Abstract
Intestinal adaptation is the process that attempts to restore total gut absorption after intestinal resection. In humans, the ileum as well as the colon can undergo adaptation without the jejunum. However, there is little evidence for the jejunum to undergo adaptation in the absence of the ileum. Here, we report the unusual case of a pre-pubertal boy who underwent total ileal resection, right hemicolectomy and jejunostomy after a motor vehicle accident. Despite ileal resection, he demonstrated evidence of successful structural and functional jejunal adaptation.
Keywords: short bowel syndrome, jejunal adaptation, diarrhea
PRESENTATION OF CASE
Dr Sam Cheng and Dr Grace Gathungu: Our patient was an 8 year-old Asian-American boy with autism who was referred to our clinic in 2005 for management of high jejunostomy output associated with short bowel. Past medical history was significant for a severe motor vehicle accident 5 months prior. He suffered extensive mesenteric injury to the distal small bowel and right colon, requiring right hemicolectomy with removal of approximately 50% proximal colon and complete ileal resection. Intra-operatively, he was noted to have 140 cm of jejunum remaining. An end jejunostomy was created along with Hartmann’s pouch. Post-operatively, he received total parenteral nutrition for 2 months before transitioning to enteral nutrition (i.e. Peptamen Jr) and then to complete oral feeds. There was no family history of celiac disease, cystic fibrosis or inflammatory bowel disease.
On physical exam at his first clinical visit to our clinic 5 months after the accident, he was thin but well-appearing. He was afebrile with normal vital signs. Weight was 24.5 kg and height 130 cm, both at the 25th percentile for age. The abdomen was benign. The mucosa at stomal opening was pink with greenish liquid effluent.
Complete blood count, liver function tests, ESR, tissue transglutaminase antibody, and electrolytes including Ca2+, phosphate and Mg2+ were normal. Prealbumin, albumin, vitamins ADEK, and B12 were also within normal limits.
Initially, stomal output averaged 1.8–2.0 L/day, being more with simple sugary foods or drinks and relatively low in volume and pasty with complex carbohydrates and fatty foods, between meals or during sleep. It was non-foulsmelling, non-mucousy, and non-bloody; it was also not responsive to empiric treatment with antibiotics. A small bowel series revealed normal-appearing jejunum without evidence of intestinal stricture. Trials of proton pump inhibitor therapy to reduce gastric acid secretion and antidiarrheal medications such as loperamide were given but output was not significantly affected.
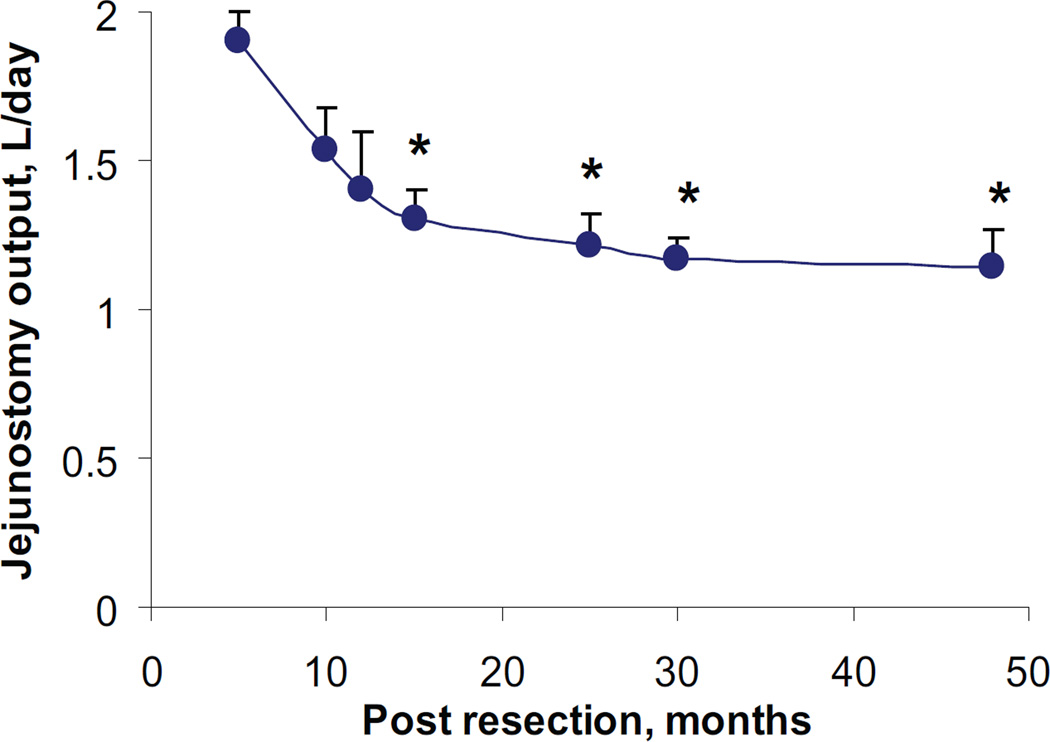
Over the next 4 years, the parents were instructed to periodically record the volume of jejunal output (Figure 1). Daily outputs, averaged over 2–5 days, were expressed as mean ± SEM L/day. On the first recording, 5 months postintestinal resection, jejunostomy output was 1.9 ± 0.1. However, 10 months later, output dropped by 32% to 1.3 ± 0.1 (P=0.05). Over the next 3 years, there was only an 8% reduction to 1.1± 0.1 (p<0.05). The overall reduction in output over the 4 year period was 40%. Moreover, stomal consistency also gradually improved from watery to pasty despite relaxing his dietary restriction on simple sugary foods and drinks.
Figure 1.
Changes in jejunostomy output as a function of time. * p<0.05 with respect to the first reading.
His appetite increased post-operatively and doubled 5 months postresection. His typical daily dietary intake includes 4 to 5 glasses (6 ounces each) of water and 1–2 glasses of lactose-free milk in addition to a standard Indo-Pakistani diet that consists of meats and a small amount of vegetables. Since juices and fruits were found to increase his jejunostomy output, these simple sugary foods and drinks were restricted within the 1st year post-resection. Such dietary restrictions were gradually liberated 12 months post-operatively. Currently, he is consuming normal amounts of simple sugary foods and drinks without any restrictions.
Ultimately, 4 years post-resection a decision was made to perform jejunocolic anastomosis, despite the possible risk of developing diarrhea and fecal incontinence in a developmentally delayed child. 2 months post-anastomosis, he had 5–6 soft stools a day without nocturnal urgency or fecal incontinence. At a 10 month follow-up, this decreased to 2–3 daily bowel movements.
Initially, he received monthly vitamin B12 injection for B12 malabsorption, which continued until 6 months post-jejuno-colic anastomosis. His serum B12 levels had remained normal until 6 months after discontinued B12 supplementation when his serum level started to drop below the normal limit and he has been required to take intranasal B12 intermittently to maintain normal serum B12 levels. Over this 4 year period, he was growing well with weight and height above the 25th percentile for age. Complete blood count and electrolytes including Ca2+, phosphate and Mg2+ were normal. Pre-albumin, albumin, and vitamins ADEK were also within normal limits.
HISTOLOGY
Dr Dhanpat Jain: The histology of the small bowel from his initial resection following motor vehicle accident was compared with that of small bowel removed during the enterostomy take-down 4 years later. These were also compared with “neo-ileal biopsies” obtained 4 months after the jeuno-colonic anastomosis was performed.
To avoid non-specific histological changes that sometimes occur in the region of mucosa in proximity to ostomy sites, the villous architecture was carefully evaluated in areas away from ostomy site. Also, many artifacts can alter the crypt:villus ratio. These include stretching of the intestinal wall and poor orientation of the mucosa. To avoid these artifacts, multiple sections were examined and the crypt:villus ratio assessed in multiple areas.
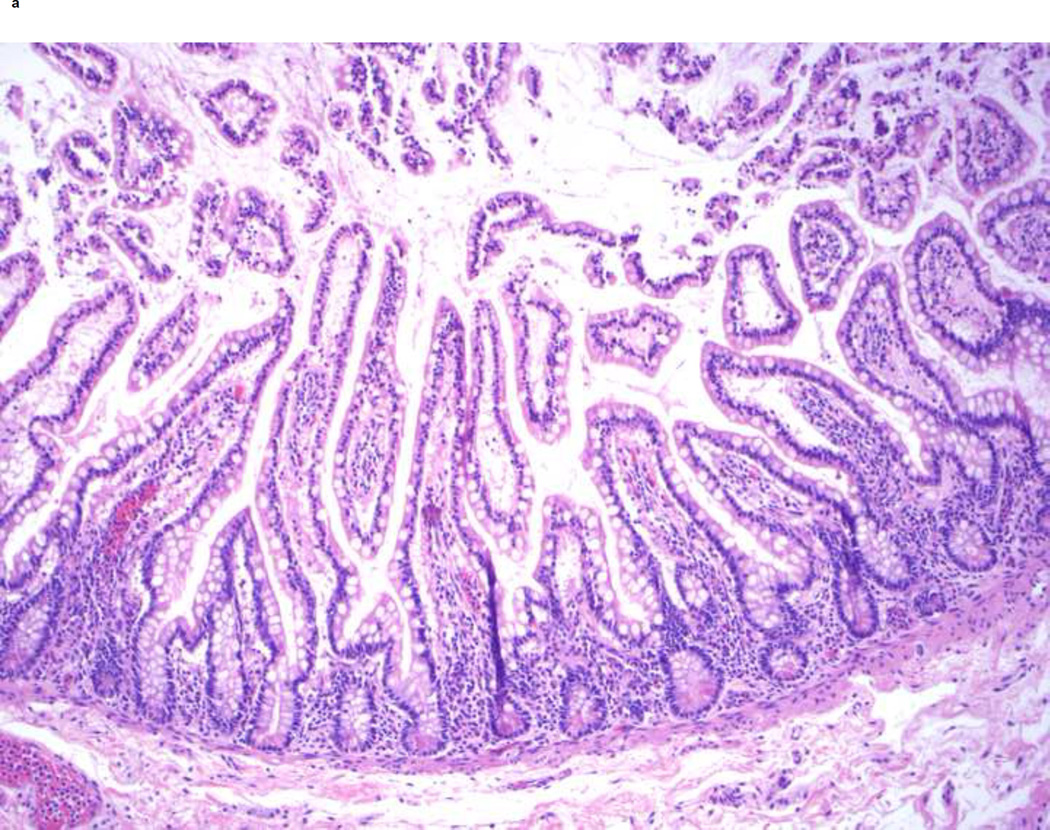
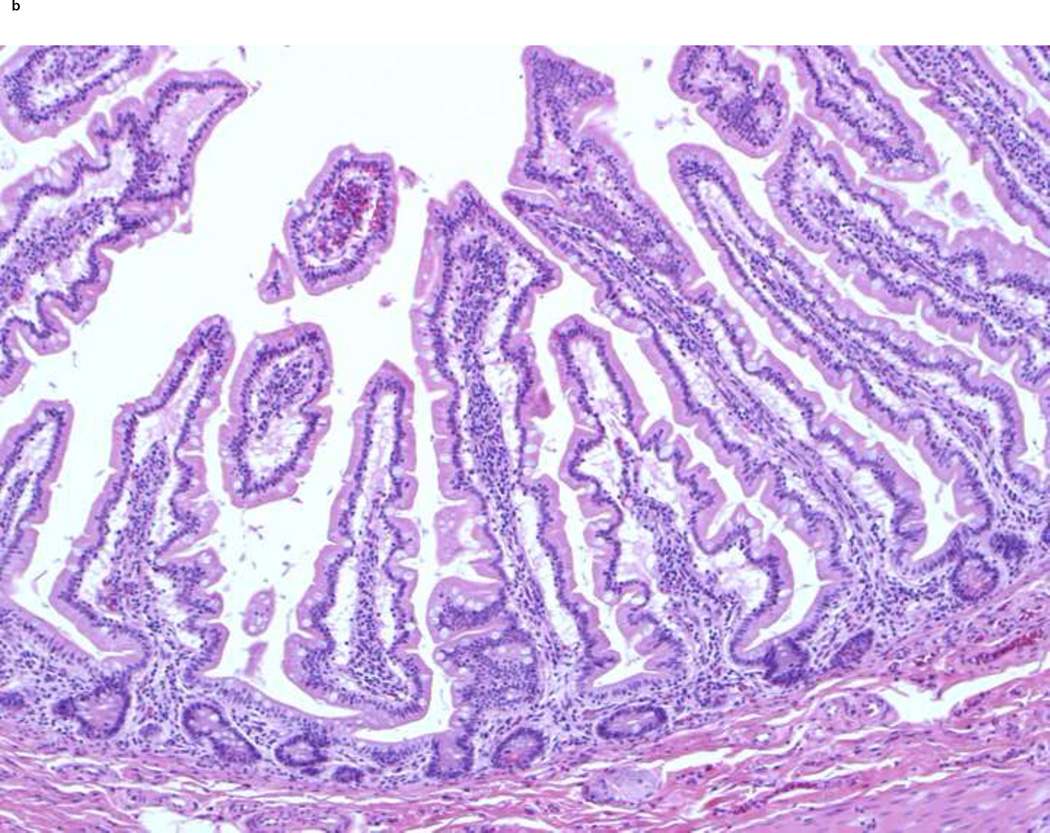
The sections from the proximal small bowel margins taken at this time represent jejunum and show tall, somewhat leaf-shaped villi with villous to crypt ratio of 5:1 (Figure 2a). Compared to this, the sections obtained from the jejunostomy take-down reveal much taller and wider villi with a villous to crypt ratio of 7–10:1 (Figure 2b). These changes in histology are striking and the findings shown are representative of these changes. The scalloping of the villous edges also increased, while the number of goblet cells appeared similar to the prior resection. These two specimens could not be compared side-by-side for any changes in the pattern of mucosal folds.
Figure 2.
Changes in jejunal histology after ilectomy and before jejuno-colic anastomosis. A shows normal structure of the jejunum from the proximal resection margin in 2005 at time of ileo-colectomy. Note the normal 5:1 ratio of villi to crypt length, leaf-like shape and scattered goblet cells in the lining epithelium. B shows hypertrophy of distal jejunal villi in 2009 before jejuno-colic anastomosis. Note that the villi are longer (with the villi to crypt ratio of 7–10:1) and wider with increased scalloping of the villous edges. They retain the leaf-like shape and the proportion of goblet cells seems unchanged. The histological findings shown are representative of these changes in multiple sections from different areas.
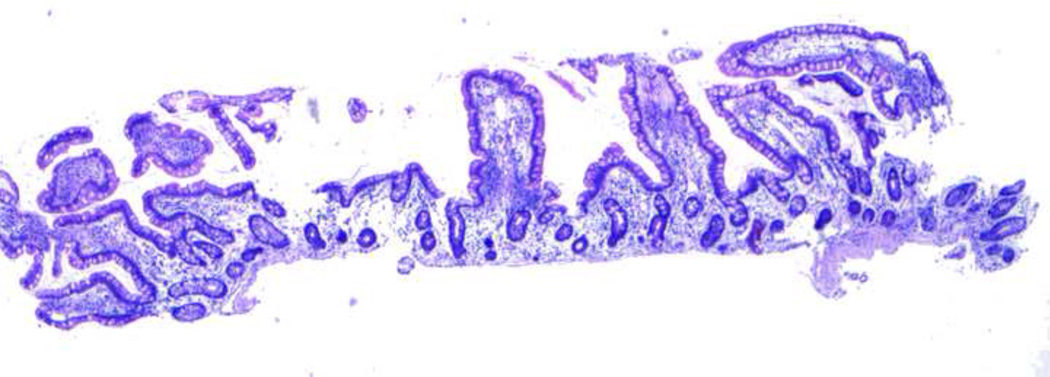
Interestingly, biopsies obtained 4 months post-jejuno-colic anastomosis showed villous structure that was typical of ileum with shorter finger-shaped villi, loss of scalloping of edges and a greater number of goblet cells (Figure 3).
Figure 3.
Changes in jejunal histology 4 months after jejuno-colic anastomosis. Note that the samples were taken from distal jejunum approximately 3 inches proximal to the anastomosis site. The histology of the “neo-ileum” is noted 4 months post-jejuno-colic anastomosis. Also noted are the decreased villous height (villi to crypt ratio of 3:1), finger-like shape, loss of scalloping of villous edges, and increased number of goblet cells – histological changes that are indistinguishable from normal ileum.
DISCUSSION
Dr Sam Cheng and Dr Sohail Husain: To our knowledge, this is the first case demonstrating that the jejunum is capable of undergoing structural and functional adaptive processes in the absence of ileum in a prepubertal child. Moreover, we illustrate that jejunal output in children can be physiologically as high as in adults. Finally, we show that, although the right colon reabsorbs the majority of small intestinal fluid, the left colon can compensate as well.
Audience: What is the normal physiology of intestinal fluid secretion and absorption?
Dr Sam Cheng: In adults the average fluid load presented to the small intestine in a 24 hour period is 9 L, which includes 2 L from dietary intake and 7 L from secretions (ie, salivary 1.5 L, gastric 2.5 L, biliary 0.5 L, pancreatic 1.5 L and small intestinal itself 1.0 L). Due to its long villi and large absorptive surface area, the jejunum normally reabsorbs the majority (ie, 5.5 L) of the fluid load with only 3.5 L remaining to be absorbed in the ileum and colon 1. After correction for differences in surface area in this child and a typical adult (0.95 m2; v.s. 1.73 m2), we can conclude that his 1.9 L jejunal output is physiologically normal. Indeed, no intestinal pathology accounting for malabsorption was identified. The function and structural differences of the three segments of the small intestine (duodenum, jejunum, and ileum) are shown in table 1.
Table 1.
Structural and functional differences between the duodenum, jejunum and ileum.
| Duodenum | Jejunum | Ileum | |
|---|---|---|---|
| Structure | |||
| Villous height | +++ | +++ | + |
| Shape | Leaf-shaped | Leaf-shaped | Finger-like |
| Goblet cells | + | + | +++ |
| Absorptive function | |||
| Macronutrients | +++ | ++ | + |
| Ca2+ | ++ | + | + |
| Fe2+ & Folate | + | − | − |
| Cobalamin | − | − | + |
| Bile acids | +/− | + | +++ |
| Water | ++ | ++ | + |
Audience: What is the capacity of the jejunum to adapt after ileal resection?
Dr Sam Cheng: Intestinal adaptation is a process that attempts to restore the total gut absorption of macronutrients, minerals, and water to that prior to intestinal resection. Adaptation is manifested by 1) structural adaptation, which increases the absorptive area of the remaining bowel, and 2) functional adaptation, which slows gastrointestinal transit and increases absorptive efficiency. Normally, the jejunum is the primary digestive and absorptive site for most nutrients 2, whereas the ileum, with shorter villi and lesser surface area, serves more as a reserve in situations in which the jejunum becomes unavailable or is resected. In the latter case, the ileum will adapt to assume the most absorptive functions.
In contrast, it seems that the human jejunum can hardly undergo adaptation without the ileum, particularly when an end jejunostomy is placed 3. No definite structural intestinal adaptation has been demonstrated so far 4, 5, although functional adaptation with slowing of gastric emptying and small bowel transit may occur in those patients with no jejunostomy and with jejunum in continuity with the colon 6. However, there is no evidence for any structural 7 or functional 8, 9 adaptation at any time in patients with a jejunostomy.
Dr Sohail Husain: There are several reported hypotheses to explain the limited capacity of the jejunum to adapt. Firstly, in contrast to the ileum, the jejunum has long villi, large absorptive surface area, highly concentrated digestive enzymes, and abundantly expressed transport carrier proteins. Thus, under normal physiological condition the digestive and absorptive capacity of the jejunum is generally thought to have reached a maximal capacity. Secondly, intestinal adaptation requires trophic gut hormones, most of which are produced in the ileum in response to luminal nutrients 10–12. Thus, when the ileum is resected, the ileum-dependent gut trophic factors and adaptation mechanism are compromised 13. Finally, whether the jejunum is capable of undergoing adaptation or not is also determined by the presence or absence of an ostomy and/or a retained colon. Normally, gastric emptying and small bowel transit for liquid is “braked” by the nutrients delivered to the ileum and colon where the sensors are normally located 14; this braking mechanism would allow the jejunum to undergo adaptation. The effect is likely mediated by the peptide YY 15 and glucagons-like peptide 2 (GLP-2) 16. Thus, jejunostomy placement bypasses the braking mechanism, and consequently reduces jejunal adaptation.
Remarkably though, our case has demonstrated that the jejunum is capable of undergoing adaptation, both structurally and functionally, to reduce net ostomy output and to maintain overall body fluid, electrolyte, and nutrient balance. The changes in the villous height, width and scalloping increased the surface area thus increasing the absorptive capacity of the remaining small intestine. It is unclear to us if the length of the small bowel also increased in this process, or if the mucosal fold pattern changed. The reason why the jejunum remnant in this child was able to adapt is unknown. The trophic gut hormones are essential for intestinal adaptation. Animal studies have shown that these adaptive gut hormones (e.g. GLP-2) can in fact be produced not only from the ileum but also from the rest of the bowel including the duodenum, jejunum and colon 10, thus providing a potential for the jejunum to adapt when necessary. Another factor would be age of the patient and the cause of resection. While intestinal development is complete by 7 years of age, growth, in particular in a child with non-diseased healthy growing intestine prior to loss of the ileum might be contributing as well 17, 18. Given this, the findings described in this case may not be applicable to adults or infants, in particular those with prior gastrointestinal diseases.
Dr Dhanpat Jain: The other interesting adaptation occurred once the continuity between small bowel and colon was restored. This time the jejunaltype villi changed to ileal-type villi with decrease in villous height, change in shape to finger-like villi, and increase in the number of goblet cells. Since neither clinical nor histological features of enteritis/colitis were evidenced, these changes are unlikely secondary to inflammatory processes. We suspect gut flora due to restoration of the luminal continuity may have played a role. It is unclear if specialized functions of the ileum (e.g. vitamin B12 and bile acid absorption) could be taken over by the jejunum. It is noted that the serum B12 levels had been normal in this patient for 6 months after discontinued B12 injections, but this most likely reflects vitamin B12 stores rather than an adaptive response as evidenced by a recent drop in his B12 serum level.
Audience: What is the capacity of the colon to adapt?
Dr Dinesh Pashankar: The primary function of the colon is to absorb sodium and water and energy from short chain fatty acids. In adults, approximately 1.3 L intestinal fluid is delivered to the colon from the ileum every day, and 1.1 L is reabsorbed 1. Normally, about 80–90% is absorbed in the proximal or right colon and only 10–20% in the distal or left colon. Thus, when the proximal colon is resected or becomes unavailable, diarrhea ensues, as occurred in this case. However, as shown in this case and others the absorptive function of proximal colon can be taken over by its distal counterpart over time and consequently, diarrhea improves.
Dr Sam Cheng: In summary, we report a child with total ileal resection, right hemi-colectomy, and jejunostomy placement. By following changes in daily stomal outputs and comparing difference in intestinal histology before and after resection, our case illustrates that 1) jejunal output even in a pre-pubertal child is comparable to a adult; 2) the jejunum can undergo adaptation, both structurally and functionally, in response to loss of the ileum; and 3) the left colon can reabsorb the majority of small intestinal fluid in the absence of right colon. It is worth noting that we did not study radiological evidence of structural adaptation nor the specialized functions (e.g. vitamin B12 and bile acid absorption) of the ileum. These are pitfalls of this case report. Although the underlying mechanisms for such an adaptation are unclear, understanding these factors could be important in developing novel therapies to promote intestinal adaptation. To our knowledge, this is the first case in a prepubertal child demonstrating that the jejunum is capable of undergoing both structural and functional adaptive processes in the absence of ileum.
Acknowledgments
The authors confirm that there is no funding source.
Footnotes
There is no conflict of interest.
References
- 1.Moore EW. Physiology of intestinal water and electrolyte absorption. AGA. 1976 [Google Scholar]
- 2.Dowling RH, Booth CC. Functional compensation after small-bowel resection in man. Demonstration by direct measurement. Lancet. 1966;2:146–147. doi: 10.1016/s0140-6736(66)92426-3. [DOI] [PubMed] [Google Scholar]
- 3.Nightingale J, Woodward JM. Guidelines for management of patients with a short bowel. Gut. 2006;55(Suppl 4):iv1–iv12. doi: 10.1136/gut.2006.091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Francesco A, Malfi G, Delsedime L, et al. Histological findings regarding jejunal mucosa in short bowel syndrome. Transplant Proc. 1994;26:1455–1456. [PubMed] [Google Scholar]
- 5.Porus RL. Epithelial hyperplasia following massive small bowel resection in man. Gastroenterology. 1965;48:753–759. [PubMed] [Google Scholar]
- 6.Nightingale JM, Kamm MA, van der Sijp JR, et al. Disturbed gastric emptying in the short bowel syndrome. Evidence for a 'colonic brake'. Gut. 1993;34:1171–1176. doi: 10.1136/gut.34.9.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Keefe SJ, Haymond MW, Bennet WM, et al. Long-acting somatostatin analogue therapy and protein metabolism in patients with jejunostomies. Gastroenterology. 1994;107:379–388. doi: 10.1016/0016-5085(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 8.Hill GL, Mair WS, Goligher JC. Impairment of 'ileostomy adaptation' in patients after ileal resection. Gut. 1974;15:982–987. doi: 10.1136/gut.15.12.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nightingale JM, Lennard-Jones JE, Gertner DJ, et al. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33:1493–1497. doi: 10.1136/gut.33.11.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson LI, Holst J, Hkanson R, et al. Distribution and properties of glucagon immunoreactivity in the digestive tract of various mammals: an immunohistochemical and immunochemical study. Histochemistry. 1975;44:281–290. doi: 10.1007/BF00490364. [DOI] [PubMed] [Google Scholar]
- 11.Liu CD, Rongione AJ, Shin MS, et al. Epidermal growth factor improves intestinal adaptation during somatostatin administration in vivo. J Surg Res. 1996;63:163–168. doi: 10.1006/jsre.1996.0241. [DOI] [PubMed] [Google Scholar]
- 12.Vanderhoof JA, McCusker RH, Clark R, et al. Truncated and native insulinlike growth factor I enhance mucosal adaptation after jejunoileal resection. Gastroenterology. 1992;102:1949–1956. doi: 10.1016/0016-5085(92)90318-s. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen PB, Hartmann B, Hansen BS, et al. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999;45:559–563. doi: 10.1136/gut.45.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferri GL, Koopmans HS, Ghatei MA, et al. Ileal enteroglucagon cells after ileal-duodenal transposition in the rat. Digestion. 1983;26:10–16. doi: 10.1159/000198863. [DOI] [PubMed] [Google Scholar]
- 15.Nightingale JM, Kamm MA, van der Sijp JR, et al. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the 'colonic brake' to gastric emptying. Gut. 1996;39:267–272. doi: 10.1136/gut.39.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wjdemann M, Wettergren A, Hartmann B, et al. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998;33:828–832. doi: 10.1080/00365529850171486. [DOI] [PubMed] [Google Scholar]
- 17.Finkel Y, Goulet O. Short bowel syndrome. In: Kleinman R, Goulet O, Mieli-Vergani G, Sanderson I, Sherman P, Shneider B, editors. Walker' Pediatric Gastrointestinal Disease. 5th edn. Shelton, CT: People's Medical Publishing House; 2008. pp. 601–612. [Google Scholar]
- 18.Poston GJ, Saydjari R, Lawrence J, et al. The effect of age on small bowel adaptation and growth after proximal enterectomy. J Gerontol. 1990;45:B220–B225. doi: 10.1093/geronj/45.6.b220. [DOI] [PubMed] [Google Scholar]