Abstract
The rhinal cortices constitute the main route for impulse traffic to and from the hippocampus. Tracing studies have revealed that the perirhinal cortex forms strong reciprocal connections with the neo- and entorhinal cortex (EC). Yet, physiological investigations indicate that perirhinal transmission of neocortical and EC inputs occurs with a low probability. In search of an explanation for these contradictory findings, we have analyzed synaptic connections in this network by combining injections of the anterograde tracer Phaseolus vulgaris-leucoagglutinin (PHAL) into the neocortex, area 36, or area 35 with GABA immunocytochemistry and electron microscopic observations. Within area 36, neocortical axon terminals formed only asymmetric synapses, usually with GABA negative spines (87%), and less frequently with GABA immunopositive (GABA+) dendrites (13%). A similar synaptic distribution was observed within area 35 except that asymmetric synapses onto GABA+ dendrites were more frequent (23% of synapses). Examination of the projections from area 36 to area 35 and from both regions to the EC revealed an even higher incidence of asymmetric synapses onto GABA+ dendrites (35% and 32% respectively) than what was observed in the neocortical projection to areas 36 and 35. Furthermore, a proportion of neocortical and perirhinal terminals containing PHAL and GABA immunolabeling formed symmetric synapses onto GABA negative dendrites in their projection sites (neocortex to area 35, 16%; area 36 to 35, 7%; areas 36–35 to EC, 12%). Taken together, these findings suggest that impulse transmission through the rhinal circuit is subjected to strong inhibitory influences, reconciling anatomical and physiological data about this network.
Keywords: perirhinal, entorhinal, GABA, interneuron, electron microscopy, tract-tracing
The perirhinal cortex is an elongated strip of cortex located in the fundus (area 35) and lateral bank (area 36) of the rhinal sulcus and is believed to play a critical role in recognition memory (reviewed in Brown and Aggleton, 2001). Medially, the perirhinal cortex abuts on the entorhinal area, with which it is reciprocally connected. Together, these cortical regions occupy a strategic position in the temporal lobe because they relay most sensory inputs from the neocortex to the hippocampus. Moreover, the rhinal cortices represent the main return path for hippocampal efferents to the neocortex (reviewed in Witter et al., 2000).
Indeed, tract-tracing studies suggest that information transfer between the neocortex and hippocampus depends on the sequential, stepwise activation of longitudinal bands of cortex within the rhinal cortices (neocortex to perirhinal area 36 to area 35 to EC to hippocampus and conversely). This stepwise progression of impulses is not absolute however as some neurons project beyond the adjoining area in the lateromedial or mediolateral directions in primates (Van Hosen and Pandya, 1975; Insausti et al., 1987; Suzuki and Amaral, 1994a,b), felines (Room and Groenewegen, 1986a; Witter and Groenewegen, 1986) and rodents (Deacon et al., 1983; Saleem and Tanaka, 1996; McIntyre et al., 1996; Burwell and Amaral, 1998a,b). In general, medially directed projections (from the neocortex and perirhinal cortex toward the entorhinal cortex) primarily originate and end in superficial layers whereas return projections toward the neocortex originate in deep layers (Deacon et al., 1983; Witter and Groenewegen, 1986; Room and Groenewegen, 1986a; Insausti et al., 1987; Burwell and Amaral, 1998a,b).
At odds with this evidence of profuse reciprocal connections however, physiological studies in the whole guinea pig brain kept in vitro have revealed that perirhinal transmission of neocortical and entorhinal inputs occurs with an extremely low probability. For instance, electrical stimulation of the lateral olfactory tract evokes massive neuronal excitation in the entorhinal cortex but no responses in area 36 (Biella et al., 2003). Similarly, stimulation of the temporal neocortex or area 36 evokes no local field responses in the entorhinal cortex (Biella et al., 2002) and analogous results were obtained in imaging studies of intrinsic (Frederico et al., 1994) and voltage-sensitive signals (de Curtis et al., 1999; Biella et al., 2003). Importantly, these results are not an artifact of the whole brain in vitro preparation since recent in vivo studies, using multisite extracellular recordings of neocortical, perirhinal, and entorhinal neurons, reached the same conclusions (Pelletier et al., 2004, 2005).
Thus, there seems to be a discrepancy between tract-tracing data, showing strong reciprocal connections between the perirhinal and entorhinal cortices, and physiological data about this pathway. Given the important role played by the rhinal cortices in memory (reviewed in Brown and Aggleton, 2001), understanding the factors that regulate neuronal excitability in this pathway emerges as an issue of critical importance. Valuable insights into this matter might be gained by considering the ultrastructural organization of the pathways linking the rhinal cortices and neocortex. However, the available studies have focused on presubicular and olfactory afferents to the entorhinal cortex as well as on its intrinsic network (for instance see Wouterlood and Nederlof, 1983; Wouterlood et al., 1985, 1995, 2000, 2004; van Haeften et al., 2003).
Accordingly, the present study was undertaken to shed light on the inhibitory mechanisms that control impulse traffic in the pathway linking the neocortex to the entorhinal cortex by way of the perirhinal cortices. To this end, we have combined iontophoretic injections of the anterograde tracer Phaseolus vulgaris-leucoagglutinin (PHAL) into the temporal neocortex or perirhinal areas 36 and 35, with pre-embedding GABA immunocytochemistry, and electron microscopic observations of anterogradely-labeled axon terminals. Our results indicate that impulse propagation from the neocortex to the entorhinal cortex is restricted by strong inhibitory influences, accounting for previous physiological observations.
MATERIALS AND METHODS
Tract-tracing
The surgical procedures used in this study were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the institutional animal care and use committee at Rutgers University. A total of 28 adult male Hartley guinea pigs weighing 250–350 g were used in this study. It should be noted that the cytoarchitectural organization of the rhinal cortices in guinea pig is similar to that of other mammals (Uva et al., 2004). The animals were kept in a 12 hour light/dark cycle and had free access to food and water. They were anesthetized with a mixture of ketamine, acepromazine, and xylazine (50 mg/kg, 2.5 mg/kg and 5 mg/kg, respectively; i.p.), placed in a stereotaxic apparatus, and then, under sterile conditions, received injections of an anterograde tracer aimed at the temporal neocortex, area 36, or area 35.
Phaseolus vulgaris-leucoagglutinin (PHAL, Vector Laboratories, Burlingame, CA), dissolved as a 2.5% solution in 0.01M phosphate buffer, pH 8.0, was injected iontophoretically into the temporal neocortex adjacent to the perirhinal cortex (7.8 AP, 9.6 ML, 11 and 11.4 DV), area 36 (7.8 AP, 9.3 ML, 11.4 DV), or area 35 of the perirhinal cortex (7.8 AP, 8.4 ML, 10.8 DV) according to a stereotaxic atlas of the guinea pig brain (Luparello, 1967). The tracer was delivered by iontophoresis through a glass pipette (tip diameter: 25–30 µm) using positive 7 µA current pulses, on and off every 7 sec for 15 min.
After a survival period of 10–12 days, the animals were deeply anesthetized with sodium pentobarbital, 50 mg/kg, i.p, and perfused through the aorta with 250 ml of 0.9% saline, followed by 500 ml of a fixative containing 1% glutaraldehyde, 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4 (PB). The brains were removed, cut into blocks containing the rhinal cortex, and post-fixed in the same fixative overnight. The tissue blocks were then sectioned at 60 µm in PB using a vibrating microtome.
Immunocytochemical procedures
For all sections, PHAL was detected by immunoperoxidase, whereas GABA immunoreactivity was detected using pre-embedding immunogold-silver. We used a monoclonal anti-GABA antibody produced in mouse (Sigma, catalog # A3010, lot # 053k4840). This antibody was prepared against purified GABA conjugated to BSA. It was evaluated by Sigma for activity and specificity by dot blot immunoassay and no cross-reaction was observed with L-alpha-aminobutyric acid, L-glutamic acid, L-aspartic acid, glycine, aminovaleric acid, L-threonine, L-glutamine, taurine, putrescine, L-alanine, and carnosine. In a previous test with this antibody, staining was completely eliminated by pretreatment of the diluted antibody with GABA (Storm-Mathisen et al., 1983). Moreover, we observed that in amygdala sections, this antibody produced a pattern of labeling identical to that seen in previous studies (Nitecka and Ben-Ari, 1987).
Briefly, sections were incubated in 1% sodium borohydride for 30 min, rinsed repeatedly in PB and 0.1M tris buffered saline, pH 7.4 (TBS), and incubated for 30 min in a blocking solution containing 1% bovine serum albumin, 3% normal goat serum, and 0.04% Triton-100X in TBS. The sections were then incubated overnight in mouse anti-GABA antibody (1:2000, Sigma) and in rabbit anti-PHAL (1:1000, Vector). These sections were then rinsed in TBS, incubated for 30 min in biotinylated goat anti-rabbit IgG (1:400, Jackson), rinsed repeatedly in TBS and then incubated for 30 min in the avidin-biotin peroxidase complex (ABC). Bound peroxidase was revealed using a solution of 0.022% diaminobenzidine (DAB) and 0.003% hydrogen peroxide in TBS. The sections were rinsed in TBS followed by rinses in 0.01 M phosphate buffered saline, pH 7.4 (PBS) and then incubated for 30 min in a buffer containing 0.8% BSA, 0.1% fish gelatin, and 3% normal goat serum in PBS. Subsequently, the sections were incubated overnight in gold-conjugated goat anti-rabbit IgG (1:50, Ted Pella, Redding, CA), rinsed in PBS, and post-fixed with 2% glutaraldehyde in PBS for 10 min at room temperature. After extensive rinses in PBS, bound gold particles were enhanced by treatment with a silver solution (Amersham Biosciences, Buckinghamshire, UK) for 6 to 8 min at room temperature.
For light microscopy, sections were processed to reveal only PHAL as above with the exception that the blocking and incubation solutions included 0.2% Triton X-100. The sections were then mounted onto glass slides, air-dried, dehydrated in a graded series of alcohol and coverslipped in Permount for examination.
Sections prepared for electron microscopy were rinsed in PB, post-fixed in osmium tetroxide (2% solution in PB) for 1 hour, and rinsed again in PB. These sections were then dehydrated in alcohol and propylene oxide, and finally embedded in Durcupan resin (Fluka, Gymea, Australia) and baked for 48 hr at 60°C. Blocks containing portions of the perirhinal or entorhinal cortices were sectioned at 60–70 nm using an ultramicrotome (Ultracut-E, Reichert-Jung/Leica Microsystems). Ultrathin sections were collected onto copper mesh grids and counterstained with 5% uranyl acetate and Reynold’s lead citrate.
Data analysis
Only tissue from injection sites confined to the regions of interest were included in the data analysis (see Results). For each successful PHAL injection site (at least three per cortical area), and in each region of interest, two to four blocks were examined, depending on the intensity of the anterograde labeling. For PHAL injections in the temporal neocortex, regions observed in the electron microscope included perirhinal areas 36 and 35 as well as the lateral entorhinal cortex. For PHAL injection sites in area 36, electron microscopic observations were performed in area 35 and the lateral entorhinal cortex. Only the latter region was examined in cases of PHAL injections in area 35.
Selected sections were photographed and the area of interest was drawn on the photographs. The tissue was then trimmed onto trapezoidal blocks at approximately the same position and in the same shape as previously drawn in the photographs. The tissue blocks were then cut in 70 nm sections that were collected onto copper mesh grids. However, we only considered the most superficial ultrathin sections to ensure proper penetration of the antibodies.
Prior to electron microscopic examination of the tissue, the tissue trapezoid was drawn at low magnification onto a square grid notebook so that each square of the copper mesh grid was depicted. The location of each PHAL labeled terminal examined was then marked onto this grid map. Within each region, all tracer-labeled profiles were noted down. The occurrence of synapses, the type of synapse (symmetric or asymmetric), and the target of the synaptic contacts (spines or dendrites, and unlabeled or GABA labeled) were registered. In addition, the presence of PHAL and GABA immunostaining within the same axonal profiles was also registered. PHAL-labeled terminals were identified by the presence of flocculent deposits resulting from the DAB reaction whereas GABA-immunopositive structures were identified by the presence of dense clusters of gold particles resulting from the immunogold reaction.
We used the criteria defined by Peters et al. (1991) to identify synapses and their post-synaptic targets. Synaptic contacts were identified by the presence of a postsynaptic density separated from the presynaptic membrane by a clear synaptic cleft and the presence of synaptic vesicles in close proximity to the presynaptic membrane. Finally, when PHAL labeled profiles were observed adjacent to GABA structures (or when double labeled by GABA), these fields were examined in 2–4 adjacent serial sections to ascertain that gold particles signaling GABA immunoreactivity were similarly distributed on the different sections.
Spines could be readily identified as such when they could be seen to emerge from the parent dendrite. When the spine and parent dendrite were not located in the same plane, spines were identified by established ultrastructural criteria (Peters et al., 1991) including the presence of spine apparatus, as well as the lack of microtubules and mitochondria. In contrast, dendrites displayed microtubules, mitochondria, endoplasmic reticulum, and were often of a larger size.
Photomicrographs were cropped into Adobe Photoshop 7.0. Brightness and contrast adjustments were performed with the same software to insure uniformity in multi-panel figures. The photographs were then imported in Adobe Illustrator 10.0 to add labels and scale bars.
RESULTS
Location of injection sites and anterograde labeling within the rhinal cortices
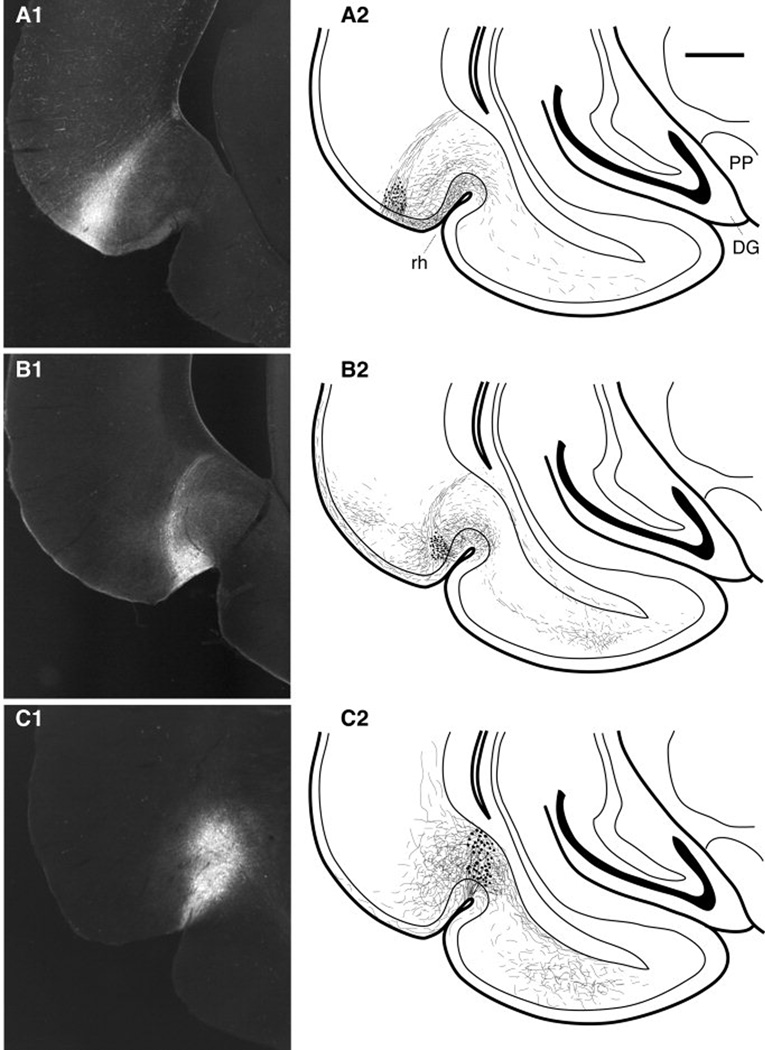
Of the 28 animals, only 12 had PHAL injections confined to the regions of interest. Thus, the following account is based on the analysis of these12 cases where the PHAL injection sites were confined to the temporal neocortex (n=3; Fig. 1A1), area 36 (n=4; Fig. 1B1), or area 35 (n=5; Fig. 1C1). All these injections involved the superficial layers of the target area although PHAL-labeled cell bodies were also present in deeper layers. These injections produced various amounts of anterograde labeling in medially located rhinal areas, as depicted in the mappings of figure 1 (right column) and the photomicrographs of figure 2. Although this labeling extended for considerable distances in the rostrocaudal axis, particularly following PHAL injections in areas 36 and 35, we only describe that seen at the same coronal level as the injection site, where all our electron microscopic observations were performed.
Fig. 1.
Iontophoretic injections of PHAL in the temporal neocortex (A1), area 36 (B1), and area 35 (C1). The right column (A2, B2, C2) shows the distribution of anterogradely labeled axons at a constant coronal plane located within one millimeter of the injection site for each case. Scale bar in A2 corresponds to 1 mm.
Fig. 2.
Low (1) and high power (2) photomicrographs showing anterograde labeling seen in (A) the perirhinal cortex following a PHAL injection in the neocortex and (B) in the lateral entorhinal cortex (layer III) following a PHAL injection in area 36. Arrows in A2 and B2 point to axonal varicosities. Roman numerals in panel A1 indicate layers. Scale bars in A1 and B1 correspond to 100 and 50 µm, respectively. Scale bar in A2 corresponds to 10 µm.
Figure 2 shows photomicrographs of anterogradely labeled fibers seen in the perirhinal cortex (Fig. 2A) and lateral entorhinal cortex (Fig. 2B) following PHAL injections in the temporal neocortex and area 36, respectively. Note that in all cases, PHAL labeled axons bore numerous varicosities (Fig. 2A2, 2B2, arrows), were generally present in all layers, but were most concentrated superficially, in layers I-III.
Following PHAL injections in the temporal neocortex (Fig. 1A2), anterograde labeling was dense in area 36, moderate in area 35 and light in the lateral entorhinal cortex. Following PHA-L injections in area 36 (Fig. 1B2), labeling was dense in area 35 and moderate in the lateral entorhinal cortex. A comparable density of anterograde labeling was seen in the lateral entorhinal cortex following PHAL injections in area 35 (Fig. 1C2). These light microscopic observations are consistent with previous results obtained in other species (Van Hoesen and Pandya, 1975; Deacon et al., 1983; Room and Groenewegen, 1986a; Witter and Groenewegen, 1986; Insausti et al., 1987; Suzuki and Amaral, 1994a,b; Saleem and Tanaka, 1996; McIntyre et al., 1996; Burwell and Amaral, 1998a,b).
Electron microscopic observations
This section reports the total numbers and proportion of different types of synapses observed following the PHAL injections described above. However, it should be pointed out that for each successful PHAL injection site, and in each target region of interest, two to four blocks were examined in the electron microscope, depending on the intensity of the anterograde labeling.
Projection of the temporal neocortex to area 36
In the electron microscope, PHAL-labeled structures were easy to differentiate from unlabeled elements because of the electron-dense amorphous DAB reaction product associated with them (Figs. 3–7). Following PHAL injections in the temporal neocortex, a large number of PHAL-positive axonal profiles could be observed in area 36, either passing fibers or synaptic boutons. In this and other pathways examined here (see below), all PHAL-positive axons were non-myelinated and ranged in diameter between 0.2 and 0.9 µm. Synaptic boutons could be distinguished by the presence of round vesicles, mitochondria, and in frequent cases the presence of synaptic specializations. In the following account, we will only consider profiles that clearly formed synapses, i.e., where the pre- and post-synaptic densities, vesicles, and synaptic cleft could be observed.
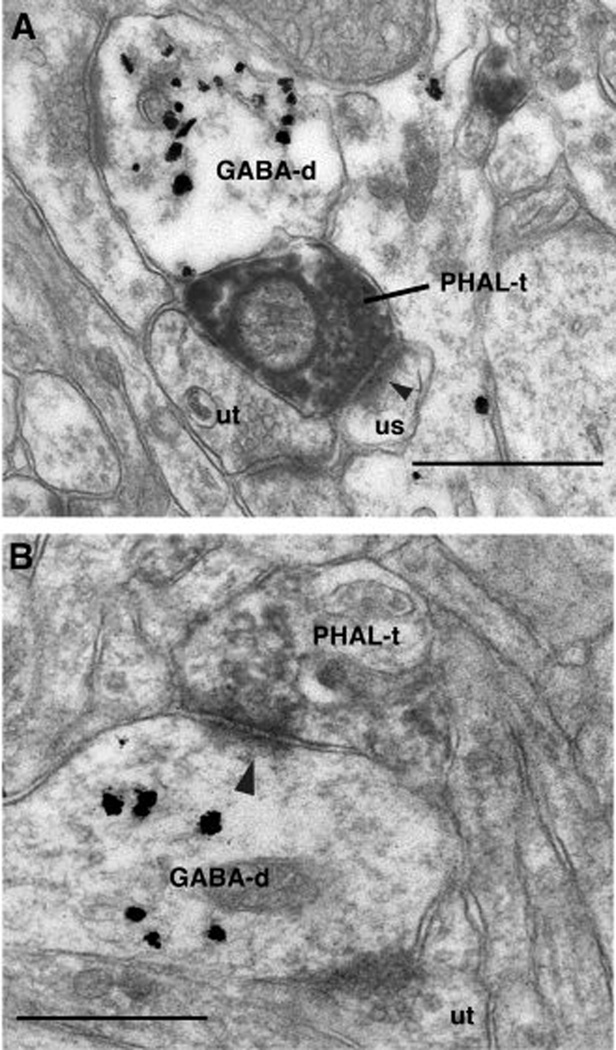
Fig. 3.
Electron-micrographs of neocortical axon terminals ending in area 36. PHAL immunoreactive axon terminals (PHAL-t) originating in the temporal neocortex and forming asymmetric synapses (arrowheads) with a dendritic spine (us, A) or GABA-positive dendritic profile (GABA-d, B) in area 36. Note heterogeneous distribution of gold particles indicating specificity of the GABA immunoreaction. Abbreviation: ut, unlabeled terminal. Scale bars correspond to 0.5 µm.
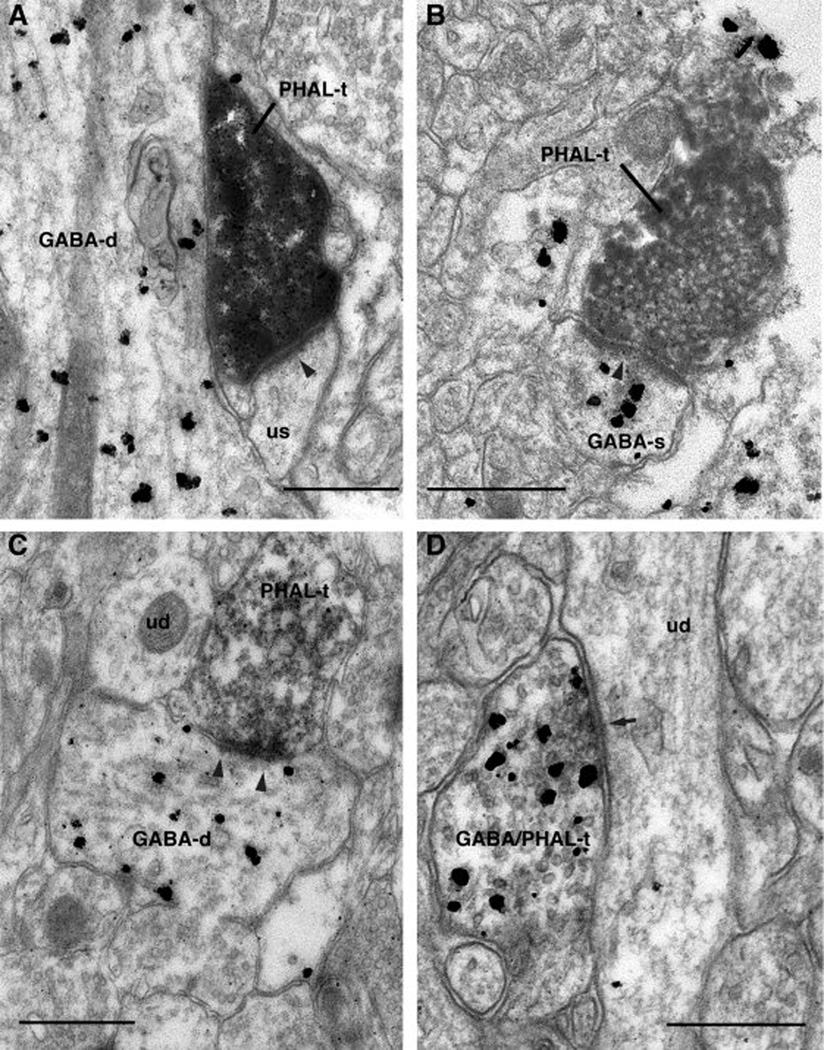
Fig. 7.
Axon terminals from perirhinal areas 36–35 and ending in the lateral entorhinal cortex. (A) PHAL-immunopositive (PHAL-t) axon terminal forming an asymmetric synapse (arrowhead) with an unlabeled spine (us). (B) PHAL-immunopositive (PHAL-t) axon terminal forming an asymmetric synapse (arrowheads) with a GABA-immunoreactive dendritic profile. (C) GABA- and PHAL-immunopositive (GABA/PHAL-t) axon terminal contacting an unlabeled spine (us). Scale bars correspond to 0.5 µm.
A total of 77 axon terminals from the temporal neocortex were observed forming synapses in area 36. All these terminals formed asymmetric synapses, typically onto GABA-immunonegative spines (87% or 67 of 77, Fig. 3A) but occasionally on dendrites (13% or 10 of 77, Fig. 3B). However, all of the dendrites in area 36 that were seen to receive synaptic inputs from the temporal neocortex were GABA immunoreactive (Fig. 3B), as indicated by the presence of concentrated gold particles.
Projection of the temporal neocortex to area 35
A total of 76 neocortical boutons forming clearly identifiable synapses within area 35 were observed. Most of these axon terminals formed asymmetric synapses (84% or 64 of 76), usually onto spines (77% or 49 of 64, Fig. 4A) but also on dendrites (23% or 15 of 64, Fig. 4B), which typically displayed GABA immunoreactivity (Fig. 4B). In addition, in contrast with projections of the temporal neocortex to area 35, a smaller group of GABA-immunoreactive terminals (16%) from the temporal neocortex formed symmetric synapses predominantly onto GABA-immunonegative dendrites (92% or 11 of 12; Fig. 5) or spines (8% or 1 of 12), suggesting the existence of a long-range inhibitory projection from the neocortex to area 35.
Fig. 4.
Electron-micrographs of neocortical axon terminals ending in area 35. PHAL immunoreactive axon terminals (PHAL-t) forming asymmetric synapses (arrowheads) with a dendritic spine (us, A) or a GABA-positive dendritic profile (GABA-d, B) in area 35. Scale bars correspond to 0.5 µm.
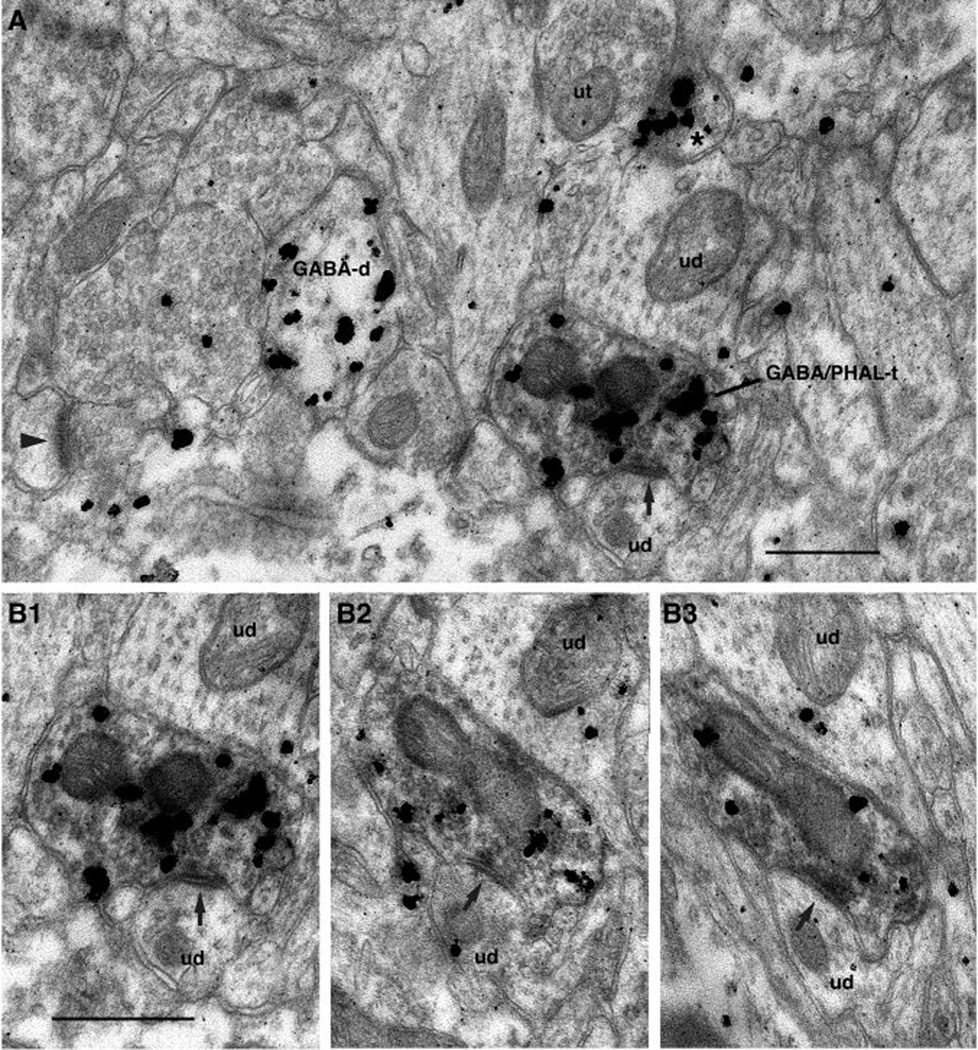
Fig. 5.
Neocortical axon terminal immunoreactive for PHAL and GABA (GABA/PHAL-t) that forms a symmetric synapse (arrows) with a GABA positive dendrite in area 35. A shows a large field containing this element whereas B shows the same axon terminal on three consecutive ultrathin sections (B1–3) at a slightly higher magnification. Scale bars correspond to 0.5 µm.
Because long-range GABAergic connections are atypical in the cerebral cortex, we routinely examined such instances of symmetrical synapses on serial sections. This analysis was performed to determine whether the co-occurrence of PHAL and GABA-immunoreactivity in the presynaptic element was due to artifactual background labeling. Figure 5 shows a representative example of such an analysis at low (Fig. 5A) and high magnifications (Fig. 5B). In the low power electron-micrograph (Fig. 5A), gold particles signaling GABA immunoreactivity can be seen to cluster within the confines of three processes, only one of which was PHAL immunoreactive (marked GABA/PHAL-t), leaving the rest of the field mostly devoid of immunolabeling. Note that that the co-occurrence of DAB and gold particles in this axon terminal was observed on three consecutive ultrathin sections (Fig. 5B1–3) and that the post-synaptic density remained thin.
Area 36 projection to area 35
A total of 131 PHAL labeled profiles from area 36 were observed forming synapses within area 35. Similar to the projection arising from the temporal neocortex, the axon terminals from area 36 predominantly formed asymmetric synapses (93% or 122 of 131), mostly onto spines (65% or 79 of 122) and less frequently onto dendritic shafts (35% or 43 of 122). All but one of these spines were GABA-immunonegative (Fig. 6A; Fig. 6B shows the exception) whereas most of these dendrites (76%) were immunoreactive for GABA (Fig. 6C).
Fig. 6.
Axon terminals from area 36 and ending in area 35. (A) PHAL positive axon terminal (PHAL-t) forming an asymmetric synapse (arrowhead) with an unlabeled dendritic spine (us). (B-C) PHAL positive axon terminals (PHAL-t) forming asymmetric synapses (arrowheads) with a spine (GABA-s, B) or dendrite (GABA-d, C), both of which were immunoreractive for GABA. (D) Axon terminal immunoreactive for GABA and PHAL (GABA/PHAL-t) forming a symmetric synapses (arrow) with an unlabeled dendrite (ud). Scale bars correspond to 0.5 µm.
In addition, a minority of PHAL-positive area 36 axon terminals (7% or 9 of 131) displayed GABA immunoreactivity and formed symmetric synapses onto GABA-immunonegative spines (30%) or dendrites (70%, Fig. 6D) in area 35.
Projection from areas 36 and 35 to the lateral entorhinal cortex
Compared to the pathways examined above, the projection from areas 36 and 35 to the lateral entorhinal cortex was of moderate intensity, reducing the yield of our electron microscopic observations. Because the PHAL positive axon terminals arising from areas 36 and 35 and ending in the lateral entorhinal cortex displayed similar morphological features, the results obtained in these two pathways were therefore pooled.
A total of 57 axon terminals forming synapses in the lateral entorhinal cortex were observed. These axon terminals typically formed asymmetric synapses (88% or 50 of 57) usually onto spines (68% or 34 of 50, Fig 7A) but also on dendrites (32% or 16 of 50, Fig 7B), a majority of which were immunoreactive for GABA (Fig. 7B). See Table 1 for a comparison of the postsynaptic targets of axons forming asymmetric synapses in the various pathways investigated in this study. The remaining PHAL-positive axon terminals (12% or 7 of 57) were GABA-immunoreactive and formed symmetric synapses onto dendritic profiles (Fig. 7C) that were devoid of GABA immunoreactivity. See supplementary figure for other examples of such synapses.
TABLE 1.
Postsynaptic targets of axons forming asymmetric synapses in the various pathways investigated in this study.
| Neocortex to area 36 |
Neocortex to area 35 |
Area 36 to Area 35 |
Areas 36–35 to entorhinal cortex |
|
|---|---|---|---|---|
| Spines | 87% | 77% | 65% | 68% |
| Dendritic profiles |
13% | 23% | 35% | 32% |
| N | 77 | 64 | 122 | 50 |
DISCUSSION
The present study was undertaken to shed light on the inhibitory influences that limit impulse traffic in the multisynaptic path linking the temporal neocortex to the entorhinal cortex by way of the perirhinal cortex. Our interest for this issue stemmed from discrepant anatomical and physiological data about this network. Indeed, despite the fact that the perirhinal cortex forms strong reciprocal connections with the temporal neocortex and entorhinal cortex (reviewed in Witter et al., 2000), perirhinal transmission of neocortical and entorhinal inputs occurs with a low probability (reviewed in De Curtis and Paré, 2004). However, our results suggest that the contradiction is only apparent, in part because an important proportion of the connectivity in this network involves excitatory inputs to GABAergic neurons as well as long-range GABAergic projections to principal neurons. The following account considers these findings in light of previous ultrastructural observations about cortical connectivity and discusses their possible functional consequences.
Feedforward inhibition in the network linking the neocortex to the rhinal cortices
Here, we analyzed the types of synapses linking the temporal neocortex to the perirhinal cortex, those linking perirhinal areas 36 and 35, as well as the projections from the perirhinal cortex to the lateral entorhinal cortex. Although asymmetric synapses between GABA-immunonegative elements constituted the most common type of connections seen in all these pathways, an important proportion of asymmetric synapses (23–35%) were formed by GABA-immunonegative axon terminals contacting GABA-positive profiles. This was true in all pathways examined with the exception of the pathway linking the temporal neocortex to area 36, where such synapses accounted for a much lower proportion of asymmetric synapses (13%).
The proportion of asymmetric synapses onto GABAergic neurons seen in the pathways linking area 36 to area 35 as well as from the perirhinal cortex to the entorhinal cortex (35% and 32%, respectively) is higher than previously reported for long-range cortico-cortical connections in other regions of the cerebral cortex. Indeed, in the primary visual cortex (Kisvarday et al., 1986; Gabbott et al., 1987; McGuire et al., 1991), primary somatosensory cortex (Elhanny et al., 1990), and prefrontal cortex (Melchitzky et al., 1998), only 8 to 20% of the synapses formed by long-range cortico-cortical axons were found to contact presumed interneurons.
Excitatory inputs onto GABAergic neurons constitute the morphological substrate of what physiologists refer to as feedforward inhibition. This form of inhibitory control ensures that inhibition rapidly follows excitatory postsynaptic potentials in order to prevent runaway excitation. Several physiological studies have documented this form of inhibition in the rhinal cortices (Finch et al., 1988; Jones, 1994; Funahashi and Stewart, 1998; Martina et al., 2001a; Garden et al., 2002).
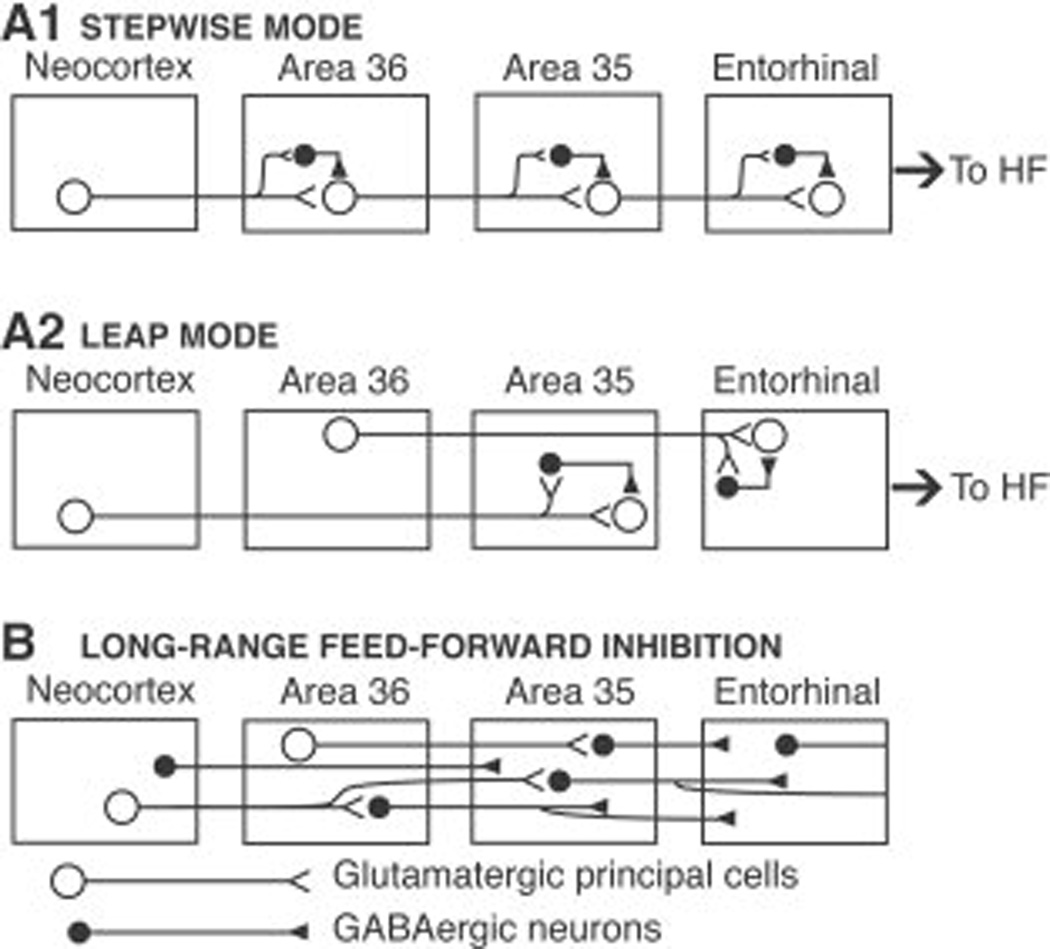
However, because the pathway linking the neocortex to the entorhinal cortex is multisynaptic, feedforward inhibition may exert an even more powerful control of excitability in this pathway. Indeed, progression of impulses through the rhinal cortices occurs in two ways (for instance see Room and Groenewegen, 1986a; Witter et al., 1986): most of the connections involve a slow stepwise progression through a sequence of adjacent cortical areas (hereafter referred to as the stepwise mode; Fig. 8A1), but some neurons project beyond the adjoining area (leap mode; Fig. 8A2). For example, the temporal neocortex projects to area 36 which projects to area 35 (stepwise mode). In parallel, some neocortical cells directly project to area 35 (leap mode) where, as shown here, they contact both principal cells and GABAergic interneurons (Fig. 8A2). Because principal cells generally have a longer membrane time constant and, according to some, a more negative membrane potential than local circuit cells (McCormick et al., 1985; Kawaguchi and Kubota, 1997; Martina et al. 2001a,b), excitatory inputs take longer to fire principal cells than interneurons. As a result, the feedforward inhibition generated by leaping projections would be triggered before P-cells receive the bulk of their excitatory drive via stepwise connections. This should reduce transfer probability through the slow path (Fig. 8A1). Here, it should be stressed that although feedforward inhibition dampens excitability at every step of this multisynaptic pathway, what truly reduces the probability of impulse transfer between the neocortex and entorhinal cortex is the cumulative effect of these inhibitory influences at multiple successive stages in the multisynaptic path linking these cortical regions.
Fig. 8.
Schematic representation of the connectivity evidenced in the present study. Glutamatergic and GABAergic neurons are represented by open and filled circles, respectively. Neocortical impulses can reach the hippocampus in two ways: stepwise (A1) and leap (A2) modes. Most neocortical inputs reach the hippocampus via a sequence of glutamatergic corticocortical connections coursing through the superficial layers of area 36, area 35, and the entorhinal cortex. At each stage of this multisynaptic pathway, principal cells form excitatory synapses with other principal cells and with GABAergic local-circuit neurons that generate feedforward inhibition. (A2) Although the bulk of the connections in this network follow a stepwise progression, some principal neurons project beyond the adjoining area where they also form excitatory synapses with other principal cells and GABAergic local-circuit neurons. (B) Some GABAergic cells have axons that extend beyond the area where their soma is located. Abbreviations: HF, hippocampal formation.
Besides feedforward inhibition, other factors, such as the intrinsic membrane properties of rhinal neurons, likely contribute to reduce the probability of impulse transfer from the neocortex to the entorhinal cortex. Indeed, there are “late-firing” neurons in the perirhinal cortex (Faulkner and Brown, 1999; McGann et al., 2001). In these cells, there is a conspicuous delay between the onset of depolarizing current pulses and spike discharges. During this delay, the membrane potential depolarizes slowly, giving rise to a ramp lasting hundreds of milliseconds. In other cell types, this behavior results from the presence of a slowly inactivating K+ conductance that activates around −65 mV, has rapid activation kinetics, inactivates slowly, and requires hyperpolarization beyond −100 mV for full deinactivation (Storm 1988; Bargas et al. 1989; Nisenbaum et al. 1994). The presence of this K+ current has important consequences for the activity of late-firing neurons. As a result, depolarizing voltage transients caused by excitatory synaptic inputs are attenuated. Moreover, in response to a sustained barrage of excitatory synaptic inputs, the membrane potential rise is delayed. Thus, in concert with feedforward inhibition, this intrinsic K+ current should further reduce perirhinal transfer of neocortical and entorhinal inputs.
At odds with these considerations however, a recent study (de Villiers-Sidani et al., 2004) reported “robust synaptic activation patterns of the perirhinal-entorhinal interconnections” (p. 255). However, upon closer examination, it becomes clear that this study addressed a different question. These authors electrically stimulated area 35 and recorded <1 mm more medial in the adjacent part of the lateral entorhinal cortex. Leaving aside the issues of current diffusion from the stimulation site and the complications related to the likely antidromic invasion of entorhinal neurons by electrical stimuli, one should not be surprised that excitatory responses were observed in the entorhinal cortex. After all, area 35 does send an excitatory projection to the entorhinal cortex in all species investigated so far including monkeys, rats, cats, and guinea pigs (see references in Introduction). However, the question is not whether area 35 projects to the lateral entorhinal cortex, but whether afferents to the perirhinal region can overcome the strong local inhibition and generate significant neuronal activation in the entorhinal cortex. The only way to test this is to stimulate afferents to the perirhinal cortex, which was not investigated in the study by de Villiers-Sidani et al. (2004).
Long-range GABAergic cells in the pathways linking the temporal neocortex to the entorhinal cortex
An unexpected observation in the present study was the occurrence of symmetric synapses formed by GABA-immunoreactive axon terminals onto GABA-negative dendritic profiles. Such synapses accounted for as many as 7–16% of contacts in all pathways examined here except in that linking the temporal neocortex to area 36. This observation implies that some of the anterograde labeling produced by PHAL injections in the temporal neocortex and the perirhinal cortex resulted from PHAL transport within GABAergic cells with axons extending beyond the area where their soma is located (Fig. 8B). Two factors suggest that this observation is not an artifact due to spread of the tracer to neighboring area. First, we only considered PHAL injections that remained confined within a particular region of interest. Second, such synapses were not only observed between adjoining areas, but also at a distance (e.g. from the temporal neocortex or area 36 to area 35 or the lateral entorhinal cortex, respectively).
These observations are inconsistent with the traditional view of cortical physiology where communication between cortical areas is thought to be entirely dependent on glutamatergic neurons. According to this model, GABAergic cells of the cerebral cortex act locally, within the area where their somata are located. As a consequence, all IPSPs generated in the course of interactions between cortical areas are believed to be generated di-synaptically, after the glutamatergic activation of local-circuit GABAergic neurons.
Although the suggestion that the rhinal cortices contain long-range GABAergic neurons might be considered heretic in some circles, much evidence supports this claim. For instance, in the visual cortex, some GABAergic neurons project through the corpus callosum to the contralateral visual cortex (Hughes and Peters, 1992). Moreover, a recent study using retrograde tracing in GAD67-green fluorescent protein knock-in mice revealed the existence of somatostatin-positive cortical interneurons with axons that extend for up to 2 mm (Tomioka et al., 2005). Interestingly, such long-range GABA cells are not only present in the neocortex but also in the hippocampal formation. For instance, one class of CA1 interneurons has long-range projections to field CA3 (Sik et al., 1994). Yet another type of GABAergic neuron located in the dentate gyrus projects to the subiculum (Ceranik et al., 1997). It was further reported that some entorhinal neurons projecting to the dentate gyrus are GABAergic (Germroth et al., 1989) and that there are GABAergic projections from the presubiculum to the entorhinal cortex (Van Haeften et al., 1997). Last, using threshold electrical as well as chemical microstimulation in brain slices in vitro, we have recently obtained physiological evidence that the perirhinal cortex contains GABAergic neurons that generate IPSPs in principal cells of the entorhinal cortex (J. Apergis-Schoute, A. Pinto, D. Paré, unpublished observations).
Functional implications
Our ultrastructural observations suggest that an important proportion of the connectivity linking the temporal neocortex to the entorhinal cortex involves excitatory inputs to GABAergic neurons as well as long-range GABAergic projections to principal neurons. These findings imply that impulse traffic in this network is subjected to powerful inhibitory influences, a conclusion supported by the fact that perirhinal transmission of neocortical inputs to the entorhinal cortex occurs with an extremely low probability (De Curtis et al., 1999; Biella et al., 2002, 2003; Pelletier et al., 2004, 2005).
However, this tight inhibitory control of impulse traffic in the transverse plane contrasts with that seen in the system of intrinsic longitudinal connections that link different rostrocaudal levels of the perirhinal and entorhinal cortices (for instance see Witter et al., 1986). Indeed, physiological studies suggest that these connections do not engage inhibitory neurons, only principal cells (Biella et al., 2001, 2002; Martina et al., 2001a). It is conceivable that this dual organization (tight inhibitory control in the transverse plane vs. unconstrained interactions longitudinally) reflects the normal mode of operation of the rhinal cortices. According to this view, perirhinal transfer of neocortical inputs in the transverse plane would only occur when neocortical impulses would coincide with activity in longitudinal connections, thus overwhelming the inhibitory influences that limit transverse propagation.
Another possibility, compatible with the above scheme, is that extrinsic inputs to the perirhinal cortex might reduce or overwhelm the local inhibition. For instance, both the medial prefrontal cortex (mPFC; Room et al., 1985; Sesack et al., 1989; Takagishi and Chiba, 1991) and basolateral complex of the amygdala (Krettek and Price, 1977a,b; Room and Groenewegen, 1986b; Smith and Paré, 1994; reviewed in Pitkänen et al., 2000) send robust excitatory projections to the rhinal cortices. Moreover, it was recently reported that amygdala inputs could promote the spread of perirhinal activity to the entorhinal cortex and hippocampus in conditions of partial GABA-A block (Kajiwara et al. 2003). Considering that the mPFC and amygdala have been implicated in the control of reward-related behavior and emotions (Baxter and Murray, 2002, Öngür and Price, 2000; Aggleton, 2000), it is possible that these inputs regulate impulse traffic through rhinal cortices as a function of the emotional significance of current environmental contingencies. Another possibility is that synchronized inputs from rostrocaudally distributed entorhinal neurons are required to overwhelm the local perirhinal inhibition. Testing these ideas constitute important challenges for future studies.
Supplementary Material
Acknowledgments
This work was supported by RO1 grants MH-073610 and MH-066856 from NIH. Thanks are due to members of the Paré laboratory for comments on an earlier version of this manuscript.
LITERATURE CITED
- Aggleton JP. The amygdala: a functional analysis. Oxford: Oxford University Press; 2000. [Google Scholar]
- Bargas J, Galarraga E, Aceves J. An early outward conductance modulates the firing latency and frequency of neostriatal neurons of the rat brain. Exp Brain Res. 1989;75:146–156. doi: 10.1007/BF00248538. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Biella G, Uva L, de Curtis M. Network activity evoked by neocortical stimulation in area 36 of the guinea pig perirhinal cortex. J Neurophysiol. 2001;86:164–172. doi: 10.1152/jn.2001.86.1.164. [DOI] [PubMed] [Google Scholar]
- Biella G, Uva L, De Curtis M. Propagation of neuronal activity along the neocortical-perirhinal-entorhinal interactions. J Neurosci. 2002;22:9972–9979. doi: 10.1523/JNEUROSCI.22-22-09972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biella JR, Gnatkovsky V, Takashima I, Kajiwara R, Iijima T, De Curtis M. Olfactory input to the parahippocampal region of the isolated guinea pig brain reveals weak entorhinal to perirhinal interactions. Eur J Neurosci. 2003;18:95–101. doi: 10.1046/j.1460-9568.2003.02730.x. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998a;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998b;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ceranik K, Bender R, Geiger JR, Monyer H, Jonas P, Frotscher M, Lubke J. A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. J Neurosci. 1997;17:5380–5394. doi: 10.1523/JNEUROSCI.17-14-05380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Curtis M, Paré D. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol. 2004;74:101–110. doi: 10.1016/j.pneurobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- De Curtis M, Takashima I, Iijima T. Optical recording of cortical activity after in vitro perfusion of cerebral arteries with a voltage-sensitive dye. Brain Res. 1999;837:314–319. doi: 10.1016/s0006-8993(99)01712-6. [DOI] [PubMed] [Google Scholar]
- Deacon TW, Eichenbaum H, Rosenberg P, Eckman KW. Afferent connections of the perirhinal cortex in the rat. Journal of Comparative Neurology. 1983;220:168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Tahvildari B, Alonso A. Synaptic activation patterns of the perirhinal-entorhinal inter-connections. Neuroscience. 2004;129:255–265. doi: 10.1016/j.neuroscience.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Elhanany E, White EL. Intrinsic circuitry: Synapses involving the local axon collaterals of corticocortical projection neurons in the mouse primary somatosensory cortex. J Comp Neurol. 1990;291:43–54. doi: 10.1002/cne.902910105. [DOI] [PubMed] [Google Scholar]
- Faulkner B, Brown TH. Morphology and physiology of neurons in the rat perirhinal-lateral amygdala area. J Comp Neurol. 1999;411:613–642. [PubMed] [Google Scholar]
- Finch DM, Tan AM, Isokawa-Akesson M. Feedforward inhibition of the rat entorhinal cortex and subicular complex. J Neurosci. 1988;8:2213–2226. doi: 10.1523/JNEUROSCI.08-07-02213.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederico P, MacVicar BA. Imaging the induction and spread of seizure activity in the isolated brain of the guinea pig: the roles of GABA and glutamate receptors. J Neurophysiol. 1994;76:3471–3492. doi: 10.1152/jn.1996.76.5.3471. [DOI] [PubMed] [Google Scholar]
- Funahashi M, Stewart M. GABA receptor-mediated post-synaptic potentials in the retrohippocampal cortices: regional, laminar and cellular comparisons. Brain Res. 1998;787:19–33. doi: 10.1016/s0006-8993(97)01384-x. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Martin KA, Whitteridge D. Connections between pyramidal neurons in layer 5 of cat visual cortex (area 17) J Comp Neurol. 1987;259:364–381. doi: 10.1002/cne.902590305. [DOI] [PubMed] [Google Scholar]
- Garden DLF, Kemp N, Bashir ZI. Differences in GABAergic transmission between two inputs into the perirhinal cortex. Eur J Neurosci. 2002;16:437–444. doi: 10.1046/j.1460-9568.2002.02096.x. [DOI] [PubMed] [Google Scholar]
- Germroth P, Schwerdtfeger WK, Buhl EH. Gabaergic neurons in the entorhinal cortex project to the hippocampus. Brain Res. 1989;494:187–192. doi: 10.1016/0006-8993(89)90162-5. [DOI] [PubMed] [Google Scholar]
- Hughes CM, Peters A. Symmetric synapses formed by callosal afferents in rat visual cortex. Brain Res. 1992;583:271–278. doi: 10.1016/s0006-8993(10)80033-2. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey. II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Jones RSG. Synaptic and intrinsic properties of neurons of origin of the perforant path in layer II of the rat entorhinal cortex in vitro. Hippocampus. 1994;4:335–353. doi: 10.1002/hipo.450040317. [DOI] [PubMed] [Google Scholar]
- Kajiwara R, Takashima I, Mimura Y, Iijima T. Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal-hippocampal circuit. J Neurophysiol. 2003;89:2176–2184. doi: 10.1152/jn.01033.2002. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF, Martin KAC, Freund TF, Magloczky Z, Whitteridge D, Somogyi P. Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res. 1986;64:541–552. doi: 10.1007/BF00340492. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol. 1977a;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. Journal of Comparative Neurology. 1977b;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Luparello TJ. Stereotaxic atlas of the forebrain of the guinea pig. Baltimore: Williams & Wilkins Co; 1967. [Google Scholar]
- Martina M, Royer S, Paré D. Propagation of neocortical inputs in the perirhinal cortex. J Neurosci. 2001a;21:2878–2888. doi: 10.1523/JNEUROSCI.21-08-02878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Royer S, Paré D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol. 2001b;86:2887–2895. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lightall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McGann JP, Moyer J, Jr, Brown TH. Predominance of late-spiking neurons in layer VI of rat perirhinal cortex. J Neurosci. 2001;21:4969–4976. doi: 10.1523/JNEUROSCI.21-14-04969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Gilbert CD, Rivlin PK, Wiesel TN. Targets of horizontal connections in macaque monkey primary visual cortex. J Comp Neurol. 1991;305:370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kelly ME, Staines WA. Efferent projections of the anterior perirhinal cortex in the rat. J Comp Neurol. 1996;369:302–318. doi: 10.1002/(SICI)1096-9861(19960527)369:2<302::AID-CNE10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol. 1998;390:211–224. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J Neurophysiol. 1994;71:1174–1189. doi: 10.1152/jn.1994.71.3.1174. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Ben-Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol. 1987;266:45–55. doi: 10.1002/cne.902660105. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Apergis J, Paré D. Low probability transmission of neocortical and entorhinal impulses through the perirhinal cortex. J Neurophysiol. 2004;91:2079–2089. doi: 10.1152/jn.01197.2003. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Apergis-Schoute J, Paré D. Interaction between amygdala and neocortical inputs in the perirhinal cortex. J Neurophysiol. in press doi: 10.1152/jn.00260.2005. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HF. The Fine Structure of the Nervous System. New York: Oxford University Press; 1991. [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Ann NY Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Room P, Groenewegen HJ. Connections of the parahippocampal cortex. I. Cortical afferents. Journal of Comparative Neurology. 1986a;251:415–450. doi: 10.1002/cne.902510402. [DOI] [PubMed] [Google Scholar]
- Room P, Groenewegen HJ. Connections of the parahippocampal cortex in the cat. II. Subcortical afferents. Journal of Comparative Neurology. 1986b;251:451–473. doi: 10.1002/cne.902510403. [DOI] [PubMed] [Google Scholar]
- Room P, Russchen FT, Groenewegen HJ, Lohman AH. Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: an anterograde tracing study in the cat. J Comp Neurol. 1985;242:40–55. doi: 10.1002/cne.902420104. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Tanaka K. Divergent projections from the anterior inferotemporal area TE to the perirhinal and entorhinal cortices in the macaque monkey. J Neurosci. 1996;16:4757–4775. doi: 10.1523/JNEUROSCI.16-15-04757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with post-embedding GABA and glutamate immunocytochemistry. J Comp Neurol. 1994;342:232–248. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994a;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994b;14:1854–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–36. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Tomioka R, Okamoto K, Furuta T, Fujiyama F, Iwasato T, Yanagawa Y, Obata K, Kaneko T, Tamamaki N. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21:1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- Uva L, Gruschke S, Biella G, De Curtis M, Witter MP. Cytoarchitectonic characterization of the parahippocampal region of the guinea pig. J Comp Neurol. 2004;474:289–303. doi: 10.1002/cne.20121. [DOI] [PubMed] [Google Scholar]
- Van Haeften T, Wouterlood FG, Jorritsma-Byham B, Witter MP. GABAergic presubicular projections to the medial entorhinal cortex of the rat. J Neurosci. 1997;17:862–874. doi: 10.1523/JNEUROSCI.17-02-00862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus. 2003;13:943–952. doi: 10.1002/hipo.10144. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Research. 1975;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ. Connections of the parahippocampal cortex in the cat. III. Cortical and thalamic efferents. J Comp Neurol. 1986;252:1–31. doi: 10.1002/cne.902520102. [DOI] [PubMed] [Google Scholar]
- Witter MP, Room P, Groenewegen HJ, Lohman AHM. Connections of the parahippocampal cortex in the cat. V. Intrinsic connections; Comments on input/output connections with the hippocampus. Journal of Comparative Neurology. 1986;252:78–94. doi: 10.1002/cne.902520105. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann NY Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Hartig W, Bruckner G, Witter MP. Parvalbumin-immunoreactive neurons in the entorhinal cortex of the rat: localization, morphology, connectivity and ultrastructure. J Neurocytol. 1995;24:135–153. doi: 10.1007/BF01181556. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Mugnaini E, Nederlof J. Projection of olfactory bulb efferents to layer I GABAergic neurons in the entorhinal area. Combination of anterograde degeneration and immunoelectron microscopy in rat. Brain Res. 1985;343:283–296. doi: 10.1016/0006-8993(85)90746-2. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Nederlof J. Terminations of olfactory afferents on layer II and III neurons in the entorhinal area: degeneration-Golgi-electron microscopic study in the rat. Neurosci Lett. 1983;36:105–110. doi: 10.1016/0304-3940(83)90250-1. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, van Denderen JC, van Haeften T, Witter MP. Calretinin in the entorhinal cortex of the rat: distribution, morphology, ultrastructure of neurons, and co-localization with gamma-aminobutyric acid and parvalbumin. J Comp Neurol. 2000;425:177–192. doi: 10.1002/1096-9861(20000918)425:2<177::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Van Haeften T, Eijkhoudt M, Baks-Te-Bulte L, Goede PH, Witter MP. Input from the presubiculum to dendrites of layer-V neurons of the medial entorhinal cortex of the rat. Brain Res. 2004;1013:1–12. doi: 10.1016/j.brainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.