Abstract
Fungal biofilm formation on healthcare materials is a significant clinical concern, often leading to medical device related infections, which are difficult to treat. A novel fungal repellent strategy is developed to control fungal biofilm formation. Methylacrylic acid (MAA) is grated onto poly methyl methacrylate (PMMA)-based biomaterials via plasma initiated grafting polymerization. A cationic polymer, trimethylchitosan (TMC), is synthesized by reacting chitosan with methyl iodide. Sodium alginate (SA) is used as an anionic polymer. TMC/SA multilayers are coated onto the MAA-grafted PMMA via layer-by-layer self-assembly. The TMC/SA multilayer coatings significantly reduce fungal initial adhesion, and effectively prevent fungal biofilm formation. It is concluded that the anti-adhesive property of the surface is due to its hydrophilicity, and that the biofilm-inhibiting action is attributed to the antifungal activity of TMC as well as the chelating function of TMC and SA, which may have acted as fungal repellents. Phosphate buffered saline (PBS)-immersion tests show that the biofilm-modulating effect of the multilayer coatings is stable for more than 4 weeks. Furthermore, the presence of TMC/SA multilayer coatings improve the biocompatibility of the original PMMA, offering a simple, yet effective, strategy for controlling fungal biofilm-formation.
Keywords: Trimethylchitosan, Alginate, Layer-by-Layer coatings, Fungal biofilm
1. Introduction
Biofilms are an accumulation of microbes, and a polymeric matrix they produce, that adheres to material surfaces bathed in fluids. Both bacteria and fungi colonize the surfaces of biomaterials used clinically and form biofilms associated with various infections. Although bacterial biofilms have been extensively studied, fungal biofilms have received much less attention.[1–8] Recently, because of the increasing use of antibiotics and indwelling devices, and the growing number of aged and/or immunocompromised patients, fungal biofilm associated infections have emerged as a significant clinical problem with devastating consequences.[2–8] A wide range of medical and dental devices such as dentures, catheters, heart valves, vascular bypass grafts, ocular lenses, artificial joints, central nervous system shunts, etc.,[1] have been reported to be colonized by fungal biofilms, resulting in serious device-related infections with high morbidity and mortality, extended hospital stays, and high costs.[2–8] One of the most troubling characteristics of these types of infections is that the fungal cells living inside the biofilm are much more resistant to antifungal drugs than their planktonic counterparts, due to the expression of multidrug efflux pumps, alterations in membrane structure, and the effect of the extracellular matrix (i.e., the biofilm) itself.[5,6,9–11]
In our continuing effort to design and fabricate antifungal materials for clinical use,[12–14] we discovered that the layer-by-layer (LBL) self-assembly of trimethylchitosan/sodium alginate (TMC/SA) multilayer surface coatings on various clinically relevant biomaterials effectively prevented fungal biofilm formation. Trimethylchitosan (TMC) is one of the most widely used water-soluble cationic derivatives of chitosan. TMC is antimicrobial, biocompatible, and bioadhesive, and has been used in tissue engineering, drug/gene delivery, and wound dressing applications.[15–17] Sodium alginate (SA) is a water-soluble anionic natural polymer isolated from seaweed and various microbial sources. It is biocompatible and mucoadhesive and has been widely used in the food industry; it has also been used in drug delivery, tissue engineering, and nerve repair applications.[18–22] LBL is a simple, yet versatile technique for assembling charged macromolecules, in which polyelectrolytes of opposite charges are placed one layer at a time alternatively on top of each other.[23–30]
This study describes the use of TMC/SA LBL coatings in inhibiting the formation of fungal biofilms. Although fungal cells readily attached to unmodified poly methyl methacrylate (PMMA) and form a biofilm, they failed to do so on the TMC/SA LBL coated PMMA surface. Thus, the TMC/SA multilayer coating acted as a fungal repellent and blocked biofilm formation. These results provide new insight into an alternative biomaterial design for reducing the risk of various device/material-related infections.
2. Results and Discussion
2.1. Fabrication of TMC/SA LBL Coatings onto PMMA-based Biomaterials
In this study, a PMMA-based polymer, Lucitone 199 (Dentsply Intl, York, PA), was used as a representative, clinically relevant material. PMMA is a versatile polymer with multiple dental and medical applications, including complete denture bases, bone cements, screws for bone fixation, fillers for bone cavities/skull defects, and vertebral stabilization in osteoporotic patients,[32] where fungal biofilm formation has become a growing concern.[1–14] To introduce anionic groups onto the PMMA surfaces for TMC binding and subsequent TMC/SA LBL coating, methacrylic acid (MAA) was grafted onto the PMMA polymer. The grafting reaction was carried out in a plasma cleaner as described by us previously.[12] Although plasma treatment alone (without MAA) also introduces anionic functional groups, this effect is short-lived (e.g., last for minutes) because the functional groups can diffuse into the bulk polymer to minimize the free energy of the surface. Thus, to maintain stable anionic groups on the surface, MAA-based anionic polymer chains were covalently bound to the PMMA surface during plasma treatment. [12]
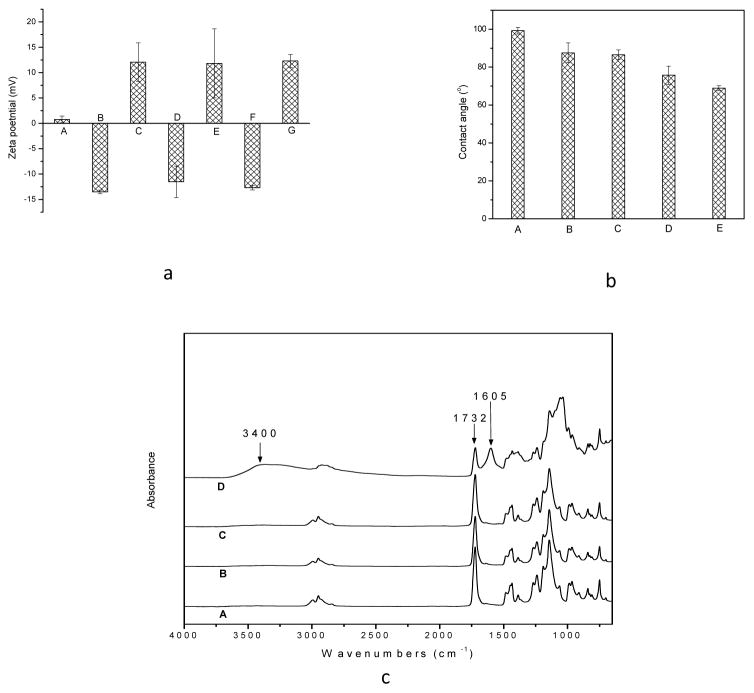
The effect of varying the reaction conditions on MAA grafting is shown in the Supporting Information (Figure S-1). TMC/SA multilayers were then readily built on the grafted PMMA (g-PMMA) surface. Surface charge was characterized with zeta potential analysis. As shown in Figure 1a, the untreated PMMA control had a zeta potential of 0.77±0.67 mV, indicative of a nearly neutral surface. After MAA grafting (grafting yield: 1.54±0.47 wt%), the zeta potential of g-PMMA changed to −13.52±0.34 mV (a negatively charged surface). Upon treatment with TMC, the zeta potential became 12.06±3.81 mV, indicating that the positively charged TMC had been successfully applied to the surface. The coating of SA onto the TMC surface reversed the surface charge (zeta potential = −11.52±3.10 mV). Predictably, it was observed that surface polarity changed with each subsequent treatment (i.e., if the final layer was TMC, a positively charged surface was obtained; if the final layer was SA, the surface carried a negative charge).
Figure 1.
Figure 1a, Zeta potential of the surface of (A) the original PMMA, (B) g-PMMA, (C) g-PMMA with one layer of TMC coating, (D) g-PMMA with one TMC/SA bilayer coating, (E) g-PMMA with 1.5 bilayer coating (one TMC/SA bilayer plus a TMC final layer), (F) g-PMMA with 10 bilayer coatings, and (G) g-PMMA with 10.5 bilayer coatings (n=4); 1b, contact angle values of (A) the original PMMA, (B) g-PMMA, (C) g-PMMA with one layer of TMC coating, (D) g-PMMA with 5.5 bilayers, and (E) g-PMMA with 10.5 bilayers (n=4); and 1c, ATR spectra of (A) the original PMMA, (B) g-PMMA, (C), PMMA-g-MAA with one layer of TMC, and (D) PMMA-g-MAA with 10.5 bilayers
The properties of the multilayer coatings were further assessed with contact angle measurements (Figure 1b). PMMA control discs had a contact angle of 99.23 ± 1.78°, indicative of a hydrophobic surface. After MAA grafting, the contact angle of g-PMMA decreased to 87.55 ± 5.29°; with one layer of TMC coating, the contact angle was 86.54 ± 2.59°. As the number of coatings increased, the contact angle significantly decreased. The sample coated with 10.5 bilayers (10 TMC/SA bilayers plus a TMC final layer) had a contact angle of 68.90 ± 1.33° (p < 0.05). The resulting decrease in contact angel (and thus increase in hydrophilicity) with additional TMC/SA coating was due to the introduction of various groups, such as -OH, -COOH, -NH2, and -N(CH3)3 onto the surface of the PMMA discs.
The presence of various functional groups on the surface was characterized using Fourier transform spectroscopy (FT-IR) in attenuated total reflection (ATR) mode (Figure 1c). The unmodified PMMA disc had a strong absorption at 1732 cm−1, which is characteristic of carbonyl groups. After MAA-grafting and one layer of TMC coating, little difference could be detected in the ATR spectra; this could be explained by the low amount of new functional groups introduced onto the surface.
With the addition of more layers, the presence of TMC and SA functional groups became detectable. For example, the spectrum of g-PMMA with 10.5 TMC/SA bilayers showed a broad band at 3200–3500 cm−1, which was related to the hydroxyl groups and amino groups of SA and TMC, in addition to the 1732 cm−1 band of the original PMMA, and a 1605 cm−1 band which was associated with the carboxylic groups of SA. All of these findings suggest that TMC/SA multilayers were successfully coated onto the PMMA-based biomaterial.
2.2. Effect of TMC/SA LBL Coatings on Initial Fungal Adhesion and Biofilm Formation
Candida albicans (C. albicans, ATCC 10231) was used for proof of principle studies. This is a diploid fungus that grows both as yeast and filamentous cells; it readily colonizes a wide range of materials used clinically, including PMMA, and forms biofilms which cause device-related infections.[1–11] The Candida adhesion study was performed for 1h in phosphate buffered saline (PBS) at 37°C and the level of adherent cells was determined by measuring general metabolic activity with the XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) assay.
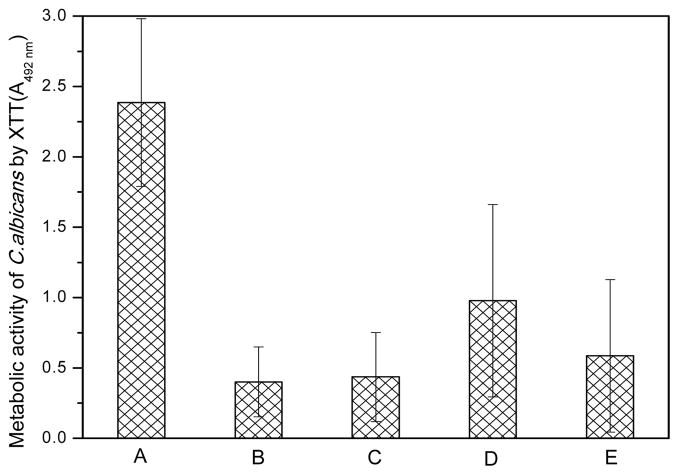
We used this assay instead of the CFU (colony forming unit) determination method because sessile Candida cells isolated from biofilms can have different modes of growth from their free-floating counterparts, making the CFU determination method inaccurate.[5] All surface-modified PMMA samples showed significantly lower amounts of Candida than the control (p < 0.05; Figure 2); differences between the modified surfaces were not statistically significant.
Figure 2.
Level of Candida initial adhesion on different samples evaluated by the metabolic activity of adherent Candida using the XTT assay on (A) the original PMMA to serve as controls, (B) g-PMMA, (C) g-PMMA with one TMC layer, (D) g-PMMA with 5.5 bilayers, and (E) g-PMMA with 10.5 bilayers, after one hour of initial adhesion in PBS (n=4)
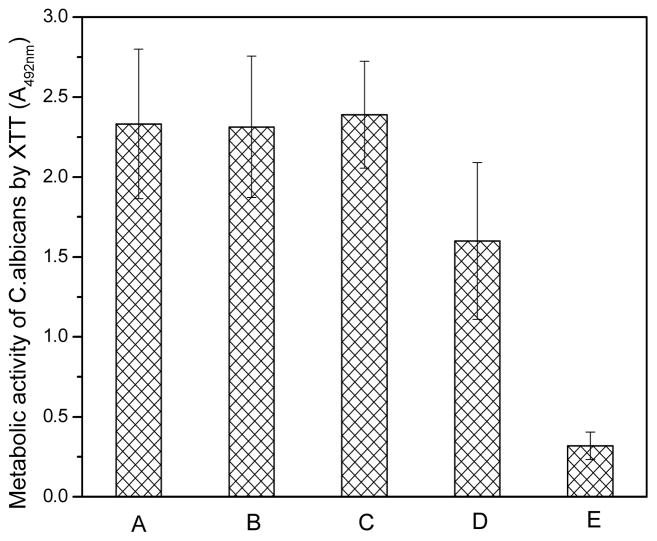
These results suggest that polar and hydrophilic surfaces reduce initial Candida adhesion. In subsequent biofilm-formation studies, materials with adherent Candida were incubated in yeast and mold (YM) broth for 2 days to allow biofilm formation. In contrast to our expectation based on the initial adhesion studies (Figure 2), g-PMMA did not provide any noticeable effect in controlling biofilm formation; in particular, the level of biofilm was very similar to that of the original PMMA control (Figure 3). Similarly, there was no apparent anti-biofilm effect observed with surfaces containing a single layer of TMC coating. However, anti-biofilm activity gradually increased as more layers of the TMC/SA coating was added, e.g., the metabolic activity of Candida on g-PMMA with 5.5 bilayers and 10.5 bilayers was 54.78% and 11.96%, respectively, of the PMMA control (p < 0.05).
Figure 3.
Level of Candida biofilm formation on different samples evaluated by the metabolic activity of adherent Candida using the XTT assay on (A) the original PMMA to serve as controls, (B) g-PMMA, (C) g-PMMA with one TMC layer, (D) g-PMMA with 5.5 bilayers, and (E) g-PMMA with 10.5 bilayers, after two days of incubation in broth solutions (n=4).
These findings suggest that different surface properties affect anti-adhesion and anti-biofilm activities. It is known that Candida favors hydrophobic surfaces for colonization and this is one of the reasons why this fungus readily forms biofilms on PMMA-based materials.[1–12] After MAA grafting and/or TMC/SA LBL coating, the PMMA surfaces became more hydrophilic, resulting in lower initial adhesion. TMC has known biocidal activity.[15–17] To further evaluate the influence of TMC on Candida adhesion and growth, a “sandwich assay” was used[12] to evaluate the effect of continuous contact with PMMA (or a PMMA-modified surface) on fungal metabolic activity (Figure 4).
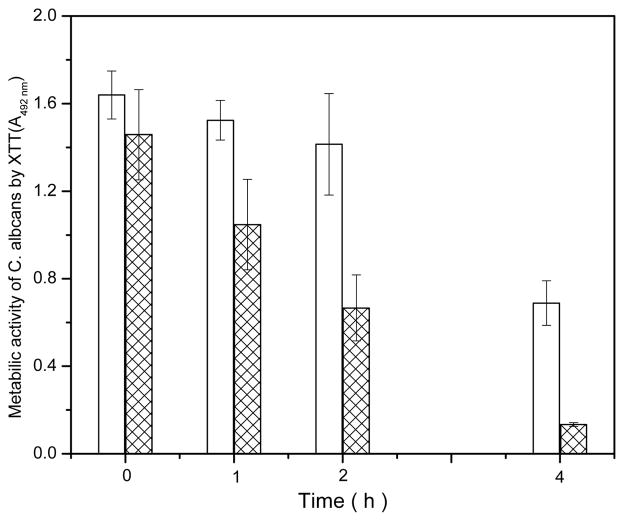
Figure 4.
Antifungal activities of the disc samples against Candida after different periods of direct contact; the figure shows the metabolic activity of Candida on the PMMA control disc (open bar) and g-PMMA with 10.5 bilayer coatings (marked bar) after different periods of direct contact (n=4). A higher XTT absorbance indicates a higher metabolic activity of Candida cells.
After one hour of direct contact with the surface coated with 10.5 bilayers (10 TMC/SA bilayers plus a TMC final layer), 77.2% of the metabolic activity of the PMMA control remained. When contact time was extended to 2h or 4h, the metabolic activity on the 10.5 bilayers decreased to 47.9% and 19.9%, respectively, of the PMMA control levels. Additional increases in contact time failed to reveal any further antifungal activity. Based on these observations, we conclude that the anti-adhesive effect of the LBL modified PMMA is primarily mediated by surface hydrophilicity (lower contact angles). Since the initial adhesion test was only for 1h, there was likely not enough time for us to observe significant antifungal effect due to the TMC coating. This explains our observation that although all the surface-modified PMMA samples showed significantly lower level of adherent Candida than the control (p < 0.05; Figure 2); differences between the modified surfaces (with different layers of TMC) were not statistically significant.
On the other hand, the hydrophilicity provided by g-PMMA appears to be ineffective at preventing Candida growth and/or biofilm formation since robust biofilm growth on g-PMMA was evident after the attached Candida had been cultured for 2 days (Figure 3). In contrast, there was a potent anti-biofilm effect displayed by the TMC/SA coated surfaces in the same assays, which was likely due to the antifungal effects of TMC. Further, both TMC and SA are polymeric chelators that can sequester divalent cations such as Ca2+ and Mg2+. [15–22] Since these cations are essential for stabilizing the polymeric extracellular matrix (ECM) structure of the biofilm synthesized by the fungus,[35] it is possible these chelating effects might have also contributed to the observed anti-biofilm properties.
To determine the anti-biofilm activity of the final coating (TMC or SA), PMMA control, g-PMMA coated with 10 bilayers (SA as final layer), and g-PMMA coated with 10.5 bilayers (TMC as final layer) were tested in biofilm-controlling studies following initial adhesion. After two days of incubation, the metabolic activity of Candida on PMMA controls was significantly higher (p < 0.05) than on the 10-bilayer and 10.5-bilayer discs (Figure 5a), indicating a significantly higher level of adherent Candida on the PMMA control. The metabolic activity on the 10.5 bilayers was slightly lower (not statistically significant) than that on the 10 bilayers, suggesting that in addition to the direct antifungal activities of TMC, the chelating effects TMC and SA could also provide the biofilm-controlling activity.
Figure 5.
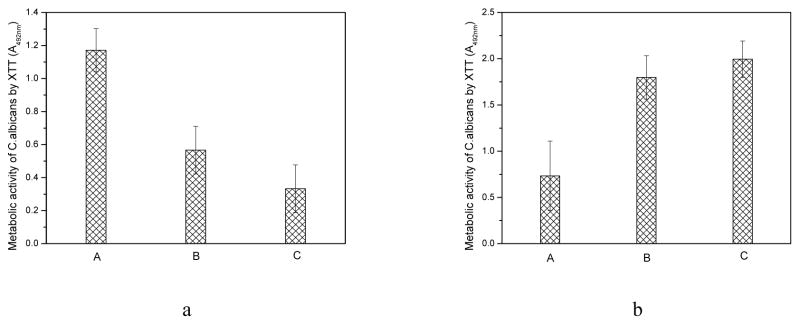
Figure 5a. Metabolic activity of adherent Candida on (A) the unmodified PMMA to serve as controls, (B) g-PMMA with 10 bilayers, and (C) g-PMMA with 10.5 bilayers, after two days of incubation in broth solutions (n=4); and 5b, metabolic activity of free floating Candida in the broth solutions with (A) the original PMMA disc, (B) g-PMMA coated with 10 bilayers, and (C) g-PMMA coated with 10.5 bilayers, after two days of incubation (n=4). A higher XTT absorbance indicates a higher metabolic activity of Candida cells.
The metabolic activity of Candida, suspended in broth solutions containing the corresponding PMMA-based samples, was also determined using the XTT assay (Figure 5b). Interestingly, the broth solutions containing PMMA control discs had significantly less Candida metabolic activity than broth solutions containing the other two types of discs (p < 0.05). Further, the fungi in the 10.5-bilayer broth showed significantly higher metabolic activity than those in the 10-bilayer broth (p < 0.05).
These results suggest that when Candida cells were given a preference between the broth and the PMMA surface (without TMC/SA coatings), the fungal cells favored the PMMA surface for growth and biofilm formation. However, if the surface of the PMMA discs is modified with the TMC/SA multilayers, biofilm formation is affected because of the antifungal effects of the TMC coating and the chelating effects of TMC/SA. In this case, the TMC/SA LBL coatings acted as fungal repellents to prevent Candida biofilm formation. Because TMC had both antifungal effects and chelating effects, using TMC as the final layer led to even more potent repelling effects than using SA as the final layer. To the best of our knowledge, this is the first demonstration of a process for modifying a surface that has fungal repelling activity, rather than fungicidal effect, to achieve anti-fungal biofilm properties, offering a new, drug-free strategy in controlling biofilm formation on healthcare materials.
Biofilm-controlling effects were also directly observed using SEM (scanning electron microscope). As shown in Figure 6, after two days of incubation, a large amount of Candida adhered to the surface of unmodified PMMA (Figure 6 A), forming layered clusters with the presence of filamentous cells (black arrows), suggesting the formation of a fungal biofilm. On g-PMMA (Figure 6 B), Candida adhered and aggregated on the surface, but no filamentous cells were observed. The introduction of TMC/SA coatings led to much less Candida; only scattered Candida cells were evident with no biofilm formation. Few scattered Candida with abnormal appearances (shapes/sizes) and its fragments were observed on the disc surfaces. These findings strongly suggest that TMC/SA multilayer coatings effectively prevented biofilm formation.
Figure 6.

Representative SEM images of biofilm formed on (A) the original PMMA control, (B) g-PMMA, (C) g-PMMA coated with a one layer of TMC, (D) g-PMMA coated with 5.5 bilayers, and (E) g-PMMA coated with 10.5 bilayers, after incubation in broth for 2 days. The scale bar is 10 μm.
The prolonged biofilm-controlling effect of TMC/SA multilayer coatings was evaluated by immersing a series of g-PMMA discs coated with 10.5 bilayers in PBS at 37 °C for up to 4 weeks. The anti-biofilm activity of the coating was essentially unchanged during this time period (Figure S-2 in Supporting Information), suggesting that this surface modification strategy has potential for controlling fungal biofilm formation on clinically relevant biomaterials over extended periods of time.
2.3. Biocompatibility of the TMC/SA LBL Coatings
It is well-established that PMMA-based materials have mild to moderate cytotoxic effects due to the leaching of residual un-polymerized monomers,[33,34] which can result in cell cycle delay and death. To determine the effects of the TMC/SA LBL coatings on cytotoxicity of the resulting materials, the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was used to evaluate the samples (Table 1). The g-PMMA coated with 10.5 bilayers had no significant effect on cell growth, as compared to the PMAA control, except on Day 7; the percent of viable cells in the multilayer conditioned media was significantly higher than that in the PMMA conditioned media (p < 0.05). The preparation of the g-PMMA discs and the subsequent coatings may reduce the content of the residual monomers in the original PMMA. Further, the presence of TMC/SA multilayer coatings may reduce residual monomer release. In both cases, improved biocompatibility would have resulted.
Table 1.
Effect of TMC/SA multilayer coating on CRL-1213™ cell viability using the MTT assay
| Days of disc conditioning | % viable cells after incubation with PMMA control conditioned media | % viable cells after incubation with the multilayer coating conditioned media |
|---|---|---|
| 1 day | 110.04±38.02 | 82.77±23.40 |
| 3 days | 111.45±24.81 | 124.45±8.61 |
| 5 days | 90.83±13.37 | 108.27±19.05 |
| 7 days | 54.40±7.59 | 74.67±1.16* |
Significant difference vs. the PMMA control.
3. Conclusions
The development of biomaterials capable of preventing fungal biofilm formation is a daunting challenge. In this report, we demonstrate that fungal biofilm-controlling functionality can be introduced into a PMMA-based biomaterial by grafting MAA onto the surface, followed by TMC/SA LBL self-assembly coatings. These modifications dramatically alter the hydrophilicity and surface charge of the resulting materials. The TMC/SA multilayers significantly reduce fungal cell initial adhesion, and effectively prevent biofilm formation. The anti-adhesion activities are likely related to surface hydrophilicity, while the biofilm-controlling functions are mediated through the antifungal activities of TMC as well as the chelating effects of TMC and SA, which act as fungal repellents to prevent Candida biofilm formation. To the best of our knowledge, this is the first demonstration of a fungal repellent, based on an LBL coating strategy, which prevents biofilm formation. The anti-adhesion and biofilm-controlling effects were stable for at least 4 weeks. In addition, the introduction of TMC/SA multilayer coatings improved the biocompatibility of the original PMMA-based materials. These results indicate the great potential of this strategy for developing new materials that can be used in a wide range of biomedical applications to prevent fungal biofilm formation.
4. Experimental Section
Materials
A PMMA-based polymer, Lucitone 199 (Dentsply Intl, York, PA), was used as a representative, clinically relevant material. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received. Candida albicans (C. albicans, ATCC 10231) and rat skin fibroblast cells (ATCC CRL-1213) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA).
Instruments
FT-IR spectra of the samples were recorded on a Nicolet iS10 Mid-IR spectrometer using the ATR mode. 1H-NMR spectra were collected on a 600 mHZ Bruker NMR spectrometer (D2O as solvent). Contact angle was measured using the VCA optima surface analysis system (AST, MA, USA); values for each surface were obtained by averaging six different measurements, under the same experimental conditions, in quadruplicate. Zeta-potential of the solid samples was determined with a DelsaNano-C particle analyzer with a flat surface cell assembly, using polystyrene (PS) latex in 10 mM sodium chloride solution as the probe particles. SEM observations were performed on a JEOL JSM 7401 FE-SEM.
Grafting of methylacrylic acid (MAA) onto PMMA-based materials
PMMA-based Lucitone 199 discs (6.0 mm of diameter and 0.5 mm of thickness) were fabricated according to the manufacturer’s instructions. The discs were dipped into acetone containing 0–20 wt% methylacrylic acid (MAA) and diurethane dimethacrylate (DUMA, 5 wt% MMA, as a crosslinker) with 1wt% azobisisobutyronitrile (AIBN) as an initiator at ambient temperature. After a certain period of time (30–120 min), the discs were taken out, air-dried for 60 min, and put in a plasma cleaner (Harrick Plasma, Ithaca, NY) at high RF level for 5–30 min of plasma treatment on each side to graft MAA onto PMMA. The resulting discs (g-PMMA) were washed with acetone and deionized water, air-dried for 24h, and stored in a desiccator for 3 days to reach an equilibrium weight. Grafting yield of MAA onto PMMA was calculated according to the following equation:
where W0 was the weight of the original disc, and W1 was the weight of the g-PMMA.
Preparation of trimethylchitosan (TMC)
TMC was synthesized following a previously described procedure[31] with slight modification. Briefly, 4.0 g chitosan were hydrated in 160 mL N-methylpyrrolidone at 90 °C for 24h. Afterwards, 4.8 g sodium iodide and 22.0 mL of 15wt % sodium hydroxide solution were added to the flask, followed by the addition of 11.5 mL methyl iodide. The mixture was stirred at 90 °C for 18h and then poured into acetone. The precipitate was collected by filtration, washed with acetone, and dried at 50°C under vacuum. The product was dissolved in 5wt% sodium chloride (aqueous) solution to transform iodide ions to chloride ions. After precipitation from ethanol, TMC was collected, dried at 50 °C under vacuum, and stored in a desiccator. The degree of quaternization (28.0%) was determined by NMR studies.[31]
Fabrication of TMC/alginate LBL coatings onto the surfaces of the PMMA-based materials
Stock solutions of TMC and SA with a concentration of 0.1% (w/v) were prepared individually in Tris buffer [0.1 M, tris(hydroxymethyl) aminomethane-hydrochloric acid, pH 7.0]. The g-PMMA discs were first immersed in the TMC solution for 20 min, rinsed thoroughly with deionized water, and then air dried. The resulting discs were designated as g-PMMA coated with one TMC layer. To build multilayers on the discs, using the LBL technique, g-PMMA-TMC discs were immersed in SA solution for 20 min, rinsed with deionized water, and air dried. The resulting discs, g-PMMA-TMC-SA, or g-PMMA coated with one bilayer (SA as the final layer), were coated with TMC again using the same method to produce g-PMMA-(TMC-SA)1-TMC, or g-PMMA coated with 1.5 bilayer (TMC as the final layer). Discs with even more layers, e.g., g-PMMA coated with 5 bilayers (SA as the final layer) or g-PMMA coated with 10.5 bilayers (TMC with the final layer), were prepared similarly.
Effect of TMC/SA multilayer coatings on fungal initial adhesion
C. albicans was used as a representative example of a fungus because of its prevalence in fungal biofilms and device-related infections. [1–11] To test the effect of the TMC/SA multilayer coatings on Candida initial adhesion, a series of PMMA, g-PMMA and g-PMMA coated with TMC/SA multilayers were immersed individually in 108–9 CFU/mL of C. albicans suspended in PBS. After shaking (30 rpm) at 30 °C for 1h, each disc was aseptically removed from the Candida suspension, and gently washed 3 times with sterile PBS to remove any non-adherent fungi. The discs were individually sonicated in 1 mL sterile PBS for 5 min and then vortexed for 60 s to release the adherent fungi into PBS. The level of recoverable Candida from each disc was determined using the XTT assay (n=4), as previously described.[5,12] Briefly, 100 μL of the resulting suspension were aseptically transferred to the well of a 96-well microplate, and 50 μL of the XTT cell proliferation Kit II reagent (prepared by mixing 5 mL of the XTT labeling reagent with 0.1 mL of an electron-coupling reagent, Roche) were added to each well. The mixture was incubated at 37 °C for 20h in dark. The absorbance of each well was measured using an Infinity M200 Pro plate reader at 490 nm. Each test was performed in triplicate.
Effect of the TMC/SA multilayer coatings on fungal biofilm formation
The effect of the TMC/SA multilayer coatings on Candida biofilm formation was evaluated in parallel with the initial adhesion study (see above). After one hour of Candida adhesion, each disc was aseptically removed from the Candida suspension, gently washed 3 times with sterile PBS to remove non-adherent fungi, and placed in YM broth solution for 2 days at 30 °C to allow biofilm formation. Afterwards, the discs were washed with sterile PBS to remove non-adherent Candida. Some of the discs were used in the XTT assay, after sonication and vortexing, to determine the level of adherent Candida on each disc as described above. The remaining discs were immersed in 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer and stored at 4 °C overnight. At the end of treatment, samples were washed 3 times with PBS, followed by dehydration in a graded ethanol series (25%, 50%, 70%, 95%, and 100%; 10 min each). The dehydrated samples were air dried, sputter coated with Pd-Au, and viewed in a JEOL JSM 7401 FE-SEM to check for the presence of adherent Candida/biofilms.
Candida content in the broth solution was also tested with the XTT assay. Briefly, before disc removal, 100 μL of the broth were aseptically transferred to the well of a 96-well microplate, and 50 μL of the XTT cell proliferation Kit II reagent were added to each well. The mixture was incubated and the absorbance was measured according to the procedure as described above.
Antifungal activities of TMC/SA multilayer coatings on PMMA-based materials
Candida were grown in YM broth, washed two times with sterile PBS using a centrifuge to pellet the cells, resuspended in PBS, and diluted to 108–109 CFU/mL. Afterwards, 2.5 μL of the Candida, suspended in 0.1% Triton X-100 in PBS, were placed onto the surface of a disc containing a TMC/SA multilayer coating. The disc was then “sandwiched” using another identical disc. Caution was used to ensure that all of C. albicans suspension was covered between the two discs during the experiments. After various periods of time, the entire “sandwich” was transferred to 10 mL sterile PBS. The mixture was sonicated for 5 min and then vortexed for 60 s. Our preliminary studies found that these treatments detached most of the Candida without affecting fungal growth. Antifungal activity was determined using the XTT assay.
Durability of the biofilm-controlling effects of the TMC/SA multilayer coatings
A series of g-PMMA discs coated with TMC/SA multilayers were immersed in PBS at 37°C under constant shaking for 4 weeks. Weekly, some of the discs were taken out, and immersed in a suspension of Candida in PBS, followed by incubation in YM broth, to reevaluate the biofilm-controlling effects of the coatings, using the same procedures as described above.
Biocompatibility of the TMC/SA multilayer coatings
Biocompatibility was evaluated using rat skin fibroblasts (ATCC CRL-1213), as a representative mammalian cell, with the MTT (3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay. A series of g-PMMA discs coated with TMC/SA multilayers were immersed in PBS at 37 °C under constant shaking for 1, 3, 5, and 7 days to prepare “disc conditioned media”. Rat skin fibroblasts were cultured in a media (DMEM with 10% FBS) at 37 °C in a humid atmosphere with 5% CO2 and 95% air. At confluence, cells were trypsinized, and 100 μL of the cell suspension (5 × 105 cells/mL) were added to the well of a 96-well plate. The fibroblast cultures were grown for 24h as above and then treated with media containing 50% fresh growth media and 50% (v/v) the “disc conditioned media” for an additional 24h. Afterwards, 10 μL of MTT reagent were added to each well and the mixture incubated in the dark for 4h. Subsequently, 100 μL DMSO (dimethyl sulfoxide) were added to each well, and the absorbance of the solution at 562 nm measured using an Infinite® 200 Pro multimode reader. Cells incubated with 50% of growth medium and 50% of pure PBS (without disc immersion) were tested under the same conditions to serve as controls.
Statistical analysis
Data shown in the figures (mean ± standard deviation) are representative of at least three independent experiments. Data were analyzed by student’s t test and p values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
This study was supported by NIDCR, NIH (R01 DE021084) and VA Merit Review (1I01BX001103). We would like to thank Dr. David Dean (Professor, Comprehensive Dentistry, UTHSCSA) for his careful review and editing of this manuscript.
Contributor Information
Dr. Fuguang Jiang, Department of Chemistry, University of Massachusetts, Lowell, MA 01854, USA
Prof. Chih-Ko Yeh, Email: yeh@uthscsa.edu, Department of Comprehensive Dentistry, University of Texas Health Science Center at San Antonio, and Geriatric Research, Education and Clinical Center, Audie L. Murphy Division, South Texas Veterans Health Care System, San Antonio, TX 78229, USA
Dr. Jianchuan Wen, Department of Chemistry, University of Massachusetts, Lowell, MA 01854, USA
Prof. Yuyu Sun, Email: yuyu_sun@uml.edu, Department of Chemistry, University of Massachusetts, Lowell, MA 01854, USA
References
- 1.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. J Bacteriol. 2001;183:5385. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blankenship JR, Mitchell AP. Curr Opin Microbiol. 2006;9:588. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Nett J, Andes D. Curr Opin Microbiol. 2006;9:340. doi: 10.1016/j.mib.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Nat Rev Microbiol. 2011;10:112. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramage G, Vandewalle K, Wickes B, Lopez-Ribot J. Antimicrob Agents Chemother. 2001;45:2475. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baillie GS, Douglas LJ. Antimicrob Agents Chemother. 1998;42:1900. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramage G, Williams C. Adv Appl Microbiol. 2013;84:27. doi: 10.1016/B978-0-12-407673-0.00002-3. [DOI] [PubMed] [Google Scholar]
- 8.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Crit Rev Microbiol. 2009;35:340. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 9.Iñigo M, Pemán J, Del Pozo JL. Int J Artif Organs. 2012;35:780. doi: 10.5301/ijao.5000170. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn DM, Ghannoum MA. Curr Opin Investig Drugs. 2004;5:186. [PubMed] [Google Scholar]
- 11.Mukherjee PK, Chandra J. Drug Resist Updat. 2004;7:301. doi: 10.1016/j.drup.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Cao Z, Yeh CK, Sun Y. Colloids Surf B Biointerfaces. 2013;110:96. doi: 10.1016/j.colsurfb.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar CC, Lin AL, Cao Z, Zhao XR, Wu LA, Chen S, Sun Y, Yeh CK. Oral Dis. 2013;19:287. doi: 10.1111/odi.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Z, Sun X, Yeh CK, Sun Y. J Dent Res. 2010;89:1517. doi: 10.1177/0022034510379604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mourya VK, Inamdar NN. J Mater Sci Mater Med. 2009;20:1057. doi: 10.1007/s10856-008-3659-z. [DOI] [PubMed] [Google Scholar]
- 16.Tan H, Ma R, Lin C, Liu Z, Tang T. Int J Mol Sci. 2013;14:1854. doi: 10.3390/ijms14011854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belalia R, Grelier S, Benaissa M, Coma V. J Agric Food Chem. 2008;56:1582. doi: 10.1021/jf071717+. [DOI] [PubMed] [Google Scholar]
- 18.Augst AD, Kong HJ, Mooney DJ. Macromol Biosci. 2006;6:623. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 19.Eiselt P, Yeh J, Latvala RK, Shea LD, Mooney DJ. Biomaterials. 2000;21:1921. doi: 10.1016/s0142-9612(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Shelton RM, Cooper PR, Lawson M, Triffitt JT, Barralet JE. Biomaterials. 2003;24:3475. doi: 10.1016/s0142-9612(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 21.Hariyadi DM, Lin SC, Wang Y, Bostrom T, Turner MS, Bhandari B, Coombes AG. J Drug Target. 2010;18:831. doi: 10.3109/1061186X.2010.525651. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad Z, Khuller GK. Expert Opin Drug Deliv. 2008;5:1323. doi: 10.1517/17425240802600662. [DOI] [PubMed] [Google Scholar]
- 23.Decher G. Science. 1997;277:1232. [Google Scholar]
- 24.Guyomard A, Dé E, Jouenne T, Malandain J-J, Muller G, Glinel K. Adv Funct Mater. 2008;18:758. [Google Scholar]
- 25.Lawrence NJ, Wells-Kingsbury JM, Ihrig MM, Fangman TE, Namavar F, Cheung CL. Langmuir. 2012;28:4301. doi: 10.1021/la2033725. [DOI] [PubMed] [Google Scholar]
- 26.Stauder M, Papetti A, Mascherpa D, Schito AM, Gazzani G, Pruzzo C, Daglia M. J Agric Food Chem. 2010;58:11662. doi: 10.1021/jf1031839. [DOI] [PubMed] [Google Scholar]
- 27.Follmann HD, Martins AF, Gerola AP, Burgo TA, Nakamura CV, Rubira AF, Muniz EC. Biomacromolecule. 2012;13:3711. doi: 10.1021/bm3011962. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Lee D, Sheng X, Cohen RE, Rubner MF. Langmuir. 2006;22:9820. doi: 10.1021/la0622166. [DOI] [PubMed] [Google Scholar]
- 29.Lichter JA, Rubner MF. Langmuir. 2009;25:7686. doi: 10.1021/la900349c. [DOI] [PubMed] [Google Scholar]
- 30.Tang Z, Wang Y, Podsiadlo P, Kotov NA. Adv Mater. 2006;18:3203. [Google Scholar]
- 31.Sieval AB, Thanou M, Kotzé AF, Verhoef JC, Brussee J, Junginger HE. Carbohydr Polym. 1998;36:157. [Google Scholar]
- 32.Frazer RQ, Byron RT, Osborne PB, West KP. J Long Term Eff Med Implants. 2005;15:629. doi: 10.1615/jlongtermeffmedimplants.v15.i6.60. [DOI] [PubMed] [Google Scholar]
- 33.Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. J Dent Res. 1995;74:1602. doi: 10.1177/00220345950740091601. [DOI] [PubMed] [Google Scholar]
- 34.Vale FM, Castro M, Monteiro J, Couto FS, Pinto R, Gião Toscano Rico JM. Biomaterials. 1997;18:1133. doi: 10.1016/s0142-9612(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland IW. Microbiol. 2001;147:3. doi: 10.1099/00221287-147-1-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.