measure forced expiratory volume in one second (FEV1) (n=7830 measurements). Regional PM2.5 was measured at a single air monitor located centrally in the study region. To assess relationships between PM2.5 and these outcomes we used linear regression with generalized estimating equations, adjusting for meteorological and temporal confounders. Effect modification by atopy was explored as well.
Results
An interquartile increase (IQR) in weekly PM2.5 of 6.7 μg/m3 was associated with an increase in reported asthma symptoms. Specific symptoms including wheezing, limitation of activities, and nighttime waking displayed the strongest associations. FEV1 as a percent of predicted decreased by 0.9% (95%CI: −1.8, 0.0) for an IQR increase in PM2.5 one day prior, and by 1.4% (95%CI: −2.7, −0.2) when restricted to children with atopic asthma.
Conclusions
This study provides evidence that PM2.5 in an agricultural setting contributes to elevated asthma morbidity. Further work on identifying and mitigating sources of PM2.5 in the area is warranted.
Keywords: PM2.5, pediatric asthma, asthma exacerbations, rural asthma, agricultural air quality
1. INTRODUCTION
Several longitudinal cohort studies have demonstrated that children with asthma experience short-term increases in symptoms as well as decrements in lung function following exposure to outdoor particulate matter with aerodynamic diameter of 2.5 μm or less (PM2.5) (Ostro et al. 2001; Slaughter et al. 2003; Delfino et al. 2004; Moshammer et al. 2006; Trenga et al. 2006; Dales et al. 2009; Gent et al. 2009). While this association has been well-described in urban settings, it remains largely unexplored in rural, agricultural communities.
Relationships between particulate matter (PM) exposure and respiratory health observed for urban children may not be generalizable to rural regions because of important differences in PM composition. Rural PM tends to contain a higher proportion of organic dust (Schenker et al. 1998), which is derived from plants, animal cells, insects, mold and fungi. In general, organic dusts are pro-inflammatory and cause airway inflammation and obstruction following inhalation (Schwartz 1999). These urban-rural differences in composition reflect varying PM2.5 sources. In cities, motor vehicle exhaust is a major source of PM2.5, and wood burning and industrial point emissions contribute to lesser degrees (Maykut et al. 2003). In contrast, rural PM2.5 often is generated by agricultural activities, residential wood burning, and natural processes. Tilling, harvesting and field burning disperse an estimated 936 thousand tons of PM2.5 into the atmosphere every year, accounting for about 16% of all outdoor PM2.5 in the US (Aneja et al. 2009). In addition, large-scale agricultural operations emit gases that indirectly increase regional PM2.5 concentrations (NRC 2003). For example, large facilities for animal confinement release substantial amounts of ammonia gas, which subsequently reacts with nitric oxides and sulfuric acid in the atmosphere to form ammonium salt aerosols, a component of PM2.5 (Aneja et al. 2009).
While past research indicated that asthma prevalence may be lower for children living on or near farms (Reidler et al. 2001; Gergen et al. 1988), recent investigations suggest that asthma morbidity in the rural US is as high or higher than in urban communities (Chrischilles et al. 2004; Pesek et al. 2010; Malik et al. 2012). Rural communities often face unique barriers to asthma diagnosis and management, such as limited access to health care, poor insurance coverage, poverty, and geographic isolation (Valet et al. 2009; Ownby 2005).
Aggravating Factors of Asthma in a Rural Environment (AFARE) is a community-based participatory research project aimed at identifying airborne asthma exacerbators in the ambient environment of an agricultural community. Here we describe relationships between community-wide temporal changes in PM2.5 and asthma morbidity for AFARE children using a longitudinal, repeated measures study design. We also explored whether atopy is associated with increased susceptibility to PM2.5 in this rural community.
2. METHODS
2.1 Study setting
The study took place in the Yakima Valley of Washington State, an area covering approximately 300 square miles and characterized by a high density of large-scale agricultural operations, including tree fruit orchards and dairy farms. The AFARE Study was conducted within El Proyecto Bienestar, a community-based participatory research partnership between the University of Washington Pacific Northwest Center for Agricultural Safety and Health; Yakima Valley Farm Worker Clinics (YVFWC), a network of federally-qualified health clinics serving migrant and seasonal farmworker families as well as other underserved populations in the region; and the Northwest Community Education Center which includes Radio KDNA, a Spanish language public radio station that provides support and education for the Latino community in the Yakima Valley.
2.2 Study subjects
AFARE subject recruitment began in August 2010 and continued throughout the first year of the study toward a goal of at least 50 participants. Subjects were invited to participate in the study if they were involved in the YVFWC Asthma Program, were of school age, had no other serious illnesses and intended to stay in the region during the two-year duration of the study. The Asthma Program is a longstanding clinical service delivered by community health workers at the patient’s home, providing education about asthma management, including proper medication use and home indoor trigger identification and control (Postma et al. 2011). In total, 59 subjects were enrolled in the study, and 10 (17%) dropped out prior to the end of AFARE data collection. One subject who dropped out immediately following enrollment was excluded from analysis, leaving a sample size of 58 participants. Data from the other 9 subjects who left the study were retained because they participated for a substantial amount of time and the decisions to end participation were unrelated to health or exposure status.
Research activities involving human subjects were approved by the University of Washington Institutional Review Board. Informed consent was obtained from all parents as well as assent from the child if child was 13 years or older.
2.3 Baseline health assessment
At enrollment, subjects and caretakers completed a health history survey to determine clinical features of asthma status including current medication use. All subjects also underwent skin prick testing to identify children with atopic asthma. Subjects were told to withhold from antihistamine use for 72 hours prior to testing. Three disposable multiple test skin prick applicators were applied to the volar aspect of the subject’s lower arm. (Multi-Test II, Lincoln Diagnostics, Decatur IL, USA). Antigens included 22 aeroallergens that comprised typical indoor inhalant allergens (mouse, cat, dog, dust mite mix, cockroach mix, mold mix) and area specific aeroallergens (cow, horse, western juniper, cottonwood, wheat, alfalfa, kochia, smut mix, sagebrush, alder, pigweed, western ragweed, johnson grass, russian thistle), as well as histamine (positive control), and saline (negative control) (ALK Technologies Inc., Princeton NJ, USA). The skin reaction was assessed 20 minutes after application, and a positive response was defined as a wheal size equal to or greater than the positive control.
2.4 Longitudinal health assessment
Longitudinal asthma morbidity was assessed with two tools: an asthma symptom survey and daily home lung function tests.
2.4.1 Biweekly asthma Symptom survey
At approximately two-week intervals, phone interviews with either the child or an adult family member were conducted. Interviewees were asked to recall the one week period prior to the interview date in their responses. The interview included five questions about asthma symptoms (nighttime waking, shortness of breath, limitation of activities, wheezing, and morning asthma symptoms) with ordinal categorical responses to indicate increasing severity and frequency. A sixth question ascertained frequency of short-acting bronchodilator use as average number of “puffs” per day.
2.4.2 Daily home lung function tests
At enrollment, each child received a PikoNET handheld peak flow meter (PFM) with digital memory (nSpire Health Inc., Longmont CO, USA) and was instructed in proper use of the device according to American Thoracic Society (ATS) guidelines. Children were asked to use the PFM twice daily on every day of the study, refraining from the use of short-acting bronchodilator medication immediately prior to PFM use. At approximately six-week intervals, a staff member from YVFWC visited participants and uploaded PFM measurements from the participant’s device. During a 12-month follow-up visit with the research team at clinics, each subject’s technique and ability to produce an error-free measurement was observed, and subjects were retrained in PFM use if necessary. Use of the PFM produced a value of FEV1, which was converted into the percent of predicted value (FEV1%) based on standard reference equations (Hankinson et al. 1999). The highest value of those recorded for a subject in a day was used in analysis.
2.5 PM2.5 measurements and meteorology
We obtained 24-hour average PM2.5 concentrations based on nephelometer measurements made at a central site air monitor in Toppenish, WA, managed by the Yakama tribe and included in the WA State Department of Ecology air monitoring network (https://fortress.wa.gov/ecy/enviwa/). Two local weather stations most central to the homes of participants (AgWeatherNet database for Toppenish and Snipes stations) provided data on 24-hour average temperature, relative humidity and precipitation.
2.6 Statistical analysis
All analyses were performed using Stata 12.0 (StataCorp LP, Texas Station TX, USA). Subject characteristics pertaining to demographics and baseline health status were summarized for the cohort overall as well for subgroups defined by atopy status. Comparisons of characteristics between atopic and nonatopic children were made using chi-squared tests of homogeneity for categorical quantities and t-tests with unequal variances for continuous values. Spearman correlation coefficients were calculated to assess pairwise correlations among meteorology variables and PM2.5.
2.6.1 Epidemiologic analysis
We modeled associations between PM2.5 and each outcome using linear regression models based on generalized estimating equations (GEE) (Diggle et al. 2002) with autoregressive-1 (AR-1) or exchangeable working correlation structures to account for the correlation among the repeated measures for each subject. (Exchangeable correlation structures were used for FEV1% models on account intermittently missing data, while AR-1 correlation was used in analysis of reported asthma symptoms. Results were qualitatively similar when correlation structures were varied in sensitivity analysis.) In all models, the exposure of interest was included in models as a continuous variable, and we present results as the mean change in outcome for an IQR increase in exposure, assuming a linear relationship. Covariates included in models as potential confounders were selected a priori based on existing evidence of relationships with both respiratory health and exposure. The effects of continuous adjustment variables such as temperature, relative humidity, precipitation, elapsed week of study, and seasonality (calendar month) were represented by cubic splines with five knots each. Other covariates used for adjustment were subject-specific characteristics associated with asthma health, including sex, age, atopy, use of inhaled corticosteroids at baseline, and BMI at baseline. In sensitivity analysis, an interaction term was added to each model to assess the presence of effect modification by atopy.
For models in which outcome was derived from biweekly symptom surveys, exposure was calculated as the average PM2.5 over the seven days prior to the interview date, referred to as the weekly average PM2.5. Associations between PM2.5 and individual symptoms types were estimated by dichotomizing responses to each question as no symptom or medication use versus any symptom or medication use. Logistic regression with GEE was used to estimate the odds ratio (OR) for report of each symptom with an IQR increase in weekly PM2.5.
For models in which daily FEV1% was the outcome of interest, the 24-hour average PM2.5 measured one day prior to FEV1% measurement was used as the primary exposure of interest, and other lags were evaluated in sensitivity analyses (0, 2, 3, and 4 day lags). Values of FEV1% that were implausibly high (above 150%) or low (below 30%) were excluded from analysis. In addition, PFM measurements that were flagged by the device as potential errors were omitted from analysis even though our overall results were qualitatively the same whether we included these ‘flagged’ measurements or not. Subjects with 10 valid PFM readings or more were included in analyses of FEV1 in order to exclude participants with very poor compliance and/or PFM technique.
2.6.1.1 Epidemiologic analysis: Model diagnostics
Model diagnostics were performed to determine whether the central assumptions of GEE were violated. Specifically, plots of residuals versus the linear predictor as well as exposure of interest were inspected to determine whether there existed a meaningful trend in the deviations of residuals from zero. The possibility of influential subjects was explored using the “leave one out” method, by which point estimates and corresponding standard errors were estimated after exclusion of each subject in turn and compared to results generated from analysis of the complete study sample. None of the results of these diagnostic tests indicated cause for concern about model assumptions. Finally, analyses were repeated using linear mixed models (LMM), which returned similar results to those obtained with GEE in all cases.
2.6.1.2 Lung function measurements: Missingness mechanisms and multiple imputation
FEV1 readings were not available for all subjects on every day of the study due to data loss (e.g. technical problems with the laptop and software used to upload PFM data during home visits), broken or lost devices, imperfect compliance, and exclusion of flagged or implausible measurements. We explored patterns of missingness by comparing subjects’ data completeness rates to characteristics associated with asthma morbidity, such as asthma symptom reports, average FEV1%, inhaled corticosteroid use at baseline, and atopy status using linear regression with robust standard errors. In addition, relationships between the odds of missing PFM data on a specific day for each subject and both daily PM2.5 and the average of nonmissing FEV1 in the same week were separately assessed using linear regression with GEE.
FEV1 analyses were performed both on available data [i.e. complete case (CC) analysis] as well as on imputed data in order to address the problem of missing data. To generate imputed datasets, multiple imputation was performed using a regression model that included all covariates used in adjusted epidemiologic models, as well as dummy variables to indicate subject, and ordinal categorical responses to each question on the symptom survey in the relevant time period. Fifty datasets with imputed values for each missing daily FEV1% measurement were generated (i.e. m=50). Analyses between FEV1% and one day prior PM2.5 were conducted on each dataset separately, and the estimated coefficients and standard errors derived from each were combined using Rubin’s rules (Rubin 1987).
3. RESULTS
3.1 Characteristics of AFARE cohort
The AFARE cohort consisted of an equal number of girls and boys with an average age of 10.4 years at the time of enrollment (Table 1). 41% (n=24) of participants were from families with annual household incomes below $15,000, and half (n=29) had one or more parent employed as a farmworker. In addition to having a diagnosis of asthma, 50% (n=29) had a BMI-for-age greater than the 85th percentile at baseline. All but 4 children self-identified as Hispanic and only 17% (n=10) were born outside the United States. At baseline, subjects and their families were asked about residential proximity to possible environmental exposures associated with asthma, and nearly half (n=27) reported living within 0.25 mile of a “busy” road or a dusty, unpaved road; 38% (n=22) reported living near farms growing crops; and 19% (n=11) of families said they lived near farms raising animals.
Table 1.
Demographics and baseline health description of AFARE cohorta.
| All subjects (n=58) | Atopy status (Skin Prick Test Result)b | ||
|---|---|---|---|
| Positive (n=42) | Negative (n=16) | ||
| Demographics | |||
| Female | 29 (50%) | 17 (41%) | 12 (75%) |
| Age at baseline (years) | 10.4 +/− 2.7 | 10.4 +/− 2.7 | 10.3 +/− 2.8 |
| Household income <$15k/year | 24 (41%) | 18 (43%) | 6 (38%) |
| Born outside US | 10 (17%) | 8 (19%) | 2 (13%) |
| Hispanic/Latino ethnicity | 54 (93%) | 38 (91%) | 16 (100%) |
| Parent(s) employed as farmworker | 29 (50%) | 19 (45%) | 10 (63%) |
| Residence within ¼ mile of: | |||
| Farms growing crops | 22 (38%) | 14 (33%) | 8 (50%) |
| Farms raising animals | 11 (19%) | 9 (21%) | 2 (13%) |
| Unpaved dusty roads | 27 (47%) | 18 (43%) | 9 (56%) |
| High traffic roadway | 24 (41%) | 19 (45%) | 5 (31%) |
| Asthma and general health | |||
| Daily controller medication use at baseline: | |||
| Inhaled corticosteroids (IC) | 41 (71%) | 32 (76%) | 9 (56%) |
| Leukotriene antagonist (LTRA) | 17 (29%) | 15 (36%) | 2 (13%) |
| Both IC and LTRA | 14 (24%) | 13 (31%) | 1 (6%) |
| Ever hospitalized with asthma | 38 (66%) | 29 (69%) | 9 (56%) |
| Unscheduled visit for asthma to urgent care or ED in 12 months prior to enrollment | 46 (79%) | 34 (81%) | 12 (75%) |
| Atopic asthmab | 42 (71%) | 42 (100%) | 0 (0%) |
| At least one adult smoker in household | 8 (14%) | 5 (12%) | 3 (19%) |
Abbreviations: US, United States; ED, Emergency department.
Categorical variables summarized as N (%) and continuous measures as mean +/− standard deviation
Indicated by positive skin prick test to at least one of 22 common inhalant allergens.
Most AFARE participants had experienced a significant asthma exacerbation in the past: two-thirds (n=38) reported being hospitalized at some point for asthma, and 79% (n=46) reported having at least one unscheduled urgent care or emergency department (ED) visit for asthma in the 12 months prior to study enrollment. The majority of subjects were taking at least one controller medication at baseline, with 71% taking inhaled corticosteroids, 29% taking leukotriene antagonists, and 24% taking both.
Children with atopic asthma were more likely to be taking inhaled corticosteroids or leukotriene antagonist at baseline, to have been hospitalized with asthma, to have reported an unscheduled clinic or emergency department visit for asthma in the year before enrollment, and less likely to live with at least one adult farmworker, but none of these differences reached statistical significance.
3.2 Longitudinal asthma morbidity
Subjects participated in AFARE for an average of 92 weeks. After exclusion of one subject that completed fewer than 8 interviews, there were 1948 interviews with complete data from 57 children available for analysis.
Reported symptom occurrence is summarized for all interviews collected in Table 2. Overall, the presence of each symptom was reported in fewer than half of the interviews, though the frequency of each symptom was higher for atopic children compared to nonatopic children. Likewise, at least some bronchodilator usage was reported during about half of all interviews for the overall cohort, while atopic children were more likely to report use of this rescue medication.
Table 2.
Summary of longitudinal health data collectiona
| All subjects (n=58) | Atopy statusb | ||
|---|---|---|---|
| Allergic (n=42) | Nonallergic (n=16) | ||
|
| |||
| Percent of interviews in which each symptom reported: | |||
| Woken by asthma | 45.4% | 51.5% | 27.8% |
| Limited in daily activities | 19.6% | 21.5% | 14.1% |
| Shortness of breath | 34.8% | 38.2% | 24.9% |
| Symptoms in morning | 38.7% | 42.7% | 27.0% |
| Wheezing | 24.5% | 28.9% | 21.7% |
| Percent of interviews in which puffs of bronchodilator reported | 50.7% | 55.3% | 37.4% |
| Subject average FEV1, % predicted | 75 +/− 15% | 75 +/− 16% | 76 +/− 11% |
Abbreviations; FEV1%, forced expiratory volume in 1 second as a percent of predicted value.
Continuous values presented as mean +/− standard deviation and categorical results as percent of responses.
Indicated by positive skin prick test to at least one of 22 common inhalant allergens.
During the study, 7830 lung function measurements were collected from children’s PFM devices, and the subject-average FEV1 as percent of predicted (FEV1%) was 75% (s.d. = 15%). Participants’ FEV1 increased across the study period, with average lung function growth estimated to be 0.26 L per year of participation; similarly, FEV1% increased by an average of 2.3% per year.
Subjects’ PFM data completeness rates (i.e. percent of days with at least one PFM measurement) ranged from 12 to 80% (mean = 35%). True compliance rates, however, are likely substantially higher because these estimates were affected by loss of data in the field. We detected no statistically significant relationships between subject PFM completeness rates and subject-specific rates of symptom reports, average FEV1%, atopy status, or use of inhaled corticosteroids at baseline. We also found no evidence that the odds of FEV1 missingness on a specific day of the study was related to average lung function during the same time period (week average FEV1%) or PM2.5 measured on the day before (results not shown).
3.3 Community PM2.5
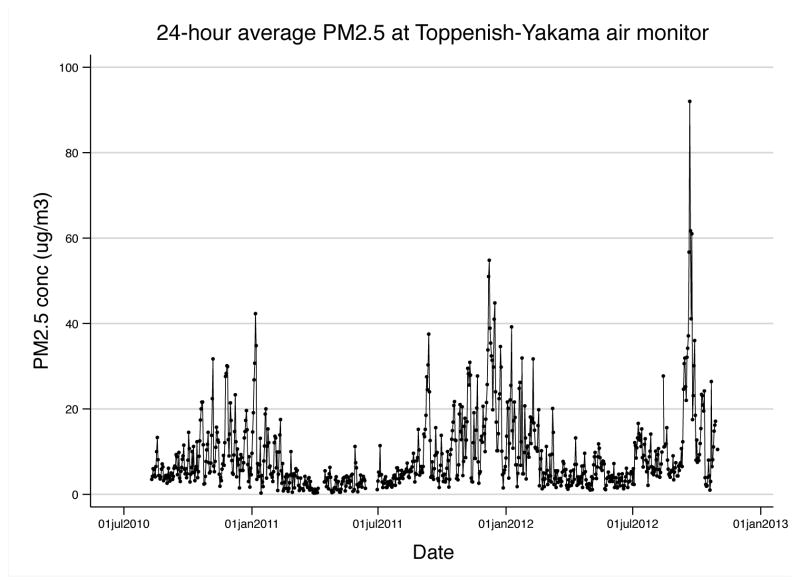
The distance between subjects’ homes to the central monitor ranged from 0.7 to 29 miles, with a median distance of 7.5 miles. Daily PM2.5 concentrations had a median (IQR) of 5.7 (7.9) μg/m3 over the entire two-year study period. In general, PM2.5 was elevated during winter months due to occurrences of stagnation events in the valley, although high PM2.5 concentrations were observed in autumn of 2011 and 2012 as a result of forest fires in the region (Figure 1). Log-transformed PM2.5 concentrations were found to be moderately and inversely correlated with windspeed (r=−0.63) and weakly correlated with relative humidity (r=0.34) and wind direction (r=−0.36).
Figure 1.
24-hour-average PM2.5 concentrations measured at the Toppenish-Yakama air monitor in the WA State Department of Ecology air monitoring network.
Abbreviations: PM2.5, particulate matter of 2.5 μm or greater in aerodynamic diameter; WA, Washington.
3.4 Associations between asthma symptoms and PM2.5
Among individual asthma symptoms assessed, the strongest association was observed with wheezing (Table 3). Specifically, with each IQR increase in PM2.5, a 31% increase in odds of wheezing was observed (95% CI: 18%, 45%). Statistically significant associations were also evident for limitations of activities and nighttime waking (OR=1.21 and 1.13, respectively, for an IQR increase in PM2.5). No effect modification by atopy was observed for any of the symptom outcomes or short acting bronchodilator use (data not shown).
Table 3.
Odds of specific asthma symptoms associated with an IQR increase in weekly PM2.5
| Symptom or medication use | OR (95%CI)a | p-value |
|---|---|---|
| Limitation of activities | 1.21 (1.00, 1.46) | 0.05 |
| Wheezing | 1.31 (1.18, 1.45) | <0.001 |
| Nighttime waking | 1.13 (1.01, 1.26) | 0.03 |
| Shortness of breath | 1.10 (0.96, 1.26) | 0.17 |
| Symptoms worse in morning | 1.00 (0.91, 1.11) | 0.97 |
| Use of short-acting “relief” medication | 1.09 (0.99, 1.20) | 0.09 |
Abbreviations: IQR, interquartile increase; PM2.5, particulate matter of 2.5 μm or greater in aerodynamic diameter.
OR is the odds ratio for report of any symptom/medication use in week prior associated with an IQR increase in weekly PM2.5 (6.9 μg/m3) after controlling for temperature, relative humidity, precipitation, seasonality and elapsed time in study (all as splines) as well as age, BMI, inhaled corticosteroid use at baseline, and sex. Results were derived from GEE model with AR1 correlation matrix.
3.5 Associations between lung function (FEV1%) and PM2.5
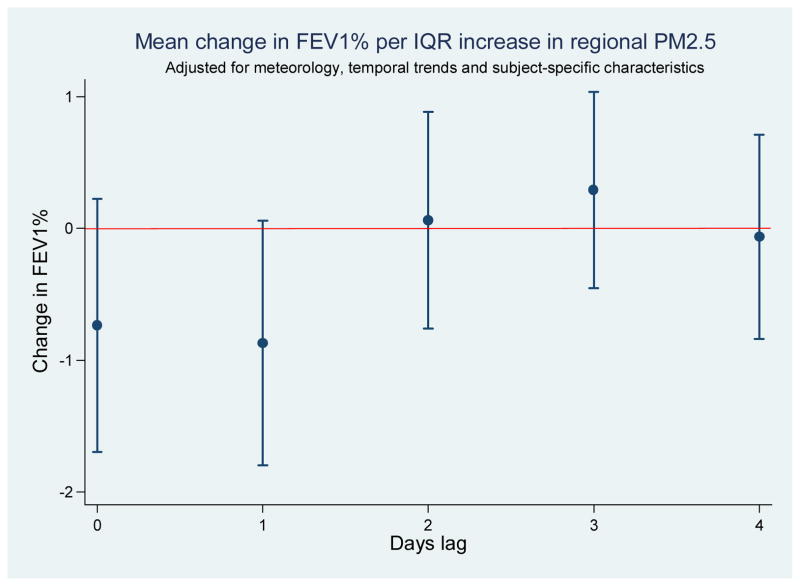
In the analysis of available FEV1% data [complete case (CC) analysis] and the analyses of 50 datasets in which missing FEV1% was imputed [multiple imputation (MI) analysis], we observed decrements in lung function associated with higher PM2.5 concentrations one day prior to FEV1% measurement (Table 4). The point estimates from CC and MI analyses were similar though results derived from the MI dataset were more precise. For CC analysis, an IQR increase in 24-hour average PM2.5 on the day prior was associated with a change in FEV1% of −0.9% (95%CI: −1.8%, 0.0%), while MI results indicated a corresponding change in FEV1% of −1.2% (95%CI: −2.0%, −0.3%). Exploratory analysis of varying lag days yielded results that were lower in magnitude and with wider 95% confidence intervals in each case (Figure 2). The decrement in FEV1% estimated for increasing PM2.5 was significantly stronger for atopic subjects in the complete case analysis (p-value for interaction = 0.014) but not when the multiply imputed dataset was analyzed (p-value for interaction = 0.55) (Table 4)
Table 4.
Association between FEV1% and IQR increase in 24-hour-average PM2.5 measured one day prior
| Complete case analysis | Multiple imputation | |||
|---|---|---|---|---|
| Subjects | Coefficient (95%CI)a | p-value | Coefficient (95%CI)a | p-value |
| All subjects (n=50)b | −0.9 (−1.8, 0.0) | 0.06 | −1.2 (−2.0, −0.3) | 0.01 |
| Atopy subgroupsc | ||||
| Atopic subjects (n= 36) | −1.4 (−2.7, −0.2) | 0.03 | −1.2 (−2.1, −0.3) | 0.01 |
| Nonatopic subjects (n= 14) | 0.5 (−0.7, 1.7) | 0.43 | −1.0 (−2.1, 0.1) | 0.10 |
Abbreviations: IQR, interquartile increase; PM2.5, particulate matter of 2.5 μm or greater in aerodynamic diameter.
Coefficient is the estimated change in FEV1% associated with an IQR increase in daily PM2.5 (7.9 μg/m3) after controlling for temperature, relative humidity, precipitation, seasonality and elapsed time in study (all as splines) as well as age, BMI, inhaled corticosteroid use at baseline, and sex. Results were derived from GEE model with exchangeable correlation matrix.
Eight subjects with fewer than 10 valid FEV1% readings on file were excluded from analysis.
Atopy was defined as at least one positive result in skin prick testing performed at baseline. p-value for interaction = 0.014 by complete case analysis and 0.55 using multiple imputation dataset.
Figure 2.
Associations between FEV1% and PM2.5 measured on multiple lag days for complete case (CC) dataa.
Abbreviations: PM2.5, particulate matter of 2.5 μm or greater in aerodynamic diameter; IQR, interquartile range.
aCoefficient is the estimated change in FEV1% associated with an IQR increase in daily PM2.5 (7.9 μg/m3) after controlling for temperature, relative humidity, precipitation, seasonality and elapsed time in study (all as splines) as well as age, BMI, inhaled corticosteroid use at baseline, and sex. Ranges represent 95% confidence intervals around each point estimate. Results were derived from GEE model with exchangeable correlation matrix.
4. DISCUSSION
Our results indicate that children with asthma in the agricultural Yakima Valley region experience short-term increases in asthma morbidity associated with increases in regional PM2.5. Adverse effects upon subjective reports of asthma symptoms (limitation of activities, more wheezing, more nighttime waking) as well as objective measures of lung function (FEV1) were observed. Our observations bear some similarities to the adverse effects of ambient PM2.5 on pediatric asthma observed in urban settings, despite likely differences in sources and composition.
Compared to monitoring sites in an urban center of WA State (i.e. Seattle), PM2.5 concentrations in the Yakima Valley were similar. A monitoring site in downtown Seattle recorded levels nearly identical to the Toppenish site (median (IQR) = 5.8 (3.2) μg/m3) during the same sampling period, while a monitor in the industrial region south of Seattle measured slightly higher PM2.5 concentrations (median (IQR) = 7.8 (4.0) μg/m3). Higher PM2.5 days (i.e. above 20 μg/m3) occurred more frequently in Toppenish than in Seattle. Forest fires in late 2012 were severe enough to cause several days of PM2.5 levels that far exceeded the EPA 24-hour National Ambient Air Quality Standards (NAAQS) for PM2.5.
This study contributes new findings to asthma research by describing relationships between respiratory health and PM2.5 exposure in a non-urban setting. Previous pediatric panel studies conducted in urban settings have yielded similar findings. For example, Ostro et al. (2001) found that African American children with asthma in Los Angeles reported a 10% increase in odds of various asthma symptoms with an IQR increase in PM2.5, even though exposures were considerably higher than those measured in our study region, with a mean PM2.5 concentration of 41 μg/m3. Dales et al. (2009) followed a cohort of children in a Canadian city characterized by high volume truck traffic, where children were exposed to PM2.5 concentrations similar to those measured in Toppenish (median (IQR) = 6.5 (6.0) μg/m3), and observed that bedtime FEV1% declined about 0.5% with each IQR increase in PM2.5 in the previous 24 hours. A small cohort (n=9) of children with asthma in Spokane, WA, a moderate sized city in Eastern Washington where some PM2.5 sources would be similar to those in the AFARE study region, were more likely to report asthma symptoms on days of higher PM2.5 with a one day lag (Mar et al. 2004). The effects measured were smaller than those observed in our study, however, with OR for symptoms generally around 1.1 for a 10 μg/m3 increase in PM2.5.
Our analyses returned mixed findings related to effect modification by atopy. Asthmatic individuals who are sensitized to one or more common aeroallergen may be more susceptible to air pollution, especially pollutants with oxidant potential (Tunnicliffe et al. 1994; Strand et al. 1998; Jenkins et al. 1999). Some investigators have hypothesized that airway inflammation following PM exposure leads to heightened permeability of airway epithelia, which in turn may enhance the allergenic potential of aeroallergens for sensitized individuals (D’Amato et al. 2005). A handful of previous panel studies provide direct evidence that atopic children with asthma do experience higher susceptibility to PM2.5. Delfino et al. (2004) measured personal PM2.5 as well as PM2.5 measured at a central site monitor and found that the observed decrements in FEV1% were stronger for atopic boys. A study of children with asthma in Fresno, CA, where PM pollution is influenced by motor vehicle traffic as well as regional agricultural activities, revealed that atopic children constituted a vulnerable subgroup in links between asthma symptoms and exposure to coarse PM (Mann et al. 2010). In contrast, other researchers hypothesize that inhalation of organic dust will stimulate airway inflammation according to nonallergic mechanisms (Schwartz 1999), implying that children with asthma will be susceptible regardless of atopy status.
There are a number of important limitations to our study. Our exposure assessment was dependent upon measurements made at a single central monitoring site, and the same daily PM2.5 concentration was assigned to each child on every day of the study. This approach captures temporal variations in regional PM2.5 but ignores any spatial variability in PM2.5 across the study area or the existence of “personal dust clouds” found in the local environment of a child, which may significantly contribute to the true PM2.5 exposure experienced by each child (Delfino et al. 2004). In using central site measurements of PM2.5 to represent exposure, we assumed that day-to-day changes in regional PM2.5 would correlate well with corresponding changes in individual-level exposures for AFARE children. If the exposure error caused by spatial variability in outdoor PM2.5 was nondifferential in the AFARE study region, this error may have the effect of biasing observed associations towards null.
Our approach to exposure assessment is further limited by the method of measuring PM2.5 concentrations. Nephelometer measurements provide mass concentration alone, affording no insight into concentrations of the specific chemical and biological PM constituents shown to be associated with respiratory effects, such as elemental carbon, wood smoke, fungal species, pollen proteins, or endotoxins. Furthermore, we cannot rule out the possibility that our observed associations between asthma morbidity and PM2.5 concentration arose due to confounding with other outdoor pollutants that frequently co-exist with PM2.5. Use of a multi-pollutant model would mitigate the influence of co-pollutant confounding, and our future analyses of pollutants measured at AFARE subject homes may permit such an analysis.
Data completeness rates for one of our outcomes, home lung function tests, were variable and relatively low. This is a common limitation in collecting subject-initiated measures such as home peak flow (Redline et al. 1996). It is important to note that factors other than subjects’ lack of participation compromised completeness of FEV1 measurements, including loss of data during the process of uploading measurements from the PFM device or long wait times before replacement of broken devices, and these mechanisms are likely completely random with respect to health and exposure. We explored patterns of missingness and subjects’ data completeness rates and found no evidence that missingness and noncompliance are related to health or exposure in this study.
Despite these limitations, our study has a number of strengths. AFARE data collection included thousands of repeated measures of two distinct asthma health metrics collected over longer periods of time than many other pediatric panel studies. Panel studies are demanding of resources but are especially well suited for the study of time-varying exposures that result in short-term, reversible health effects. Because each subject is observed repeatedly during periods of relatively high and low exposure, within-subject associations between exposure and health can be analyzed, and the influence of between-subject confounding is thereby mitigated. We chose to control for temporal and meteorological variables aggressively in analysis in order to minimize the possibility that associations between PM2.5 and asthma morbidity are confounded by other covariates, a common concern in air pollution epidemiology (Lumley and Sheppard 2003). We selected regression with GEE as our primary statistical method to account for correlation of measurements within subjects, and we compared the results to those obtained using linear mixed models. Finally, we recognized the fact that missing lung function tests data could introduce bias to GEE results if the missingness mechanism was not completely at random (Diggle et al. 2002), and we used multiple imputation to impute informed guesses for missing values. Our multiple imputation model was strengthened by inclusion of asthma symptom responses within the relevant time periods.
5. CONCLUSIONS
Our results contribute evidence that PM2.5 pollution in this agricultural setting impacts the health of children with asthma, a significant finding in light of increasing PM2.5 levels in this region, which have been approaching nonattainment of EPA NAAQS in recent years. Further work on identifying and mitigating the dominant sources of PM2.5 in this area as well as similar agricultural settings is warranted, especially given the vulnerability of rural communities to adverse effects of pediatric asthma.
Funding sources
This research was supported with grant number 5R21ES17906-2 from the National Institute of Environmental Health Sciences (NIEHS). CL was supported by training grant number HD052462-01 from the NIH.
Highlights.
Little is known about environmental triggers of asthma in rural, agricultural settings
Children with asthma in an agricultural area had lower FEV1 after elevated PM2.5
Children also reported asthma symptoms more frequently after elevated weekly PM2.5
Results warrant increased attention to rural air pollution and pediatric asthma
Acknowledgments
We are grateful for the participation of research subjects and their families, and for the work of Ginger Ellingson, project coordinator. Two companies (ALK and Lincoln Diagnostics) donated supplies for skin prick testing.
Abbreviations and definitions
- AFARE
Aggravating Factors of Asthma in a Rural Environment
- CC
complete case
- ED
emergency department
- FEV1
forced expiratory volume in 1 second
- FEV1%
forced expiratory volume in 1 second as a percentage of predicted value
- GEE
generalized estimating equations
- IQR
interquartile range
- LMM
linear mixed models
- MI
multiple imputation
- NAAQS
National Ambient Air Quality Standards
- PFM
peak flow meter
- PM
particulate matter
- PM2.5
particulate matter of 2.5 μm or less
- YVFWC
Yakima Valley Farm Workers Clinic
Footnotes
Competing financial interests
The authors declare that they have no competing financial interests.
Human subjects approval
All work was reviewed and approved by the University of Washington Institutional Review Board prior to the start of research involving human subjects.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aneja VP, Schlesinger WH, Erisman JW. Effects of agriculture upon the air quality and climate: Research, policy, and regulations. Environmental Science & Technology. 2009;43:4234–4240. doi: 10.1021/es8024403. [DOI] [PubMed] [Google Scholar]
- Chrischilles E, Ahrens R, Kuehl A, Kelly K, Thorne P, Burmeister L, et al. Asthma prevalence and morbidity among rural Iowa schoolchildren. Journal of Allergy and Clinical Immunology. 2004;113:66–71. doi: 10.1016/j.jaci.2003.09.037. [DOI] [PubMed] [Google Scholar]
- D’Amato G, Liccardi G, D’Amato M, Holgate S. Environmental risk factors and allergic bronchial asthma. Clinical and Experimental Allergy. 2005;35:1113–1124. doi: 10.1111/j.1365-2222.2005.02328.x. [DOI] [PubMed] [Google Scholar]
- Dales R, Chen L, Frescura AM, Liu L, Villeneuve PJ. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: A panel study of schoolchildren with asthma. European Respiratory Journal. 2009;34:316–323. doi: 10.1183/09031936.00138908. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Quintana PJE, Floro J, Gastanaga VM, Samimi BS, Kleinman MT, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environmental Health Perspectives. 2004;112:932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle Peter J, Heagerty Patrick, Liang Kung-Yee, Zeger Scott L. Oxford Statistical Science Series. 2002. Analysis of Longitudinal Data. [Google Scholar]
- Gent JF, Koutrakis P, Belanger K, Triche E, Holford TR, Bracken MB, et al. Symptoms and medication use in children with asthma and traffic-related sources of fine particle pollution. Environmental Health Perspectives. 2009;117:1168–1174. doi: 10.1289/ehp.0800335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen PJ, Mullally DI, Evans R. National survey of prevalence of asthma among children in the United States, 1976 to 1980. Pediatrics. 1988;81:1–7. [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. American Journal of Respiratory and Critical Care Medicine. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Jenkins HS, Devalia JL, Mister RL, Bevan AM, Rusznak C, Davies RJ. The effect of exposure to ozone and nitrogen dioxide on the airway response of atopic asthmatics to inhaled allergen - dose- and time-dependent effects. American Journal of Respiratory and Critical Care Medicine. 1999;160:33–39. doi: 10.1164/ajrccm.160.1.9808119. [DOI] [PubMed] [Google Scholar]
- Lumley T, Sheppard L. Time series analyses of air pollution and health: straining at gnats and swallowing camels? Epidemiology. 2003;14:13–14. doi: 10.1097/00001648-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Malik HU, Kumar K, Frieri M. Minimal difference in the prevalence of asthma in the urban and rural environment. Clin Med insights Pediatr. 2012;6:33–39. doi: 10.4137/CMPed.S9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JK, Balmes JR, Bruckner TA, Mortimer KM, Margolis HG, Pratt B, et al. Short-term effects of air pollution on wheeze in asthmatic children in Fresno, California. Environmental Health Perspectives. 2010;118:1497–1502. doi: 10.1289/ehp.0901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhalation Toxicology. 2004;16:809–815. doi: 10.1080/08958370490506646. [DOI] [PubMed] [Google Scholar]
- Maykut NN, Lewtas J, Kim E, Larson TV. Source apportionment of PM2.5 at an urban IMPROVE site in Seattle, Washington. Environmental Science & Technology. 2003;37:5135–5142. doi: 10.1021/es030370y. [DOI] [PubMed] [Google Scholar]
- Moshammer H, Hutter HP, Hauck H, Neuberger M. Low levels of air pollution induce changes of lung function in a panel of schoolchildren. European Respiratory Journal. 2006;27:1138–1143. doi: 10.1183/09031936.06.00089605. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Air emissions from animal feeding operations: Current knowledge, future needs. National Academies Press; Washington, DC: 2003. [Google Scholar]
- Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001;12:200–208. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Ownby DR. Asthma in rural America. Annals of Allergy Asthma & Immunology. 2005;95:S17–S22. doi: 10.1016/s1081-1206(10)61005-8. [DOI] [PubMed] [Google Scholar]
- Pesek RD, Vargas PA, Halterman JS, Jones SM, McCracken A, Perry TT. A comparison of asthma prevalence and morbidity between rural and urban schoolchildren in arkansas. Annals of Allergy Asthma & Immunology. 2010;104:125–131. doi: 10.1016/j.anai.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JM, Smalley K, Ybarra V, Kieckhefer G. The feasibility and acceptability of a home-visitation, asthma education program in a rural, Latino/a population. Journal of Asthma. 2011;48:139–146. doi: 10.3109/02770903.2010.529221. [DOI] [PubMed] [Google Scholar]
- Redline S, Wright EC, Kattan M, Kercsmar C, Weiss K. Short-term compliance with peak flow monitoring: results from a study of inner city children with asthma. Pediatr Pulmonol. 1996;21:203–10. doi: 10.1002/(SICI)1099-0496(199604)21:4<203::AID-PPUL1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. J. Wiley & Sons; New York: 1987. [Google Scholar]
- Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman J, et al. Respiratory health hazards in agriculture. American Journal of Respiratory and Critical Care Medicine. 1998;158:S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- Schwartz DA. Etiology and pathogenesis of airway disease in children and adults from rural communities. Environmental Health Perspectives. 1999;107:393–401. doi: 10.1289/ehp.99107s3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Annals of Allergy Asthma & Immunology. 2003;91:346–353. doi: 10.1016/S1081-1206(10)61681-X. [DOI] [PubMed] [Google Scholar]
- Strand V, Svartengren M, Rak S, Barck C, Bylin G. Repeated exposure to an ambient level of NO2 enhances asthmatic response to a nonsymptomatic allergen dose. Eur Respir J. 1998;12:6–12. doi: 10.1183/09031936.98.12010006. [DOI] [PubMed] [Google Scholar]
- Trenga CA, Sullivan JH, Schildcrout JS, Shepherd KP, Shapiro GG, Liu LJS, et al. Effect of particulate air, pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest. 2006;129:1614–1622. doi: 10.1378/chest.129.6.1614. [DOI] [PubMed] [Google Scholar]
- Tunnicliffe WS, Burge PS, Ayres JG. Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. Lancet. 1994;344(8939–8940):1733–1736. doi: 10.1016/s0140-6736(94)92886-x. [DOI] [PubMed] [Google Scholar]
- Valet RS, Perry TT, Hartert TV. Rural health disparities in asthma care and outcomes. Journal of Allergy and Clinical Immunology. 2009;123:1220–1225. doi: 10.1016/j.jaci.2008.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]