Abstract
Drugs targeting the glutamate N-methyl-D-aspartate receptor (NMDAR) may be efficacious for treating mood disorders, as exemplified by the rapid antidepressant effects produced by single administration of the NMDAR antagonist ketamine. Though the precise mechanisms underlying the antidepressant-related effects of NMDAR antagonism remain unclear, recent studies implicate specific NMDAR subunits, including GluN2A and GluN2B, as well as the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) subunit glutamate receptor interacting molecule, PSD-95. Here, integrating mutant and pharmacological in mice, we investigated the contribution of these subunits and molecules to antidepressant-related behaviors and the antidepressant-related effects of the GluN2B blocker, Ro 25-6981. We found that global deletion of GluA1 or PSD-95 reduced forced swim test (FST) immobility, mimicking the antidepressant-related effect produced by systemically administered Ro 25-6981 in C57BL/6J mice. Moreover, the FST antidepressant-like effects of systemic Ro 25-6981 were intact in mutants with global GluA1 deletion or GluN1 deletion in forebrain interneurons, but were absent in mutants constitutively lacking GluN2A or PSD-95. Next, we found that microinfusing Ro 25-6981 into the medial prefrontal cortex (mPFC), but not basolateral amygdala, of C57BL/6J mice was sufficient to produce an antidepressant-like effect. Together, these findings extend and refine current understanding of the mechanisms mediating antidepressant-like effects produced by NMDAR-GluN2B antagonists, and may inform the development of a novel class of medications for treating depression that target the GluN2B subtype of NMDAR.
Keywords: NMDA, glutamate, PSD-95, prefrontal cortex, GluN2B, GluA1, depression
Introduction
There is growing evidence that drug treatments targeting the glutamate NMDAR may be efficacious in mood and anxiety-related disorders [1]. A single administration of non-specific NMDAR antagonists, such as ketamine, produces rapid antidepressant effects in both clinical [1–5] and relevant preclinical assays, such as the forced swim test (FST) [2–13]. Systemic administration of NMDAR antagonists, including ketamine, has been shown to increase extracellular levels of glutamate and glutamate cycling in the rodent medial prefrontal cortex (mPFC), at doses similar to those producing antidepressant-like actions [14, 15]. The effect of these drugs may be to increase glutamate transmission through synaptic proteins on excitatory cells and exert an excitatory postsynaptic effect on pyramidal neurons – possibly due to antagonism of interneuronal NMDARs that normally exert tonic inhibition over pyramidal cell excitability [16, 17]. This scheme suggests that NMDARs on inhibitory cells, as well as various other synaptic proteins on excitatory cells, are necessary for the antidepressant-like response to NMDAR blockers. However, the precise mechanisms by which NMDAR blockers produce antidepressant-related effects remain to be elucidated.
Another outstanding question relates to the contribution of specific NMDAR subunits to antidepressant-related effects. The NMDAR is a heteromeric receptor complex comprised of a functionally obligatory GluN1 subunit and combinations of GluN2-3 subunits. Constitutive deletion of GluN2A in mice mimics the antidepressant-like profile of NMDAR antagonists [18]. Furthermore, systemic administration of compounds that selectively block GluN2B, such as CP-101-606, ifenprodil and Ro 25-6981, produces antidepressant-like effects in rodents [7, 9, 13, 19–23] and therapeutic efficacy in human depression [24]. In mice, deletion of GluN2B in corticohippocampal pyramidal neurons produce an antidepressant-like phenotype when the deletion extends to the ventral hippocampus [13] but only after repeated stress when deletion is restricted to the dorsal CA1 hippocampal subregion [20]. GluN1 deletion in forebrain interneurons also produces a trend for an antidepressant-like effect in some studies [25, 26]. Together, these data support an antidepressant-related role for the GluN2A and GluN2B subunits, but show that this contribution may be dependent on the brain region and cell type where the subunits are expressed or require additional conditions, such as the concurrent increase in glutamate transmission.
In addition to direct pharmacological effects on NMDARs, NMDAR antagonists cause downstream increases in the synaptic mPFC and hippocampal expression of the AMPAR GluA1 subunit, the NMDAR scaffolding protein, PSD-95, and various plasticity-related proteins including neurotrophins, mTOR and GSK-3β [3, 4, 9, 11, 23, 27]. These findings have led to a ‘synaptogenic model’ of the antidepressant-related efficacy of NMDAR antagonists, in which the drugs exert their behavioral effects by promoting the formation of synapses in key neural circuits [28]. In support of this model, recent studies have found that blockade or gene deletion of AMPARs [6, 7, 23, 29–31], mTOR [23], GSK-3β [11], or a loss-of-function brain-derived neurotrophic factor (BDNF) mutation [10] prevent the antidepressant-like effects of ketamine. There is also some evidence that these antidepressant-related effects are localized to the mPFC: an mTOR inhibitor infused into the mPFC is sufficient to occlude ketamine’s antidepressant-like effect [23].
Collectively, the current literature shows that NMDAR antagonists recruit multiple glutamate receptors and downstream signaling pathways to produce their antidepressant-related effects, although a clear and comprehensive model is yet to be defined. The goal of the current study was to build upon these prior findings to explore how loss of GluA1, GluN1, GluN2A, GluN2B, or PSD-95 affects antidepressant-related behavior, and the antidepressant-related effects of an NMDAR antagonist - using a combination of mutant, pharmacological and virus-based approaches. We focused on a selective GluN2B antagonist (Ro 25-6981), given the aforementioned evidence that this class of drugs has antidepressant-like effects in rodents [7, 9, 19–23] and humans [24]. Antidepressant-related activity was assayed in mice using the FST, a simple behavioral assay sensitive to antidepressant drug treatment [32, 33]. We first assessed whether loss-of-function mutation of GluN1, GluA1 and PSD-95 affected baseline FST depression-related behavior or modified the antidepressant-like effects of the GluN2B antagonist, Ro 25-6981. Next, we sought to localize the antidepressant-like effects of GluN2B antagonism by testing the antidepressant-like effects of microinfusing Ro 25-6981 into either the mPFC or another key emotion-mediating region, the basolateral amygdala (BLA).
Material and methods
Subjects
Male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at ~2 months of age, housed 2 per cage in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 0600 h). Mice were given at least 1 week of acclimation to the local housing conditions before testing. For all experiments testing was conducted during the light phase. Mice were at least 8 weeks of age unless stated otherwise.
GluN1INTER-KO mice were generated as described previously [26]. GluN1 was postnatally deleted by crossing floxed-GluN1 mutants with mutants expressing Cre recombinase driven by the Ppp1r2 promoter (protein phosphatase 1, regulatory inhibitor subunit 2). The position of the loxP sites in the floxed mutants (referred to as the ‘Flox B line’ in [26]) causes delayed recombination at a post-adolescent age. There is a resultant loss of GluN1 in 40–50% of, primarily parvalbumin-positive, GABAergic interneurons in corticolimbic regions [26]. GluN1INTER-KO and floxed-GluN1 controls were littermates bred from floxed-GluN1/Cre-positive dam and floxed-GluN1/Cre-negative sire at the NIH. Mice were tested after 20 weeks of age, when GluN1 deletion is expected to be restricted to interneurons in corticolimbic regions [26]. The mutants were backcrossed onto a C57BL/6J for 5–7 generations. Males and females were used.
GluN2AKO mice were generated as previously described and have a constitutive deletion of GluN2A [34–38]. The mutant line was backcrossed onto a C57BL/6J background for >10 generations. GluN2AKO and non-mutant wild-type (WT) controls were bred from GluN2A heterozygous parents at the NIH. Males and females were used.
GluA1KO mice were generated as previously described and have a constitutive deletion of GluA1 [29, 36, 39–42]. The mutant line was backcrossed onto a C57BL/6J background for >10 generations. GluA1KO and non-mutant WT controls were bred from GluA1 heterozygous parents at the NIH. Males and females were used.
PSD-95KO mice were generated as previously described and have a constitutive deletion of PSD-95 [43–45]. The mutant line was backcrossed onto a C57BL/6J background for >10 generations. PSD-95KO and non-mutant WT controls were bred from PSD-95 heterozygous parents at The Jackson Laboratory (Bar Harbor, ME, USA) and transported to NIH at 8 weeks of age. Males and females were used.
Separate groups of mice were used in each FST experiment. The number of mice used in each experiment is given in the figure legends. Experimental procedures were performed in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved by the local NIAAA Animal Care and Use Committee.
Depression-related phenotype of loss-of-function NMDAR, AMPAR and PSD-95 mutations
The FST was conducted based on previously described methods [46]. Mice were gently lowered into a 20 cm-diameter cylinder, filled with 24 ± 1.0 °C water, for a 6-minute test. Immobility (cessation of limb movements except minor movement necessary to keep the mouse afloat) was scored every five seconds. Immobility during the final 4 minutes of the test was calculated and converted to a percentage.
The basal FST phenotype of GluN1INTER-KO, GluA1KO and PSD-95KO mutants was tested. In addition, Ro 25-6981-treated C57BL/6J mice, and the GluN1INTER-KO, GluN2AKO and GluA1KO mutants were assessed for responses to repeated forced swim. The mouse was gently lowered into a 30 cm-diameter water-filled cylinder for 10 consecutive days [20]. After 60 seconds elapsed, a platform (plastic wiffle ball) was remotely released and floated up to beneath a 4 × 4 cm escape hole in the side of the cylinder. The platform sank when the mouse attempted to climb onto it to escape through the hole. Each trial ended 20 seconds after platform release. Immobility during the trial was calculated (i.e., times immobile during 120 × 5-second intervals) and converted to a percentage.
Antidepressant-like effects of Ro 25-6981 in NMDAR, AMPAR and PSD-95 loss-of-function mutants
To first confirm that Ro 25-6981 showed an antidepressant-like effect in the FST of non-mutants, mice were intraperitoneally injected (10 mL/kg body weight) with 10 mg/kg Ro 25-6981 (Tocris, Ellisville Missouri) or 0.9% saline vehicle 30 minutes prior to the FST. This dose was chosen based on previous studies in the FST [7, 20, 23]. Using the same procedure, GluA1KO, GluN2AKO, GluN1INTER-KO, and PSD-95 mutants were tested in the FST after injection of Ro 25-6981 or vehicle. One week later, treatment assignments were reversed for each mouse and mice were tested in the open field test 30 minutes after injection of Ro 25-6981 or vehicle. Mice were place in a 40 × 40 × 35 cm square arena (60 lux) constructed of white Plexiglas for 30 minutes, as previously described [47]. Total distance traveled was measured by the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA) and expressed in meters.
Regionally localized antidepressant-like effects of Ro 25-6981
To localize the FST-effect of GluN2B antagonism to a specific brain region, Ro 25-6981 was infused into one of two structures critical to emotional regulation, the mPFC or BLA. C57BL/6J mice were anesthetized with isoflurane and the head was fixed into a stereotaxic device (Kopf Instruments, Tujunga, CA, USA) and implanted with 26-gauge guide cannula (Plastics One, Roanoke, VA, USA) bilaterally targeting either the mPFC or BLA. The coordinates for mPFC were anteroposterior (AP) +2.00 mm, mediolateral ±0.40 mm, dorsoventral (DV) −2.00 mm relative to bregma, based on our previous studies [48, 49]. The coordinates for BLA were −1.40 mm AP, ±3.30 mm ML, and −3.90 mm DV relative to bregma, again based on our previous studies [50]. Mice were given at least one week of post-surgery recovery before testing.
Mice were tested in the FST 15 minutes following infusion of saline or 1.0 μg/μL Ro 25-6981 (Tocris, Ellisville Missouri) in a volume of 0.30 μL per hemisphere via 33-gauge infusion cannulas (Plastics One, Roanoke, VA, USA) attached with polyurethane tubing to a 2 μL Hamilton syringe (Hamilton, Reno NV). Solutions were slowly infused at a rate of 0.05 μL/min using a microinfusion pump (Harvard Apparatus PHD 22/2000, Holliston, MA, USA) and injectors remained in place for an additional 2 minutes to ensure diffusion into the tissue. The concentration of Ro 25-6981 was based on previous work showing effects of intra-cranial infusion on anxiety-related tasks [51].
To compare the effects of intra-mPFC and intra-BLA Ro 25-6981 infusion in the FST with performance on another behavior known to be modulated by GluN2B [20], we tested mice in the light/dark exploration test for anxiety-like behavior. This test was conducted one week prior to FST to avoid potential effects of the swim stressor on anxiety-like behavior. A Latin square design was used in which drug treatments were switched for each mouse for the two tests. The FST was conducted based on previously described methods [52]. Mice began the test in an opaque black Plexiglas shelter (39 × 13 × 16 cm) with a 13 × 8 cm opening at floor level that opened onto a large white Plexiglas square arena (39 × 39 × 35 cm) illuminated to ~90 lux. Entries into and percent time spent in the light compartment over a fifteen minute session was scored automatically by the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA), with the first 5 minutes used for analysis, as previously described [53].
On completion of testing, mice were given an overdose of anesthetic, infused with 4% methylene blue through the guide cannula and then transcardially perfused with 4% paraformaldehyde. Coronal sections 50-μm thick were cut with a vibratome (Classic 1000 model, Vibratome, Bannockburn, IL, USA) and stained with cresyl violet. The localization of cannula tips was estimated with the aid of an Olympus (Center Valley, PA, USA) BX41 microscope.
Statistical analysis
The effects of drug, genotype or virus-group were analyzed using Student’s t-test or two-factor analysis of variances (ANOVA), followed by Newman Keuls post-hoc analysis. The effects of drug/genotype and trial were analyzed using two-factor ANOVA, with repeated measures for trial. The statistical threshold was set at P<.05.
Results
Depression-related phenotype of loss-of-function NMDAR, AMPAR and PSD-95 mutations
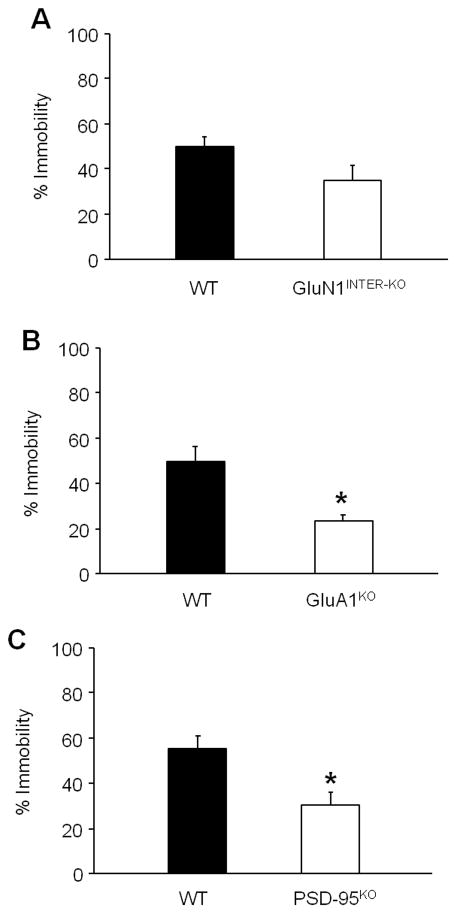
Phenotyping for basal FST behavior found that GluN1INTER-KO mutants did not differ from floxed controls (Figure 1A). The GluA1KO (t(13)=11.13, P<.01) (Figure 1B) and PSD-95 (t(19)=3.09, P<.01) mutants showed significantly less immobility than their respective WT controls. (Figure 1C).
Figure 1. FST phenotype of loss-of-function NMDAR, AMPAR and PSD-95 mutations.
(A) GluN1INTER-KO mutants showed similar immobility to floxed controls (n=7–18 per genotype). (B) GluA1KO mutants showed less immobility than WT controls (n=7–8). (C) PSD-95 mutants showed significantly less than WT controls (n=10–11 per genotype). Data are means ± SEM. *P<.05 WT versus KO
In the repeated forced swim test, Ro 25-6981 significantly reduced immobility relative to vehicle controls, in C57BL/6J mice (t(14)=3.09, P<.01) (Figure S1A), whereas the GluN1INTER-KO, GluN2AKO and GluA1KO mutants showed similar immobility to floxed controls (Figure S1B–D).
Antidepressant-like effects of Ro 25-6981 in NMDAR, AMPAR and PSD-95 loss-of-function mutants
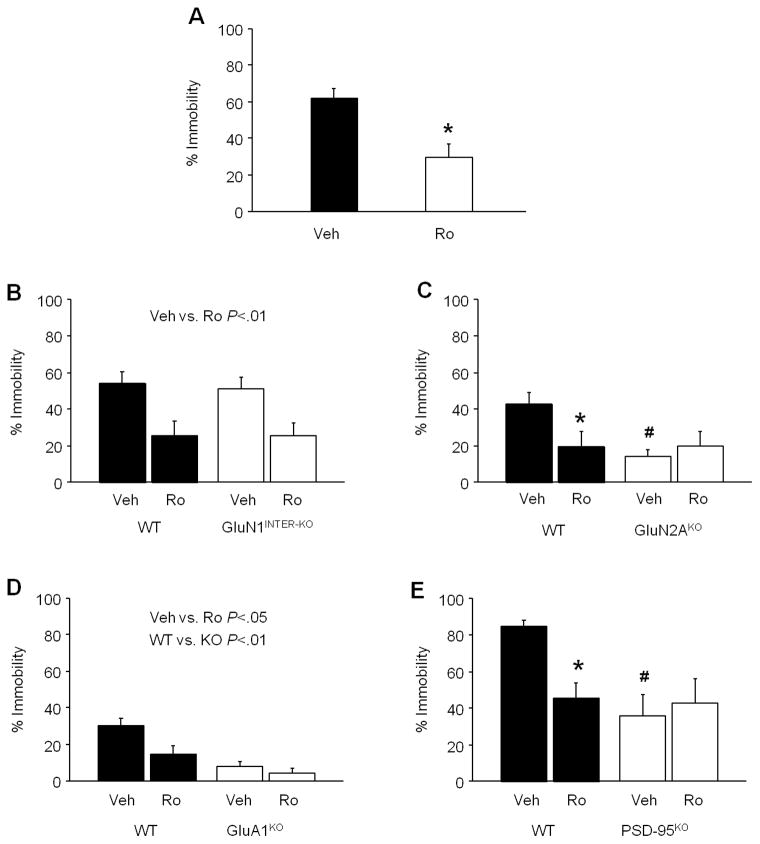
Systemic administration of Ro 25-6981 significantly reduced immobility in C57BL/6J mice, as compared to vehicle (t(10)=3.64, P<.01) (Figure 2A). In the GluN1INTER-KO mutants, Ro 25-6981 significantly decreased immobility regardless of genotype (drug effect: F1,44=15.18, P<.01) (Figure 2B). By contrast, in the GluN2AKO mutants, Ro 25-6981 significantly decreased immobility in WT controls, but not the mutant mice, and the vehicle-treated mutants were less immobile than vehicle-treated WT counterparts (drug x genotype interaction: F1,42=4.74, P<.05, followed by post hoc tests) (Figure 2C). In the GluA1KO mutants, both Ro 25-6981 (F1,20=6.79, P<.05) and genotype (F1,20=20.90, P<.01) significantly reduced immobility (Figure 2D). Finally, Ro 25-6981 significantly decreased immobility in WT controls, but not the PSD-95KO mutants, and there was significantly lower immobility in the vehicle-treated mutants than WT controls (drug x genotype interaction: F1,18=4.87, P<.05) (Figure 2E).
Figure 2. FST antidepressant-like effects of Ro 25-6981 in NMDAR, AMPAR and PSD-95 loss-of-function mutants.
(A) Systemic Ro 25-6981 reduced FST immobility in C57BL/6J mice, relative to vehicle (n=6 per treatment). (B) In GluN1INTER-KO mutant mice, Ro 25-6981 decreased FST immobility regardless of genotype (n=12 per genotype/treatment). (C) Ro 25-6981 decreased FST immobility in WT controls but not GluN2AKO mutant mice, and vehicle-treated mutants were less immobile than vehicle-treated WT controls (n=10–14 per genotype/treatment). (D) In GluA1KO mutant mice, Ro 25-6981 decreased FST immobility regardless of genotype, and mutants were less immobile than WT controls (n=6 per genotype/treatment). (E) Ro 25-6981 decreased FST immobility in WT controls but not PSD-95KO mutant mice, and vehicle-treated mutants were less immobile than vehicle-treated WT controls (n=5–6 per genotype/treatment). Veh=vehicle, Ro=Ro 25-6981. Data are means ± SEM. *P<.05 Ro versus Veh, #P<.05 KO versus WT
In the open field, Ro 25-6981 increased distance travelled, regardless of genotype, in the GluN1INTER-KO mutants (drug effect: F1,41=16.70, P<.01) (Figure S2), but had no effect in the GluN2AKO or GluA1KO mutants (Figure S2B–C), and decreased distance travelled, irrespective of genotype, in the PSD-95KO mutants (drug effect: F1,18=5.37, P<.05) (Figure S2D). The GluN2AKO (genotype effect: F1,29=6.27, P<.05) and GluA1KO (genotype effect: F1,19=6.62, P<.05) mutants travelled farther than their respective WT controls.
Regionally localized antidepressant-like effects of Ro 25-6981
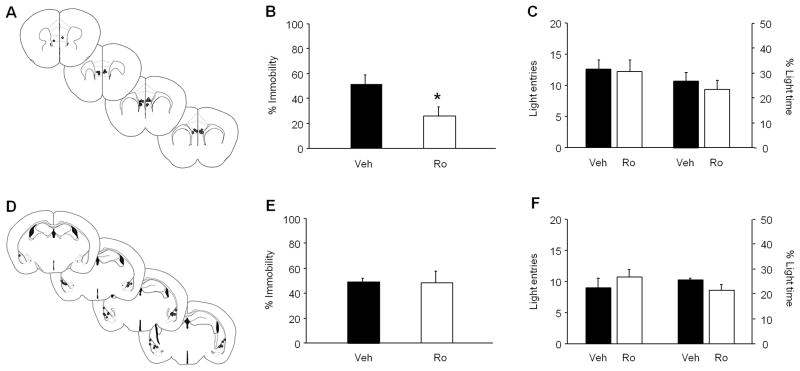
Microinfusion of Ro 25-6981 into the mPFC significantly reduced immobility in the FST, as compared to vehicle-infusion (t(15)=2.42, P<.05) (Figure 3A,B), but had no effect on light compartment entries or time in the light/dark exploration test (Figure 3C). In contrast, infusion of Ro 25-6981 into the BLA did not significantly alter FST immobility (Figure 3D,E) or light compartment exploration (Figure 3F).
Figure 3. Regionally localized FST antidepressant-like effects of Ro 25-6981.
(A) Estimated cannula placements in the mPFC. (B) Microinfusion of Ro 25-6981 into the mPFC reduced FST immobility, as compared to vehicle-infusion (n=8–9 per treatment). (C) Intra-mPFC infusion of Ro 25-6981 did not affect entries or time spent in the light compartment of the light/dark exploration test (n=7–9 per treatment). (D) Estimated cannula placements in the BLA. (E) Microinfusion of Ro 25-6981 into the BLA did not alter FST immobility (n=7–9 per treatment). (F) Intra-BLA infusion of Ro 25-6981 did not affect entries or time spent in the light compartment of the light/dark exploration test (n=8–9 per treatment). mPFC=medial prefrontal cortex, BLA=basolateral amygdala, Veh=vehicle, Ro=Ro 25-6981. Data are means ± SEM. *P<.05 Ro versus Veh
Discussion
The goal of the current study was to illuminate the mechanisms underlying the antidepressant-related effects of GluN2B antagonism by assessing the contribution of various glutamate receptor subunits and the glutamate anchoring protein, PSD-95, to these effects.
Systemic administration of the selective GluN2B antagonist, Ro 25-6981, reliably reduced immobility in C57BL/6J mice. This is in line with multiple prior studies in rats and mice reporting FST antidepressant-like effects of Ro 25-6981 [7, 9, 13, 19–23] or the non-selective NMDAR antagonist, ketamine [2–6, 8–13, 23, 27]. It also resembles the acute FST antidepressant-like phenotype produced by conditional gene deletion of GluN2B on pyramidal neurons throughout the cortex and hippocampus [13], but not by more restricted cortical, plus dorsal CA1 hippocampal, deletion, which only affects FST antidepressant-like behavior after repeated FST [20]. Here, the antidepressant-like effects of Ro 25-6981 were phenocopied by constitutive, global loss of the NMDAR GluN2A subunit or AMPAR GluA1 subunit, as previously reported [18, 29, 39], suggesting the antidepressant-like actions of NMDAR antagonists may be produced by blockade of these subunits. Indeed, the loss of the drug’s efficacy in the GluA1 mutants fits the earlier observation that treatment with an AMPAR antagonist (NBQX) occludes the antidepressant-like effects of Ro 25-6981 [7, 31]. However, we cannot exclude the possibility that the failure of Ro 25-6981 to reduce immobility could reflect a ‘floor’ effect of low baseline immobility in the GluA1 mutant. A floor effect could also have contributed to the apparent loss of Ro 25-6981’s effects in the GluN2A mutants.
Restricted deletion of the NMDAR GluN1 subunit in forebrain interneurons did not significantly affect FST behavior, though a modest trend for reduced immobility was evident (similar to that reported with postnatal GluN1 deletion in prior work [25, 26]). The lack of effect of loss of inhibitory GluN1 argue against the hypothesis that antidepressant-like effects are produced by loss of interneuronal NMDAR activity, leading to the disinhibition of pyramidal neuronal firing. However, this conclusion is tempered by the fact that GluN1 is only deleted on a subset of (primarily parvalbumin) interneurons [26] – leaving open the possibility that the remaining interneuronal receptors were sufficient to maintain normal FST behavior in these mutants.
A caveat to the FST data in these the GluA1 and GluN2A mutants is that both lines traveled farther than WT controls in an open field test, suggesting increases in locomotor activity phenotype could have accounted for their reduced FST immobility. Previous work has also found both of these mutants show hyperactivity, albeit in novel and not familiar or low-stress environments [18, 36, 41]. A similar caveat could be levelled at the antidepressant-like effects of Ro 25-6981. However, across experiments, Ro 25-6981 treatment only caused open field locomotor hyperactivity in the GluN1 mutant line (as previously reported with ketamine, but not MK-801 [25, 26, 54]); with no effects evident in the GluA1 or GluN2A lines and a significant decrease in locomotor activity in the PSD-95 mutant line. The variability in this effect echoes previous findings with Ro 25-6981 and ketamine, where increases, decreases and no change [13, 20, 21, 55–57] in open field activity have all been reported. The reason for this variability is currently unclear, but does suggest that locomotor activating effects of these drugs are unlikely to solely account for the ability to produce an antidepressant-like reduction in immobility.
Mutant mice lacking the glutamate receptor interacting protein, PSD-95, showed a basal antidepressant-like phenotype and insensitivity to antidepressant-like effects of Ro 25-6981. A recent study found that pharmacological inhibition of nitric oxide synthase coupling to PSD-95 also produced an antidepressant-like effects in the rat FST [58]. The loss of Ro 25-6981’s antidepressant-like effects following PSD-95 deletion is also notable in view of the finding that synaptic PSD-95 is increased in the mPFC after ketamine treatment [9, 23]. Together, these data suggest that dynamic alterations in PSD-95 may be a critical mechanism mediating antidepressant-related behavior in the FST. The precise nature of this contribution remains unclear, however. On the one hand, functional loss/inhibition of PSD-95 could produce an antidepressant-like effect due to disruption of synaptic NMDAR signaling, thereby mimicking the action of NMDAR blockers. On the other hand, increases in synaptic PSD-95 might be expected to support antidepressant-like efficacy of NMDAR antagonism by promoting dendritic spine formation and plasticity in critical circuits within the mPFC [28].
While parsing these potential roles awaits additional work, the current study provides some additional insights into the role of the mPFC and PSD-95 expressed in the mPFC. Infusion of Ro 25-6981 directly into the mPFC, but not the BLA, was sufficient to mimic the antidepressant-like effect of systemic Ro 25-6981 treatment. This effect was behaviorally selective to the FST – given that mPFC-infusion of Ro 25-6981 did not alter behavior in the light/dark exploration test for anxiety-like behavior. These data demonstrate a key contribution of the mPFC to the antidepressant-like effects of Ro 25-6981, consistent with prior evidence that the antidepressant-like effects of Ro 25-6981 and ketamine are associated with synaptogenesis and increases in synaptic excitability and protein expression, including GluA1, PSD-95 and Arc, in the mPFC (and dorsal hippocampus), and with the observation that intra-mPFC inhibition of mTOR blocks the antidepressant-like of systemic ketamine [3, 4, 9, 11, 13, 23]. The current finding that the antidepressant-like effects of Ro 25-6981 are localized to the mPFC, together with the earlier evidence of antidepressant-induced increases in synaptic mPFC PSD-95, suggests that the antidepressant-like effects of Ro 25-6981 may depend on PSD-95 in the mPFC.
In summary, the current study provides further evidence of antidepressant-like activity of GluN2B antagonism and offers novel insight into the glutamate receptor subunits, interacting molecules and brain regions mediating these effects. Loss-of-function mutation of GluA1 and PSD-95 mimicked the antidepressant-related effect of Ro 25-6981 by reducing FST immobility. In addition, the antidepressant-like effects of Ro 25-6981 were unaltered in mutants with global GluA1 deficiency or interneuron-specific GluN1 deletion, but absent in mutants with global deletion of GluN2A or PSD-95. Moreover, pharmacological blockade of GluN2B in the mPFC was sufficient to produce an antidepressant-like action and global PSD-95 deletion prevented the antidepressant-like effects of systemic GluN2B antagonism. These findings help extend and refine current understanding of the mechanisms mediating antidepressant-like effects of NMDAR antagonists, and help inform the development of a novel class of potential medications for treating depression.
Supplementary Material
Research Highlights.
We studied depressive behaviors with mutant, pharmacological, and viral strategies
We studied the mechanisms underlying antidepressant activity of NMDAR antagonists
The effect NMDAR antagonists was not blocked by deletion of interneuronal NMDARs
Loss of the synaptic protein PSD-95 decreased depression-related behaviors in mice
Infusion of NMDAR antagonist to the PFC was sufficient for antidepressant response
Acknowledgments
We are grateful to Guoxiang Luo for genotyping the GluA1 mutants. Research supported by the National Institute of Alcohol Abuse and Alcoholism Intramural Research Program (Z01-AA000411) and NIH grant K22MH099164 (K.N.). R.S. receives grant support from the Deutsche Forschungsgemeinschaft SFB636/A4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–37. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–4. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- 3.Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology (Berl) 2013;230:291–8. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 2013;228:157–66. doi: 10.1007/s00213-013-3024-x. [DOI] [PubMed] [Google Scholar]
- 6.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–11. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Parise EM, Alcantara LF, Warren BL, Wright KN, Hadad R, Sial OK, et al. Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol Psychiatry. 2013;74:750–9. doi: 10.1016/j.biopsych.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 Inhibition Potentiates the Synaptogenic and Antidepressant-Like Effects of Subthreshold Doses of Ketamine. Neuropsychopharmacology. 2013;38:2268–77. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Jr, Cohen BM, et al. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology (Berl) 2011;215:689–95. doi: 10.1007/s00213-011-2169-8. [DOI] [PubMed] [Google Scholar]
- 13.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3 doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–5. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, et al. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39:1603–13. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–14. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- 19.Brown DG, Maier DL, Sylvester MA, Hoerter TN, Menhaji-Klotz E, Lasota CC, et al. 2,6-Disubstituted pyrazines and related analogs as NR2B site antagonists of the NMDA receptor with anti-depressant activity. Bioorg Med Chem Lett. 2011;21:3399–403. doi: 10.1016/j.bmcl.2011.03.117. [DOI] [PubMed] [Google Scholar]
- 20.Kiselycznyk C, Svenningsson P, Delpire E, Holmes A. Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm. Neuroscience. 2011;193:259–68. doi: 10.1016/j.neuroscience.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima-Ojeda JM, Vogt MA, Pfeiffer N, Dormann C, Kohr G, Sprengel R, et al. Pharmacological blockade of GluN2B-containing NMDA receptors induces antidepressant-like effects lacking psychotomimetic action and neurotoxicity in the perinatal and adult rodent brain. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:28–33. doi: 10.1016/j.pnpbp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Poleszak E, Wosko S, Serefko A, Szopa A, Wlaz A, Szewczyk B, et al. Effects of ifenprodil on the antidepressant-like activity of NMDA ligands in the forced swim test in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:29–35. doi: 10.1016/j.pnpbp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–7. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 25.Pozzi L, Dorocic IP, Wang X, Carlen M, Meletis K. Mice Lacking NMDA Receptors in Parvalbumin Neurons Display Normal Depression-Related Behavior and Response to Antidepressant Action of NMDAR Antagonists. PLoS One. 2014;9:e83879. doi: 10.1371/journal.pone.0083879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2013;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald PJ, Barkus C, Feyder M, Wiedholz LM, Chen YC, Karlsson RM, et al. Does gene deletion of AMPA GluA1 phenocopy features of schizoaffective disorder? Neurobiol Dis. 2010;40:608–21. doi: 10.1016/j.nbd.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freudenberg F, Marx V, Mack V, Layer LE, Klugmann M, Seeburg PH, et al. GluA1 and its PDZ-interaction: a role in experience-dependent behavioral plasticity in the forced swim test. Neurobiol Dis. 2013;52:160–7. doi: 10.1016/j.nbd.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 33.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 34.Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–4. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debrouse L, Hurd B, Kiselycznyk C, Plitt A, Todaro A, Mishina M, et al. Probing the Modulation of Acute Ethanol Intoxication by Pharmacological Manipulation of the NMDAR Glycine Co-Agonist Site. Alcohol Clin Exp Res. 2013;37:223–33. doi: 10.1111/j.1530-0277.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–67. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–5. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 38.Palachick B, Chen YC, Enoch AJ, Karlsson RM, Mishina M, Holmes A. Role of major NMDA or AMPA receptor subunits in MK-801 potentiation of ethanol intoxication. Alcohol Clin Exp Res. 2008;32:1479–92. doi: 10.1111/j.1530-0277.2008.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–72. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feyder M, Wiedholz L, Sprengel R, Holmes A. Impaired Associative Fear Learning in Mice with Complete Loss or Haploinsufficiency of AMPA GluR1 Receptors. Front Behav Neurosci. 2007;1:4. doi: 10.3389/neuro.08.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K, et al. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–40. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- 42.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–11. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 43.Camp MC, Feyder M, Ihne J, Palachick B, Hurd B, Karlsson RM, et al. A novel role for PSD-95 in mediating ethanol intoxication, drinking and place preference. Addict Biol. 2011;16:428–39. doi: 10.1111/j.1369-1600.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, et al. Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addict Biol. 2014 doi: 10.1111/adb.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzgerald PF, Pinard CR, Camp MC, Feyder M, Sah A, Bergstrom HC, et al. Durable fear memories require PSD-95. Mol Psychiatry. 2014 doi: 10.1038/mp.2015.44. [DOI] [PubMed] [Google Scholar]
- 46.Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33:2595–604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, et al. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 2008;195:547–57. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- 48.Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, et al. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14:1507–9. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes A, Fitzgerald PJ, Macpherson KP, Debrouse L, Colacicco G, Flynn SM, et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–61. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunduz-Cinar O, Macpherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–23. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–72. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, et al. Effects of Mild Early Life Stress on Abnormal Emotion-related Behaviors in 5-HTT Knockout Mice. Behav Genet. 2007;37:214–22. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- 53.Ihne JL, Fitzgerald PJ, Hefner KR, Holmes A. Pharmacological modulation of stress-induced behavioral changes in the light/dark exploration test in male C57BL/6J mice. Neuropharmacology. 2012;62:464–73. doi: 10.1016/j.neuropharm.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–48. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–69. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 56.Haller J, Nagy R, Toth M, Pelczer KG, Mikics E. NR2B subunit-specific NMDA antagonist Ro25-6981 inhibits the expression of conditioned fear: a comparison with the NMDA antagonist MK-801 and fluoxetine. Behav Pharmacol. 2011;22:113–21. doi: 10.1097/FBP.0b013e328343d7b2. [DOI] [PubMed] [Google Scholar]
- 57.Mathur P, Graybeal C, Feyder M, Davis MI, Holmes A. Fear memory impairing effects of systemic treatment with the NMDA NR2B subunit antagonist, Ro 25-6981, in mice: Attenuation with ageing. Pharmacol Biochem Behav. 2009;91:453–60. doi: 10.1016/j.pbb.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doucet MV, Levine H, Dev KK, Harkin A. Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacology. 2013;38:1575–84. doi: 10.1038/npp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.