Abstract
Background
Scarring represents a significant biomedical burden in clinical medicine. Mechanomodulation has been linked to scarring through inflammation, but until now a systematic approach to attenuate mechanical force and reduce scarring has not been possible.
Methods
The authors conducted a 12-month, prospective, open-label, randomized, multicenter clinical trial to evaluate abdominoplasty scar appearance following postoperative treatment with the embrace Advanced Scar Therapy device to reduce mechanical forces on healing surgical incisions. Incisions from 65 healthy adult subjects were randomized to receive embrace treatment on one half of an abdominoplasty incision and control treatment (surgeon's optimal care methods) on the other half. The primary endpoint for this study was the difference between assessments of scar appearance for the treated and control sides using the visual analogue scale scar score.
Results
Final 12-month study photographs were obtained from 36 subjects who completed at least 5 weeks of dressing application. The mean visual analogue scale score for embrace-treated scars (2.90) was significantly improved compared with control-treated scars (3.29) at 12 months (difference, 0.39; 95 percent confidence interval, 0.14 to 0.66; p = 0.027). Both subjects and investigators found that embrace-treated scars demonstrated significant improvements in overall appearance at 12 months using the Patient and Observer Scar Assessment Scale evaluation (p = 0.02 and p < 0.001, respectively). No serious adverse events were reported.
Conclusions
These results demonstrate that the embrace device significantly reduces scarring following abdominoplasty surgery. To the authors’ knowledge, this represents the first level I evidence for postoperative scar reduction.
Scarring and fibrosis following tissue injury represent an enormous medical burden.1,2 As humans have evolved, our response to injury has put a premium on the rapid restoration of tissue integrity by means of scar or fibrosis at the expense of form, function, and appearance. Virtually all tissue in the body, when injured, will repair with a scar.3 Although the scar does not appear or function like normal uninjured tissue, it is evolutionarily preferable to a chronic or nonhealing wound. Examples of fibrosis/scarring in medicine include chronic conditions such as pulmonary fibrosis, hepatic cirrhosis, and stromal reaction around a tumor, in addition to scarring after an acute injury such as a traumatic laceration, elective surgical procedure, or a myocardial infarction.
A frequent example of scarring or fibrosis occurs after cutaneous injury. It is estimated that there are approximately 80 million operations per year in the United States and upward of 250 million worldwide.4–6 In addition to surgical incisions, there are also more than 12 million traumatic skin lacerations treated in emergency departments annually in the United States alone.7 Whether the origin of the injury occurs in the operating room or is the result of trauma, once the wound is closed, the body goes through a predictable series of wound healing phases, ultimately resulting in a mature scar.3 Although much is known about the reepithelialization, extracellular matrix deposition, and remodeling phases of wound healing, there have still been very few advances in scar modulation.
Many products on the market are used for post-surgical improvement of scarring, but evidence supporting the efficacy of these products is limited. Products currently used to improve scar appearance include silicone gels, sheets and tapes,8–10 and topical creams containing agents such as retinoic acid and onion extract.11 Although there have been multiple randomized controlled trials evaluating the efficacy of silicone gels,12–14 the overall quality of evidence is limited.15 In terms of level I evidence, there have been few studies documenting a signifi-cant reduction in scarring. The most recent example, Juvista (Renovo Group Plc, Bristol, United Kingdom), did not meet the study endpoint in a phase 3 trial using an injectable biologic approach to minimize cutaneous scarring after an incision.16
This article reports on a randomized controlled trial using a simple device that is applied postoperatively to minimize scar formation. We sought to evaluate whether the embrace Advanced Scar Therapy device (Neodyne Biosciences, Inc., Menlo Park, Calif.) improved scar appearance following postoperative treatment. The device offloads tension, which is known to be a causative factor for the development of wide and hypertrophic scars in humans.17 The clinical indication for this randomized controlled trial was abdominoplasty, which requires a long incision such that one half of the scar may be used for the active treatment and one half for a control treatment, allowing each subject to serve as their own control. This is a challenging site prone to thick and cosmetically poor scars. In spite of the challenging nature of this indication, at 1 year follow-up, the results demonstrated a statistically significant improvement of the scar on the embrace-treated side compared with the control-treated side.
SUBJECTS AND METHODS
The Scar Prevention and the Clinical Effectiveness of a Novel Mechanomodulating Polymer (REFINE) trial was a prospective, open-label, randomized (with subjects as their own control), multicenter study to evaluate the use of the Neo-dyne embrace device to improve the aesthetic outcome of scars following abdominoplasty surgery. Procedures were performed by 12 surgeons at 12 surgery centers between June of 2011 and May of 2012. No important changes were made to the methods after trial commencement. This trial was approved by the University of Texas Southwestern Medical Center Institutional Review Board (Dallas, Texas), the Brooke Army Medical Center Institutional Review Board (Fort Sam Houston, Texas), and the Schulman Associates Institutional Review Board, Inc. (Cincinnati, Ohio). The study sponsor, Neodyne Biosciences, Inc., which provided the embrace devices, received funding for this study from the Armed Forces Institute of Regenerative Medicine (no. W81XWH-08-2-0032, Department of Defense).
Subjects
Male and female subjects aged 18 to 65 years who had undergone a de novo abdominoplasty within 1 week (4 to 8 days) of treatment application were eligible if their incision was aesthetically similar throughout its full length. Exclusion criteria included subjects with a history of collagen vascular disease, cutis laxa, connective tissue disease, psoriasis, or lupus; subjects diagnosed with scleroderma; subjects with known adverse reactions to Steri-Strip tapes (3M, St. Paul, Minn.), medical tapes, or adhesives; subjects with oozing, dehiscence, nonclosed/healed incisions at the time of first application; subjects unable to maintain adequate care of incision; subjects with a body mass index greater than 30 or weight loss of greater than 100 lb within 6 months from the date of abdominoplasty; subjects that currently smoke; subjects taking steroid therapy within 2 months from the date of study enrollment; and subjects that did not qualify in the opinion of the investigators. Basic demographic and clinical variables were collected for each subject and did not affect enrollment except as described above. Seventy-three subjects were initially assessed for eligibility, with four excluded because of a body mass index in excess of 30 and two excluded after canceling surgery.
Device
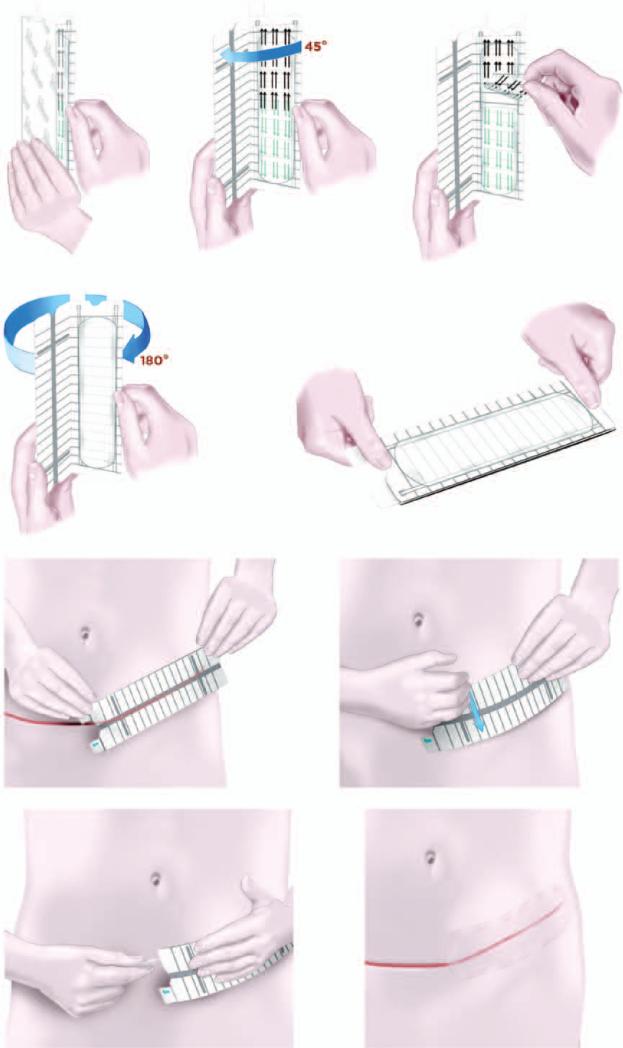
The embrace device consists of an applicator that holds and, on activation, strains a 16 × 5-cm or 6 × 4-cm silicone elastomeric dressing. On application, the dressing adheres to the skin by means of a pressure-sensitive silicone adhesive (Fig. 1). This device is U.S. Food and Drug Administration cleared for commercial use.
Fig. 1.
Schematic depiction of the embrace device. (Above, left) The embrace device used in the clinical trial is a 16x5-cm silicone elastomeric dressing that adheres to the skin using a pressure-sensitive silicone adhesive. (Above, center) The user initially opens the applicator approximately 45 degrees and (above, right) the protective liner is peeled back from the adhesive dressing. (Second row, left) The applicator is then fully opened 180 degrees to strain the dressing. (Second row, right) Once fully opened, the device is ready for application. (Third row, left) The dressing is applied directly over the center of the closed incision, and (third row, right) the user firmly presses or rubs the applicator to activate the adhesive. (Below, left) Tabs at the end of the applicator are pulled away to release the dressing and the applicator is discarded, (below, right) after which the dressing remains on for approximately 7 days.
Treatment
After meeting all eligibility criteria, 67 subjects were enrolled. Each subject underwent routine abdominoplasty surgery under general anesthesia, and wounds were closed using suture techniques according to the surgeon's standard of care. (See Table, Supplemental Digital Content 1, which shows incision closure technique, http://links.lww.com/PRS/B68.) Following surgery, one half of the newly closed wound was randomized to be treated with the embrace device and one half was randomized to treatment according to the physician's optimal postsurgical method. (See Table, Supplemental Digital Content 2, which shows standard of care for control incisions, http://links.lww.com/PRS/B69.) Randomization was accomplished by opening a sealed envelope containing instructions to assign embrace treatment to the left or right portion of the incision. The randomization list and envelopes were generated randomly by the study statistician before enrollment. The selected side was treated with the embrace device throughout the duration of the study. The embrace device was initially applied to approximately half of each incision by the health care provider at 1 week (4 to 8 days) after surgery. Subjects returned to the investigator's office weekly for removal of the embrace device and reapplication of a new device for up to 13 weeks or visits, with additional visits at 6 and 12 months from the date of the procedure for photographic evaluation and study exit.
Assessment and Outcomes
Performance was evaluated by comparing Patient and Observer Scar Assessment Scale and visual analogue scale scar scores as described below for the embrace-treated and control-treated incision sites at the 6- and 12-month study endpoints. Additional Patient and Observer Scar Assessment Scale data were recorded from subjects and investigators at 7, 9, 11, and 13 weeks. No changes to trial outcomes were made after the trial commenced.
The Patient and Observer Scar Assessment Scale system was defined and validated by van de Kar et al. in 200518 and Truong et al. in 2007.19 The evaluation consists of a 10-point numerical rating scale, ranging from a score of 1 for normal skin to a score of 10 for the worst scar imaginable, as described previously.18 The visual analogue scale scar scoring system was developed by Duncan et al. in 2006.20 This evaluation consists of a 10-cm line representing scar quality, with 0 representing normal skin and 10 indicating a poor scar. The embrace-treated portion and control-treated portion were evaluated independent of each other by three blinded board-certified plastic surgeons who are not otherwise affiliated with this study or the study sponsor.
Statistical Analysis
Results from our earlier study indicate that the standard deviation of the paired differences in visual analogue scale scores would not be expected to exceed 1.46. Should the embrace dressing, on average, show an improvement of at least 0.58 point over the control treatment, 52 subjects would provide 80 percent power to test the study hypothesis at the two-sided 0.05 alpha level.
The visual analogue scale results are expressed as mean values for the treatment and control incisions, and the mean difference is presented along with 95 percent confidence intervals. Statistical analysis of visual analogue scale scores was carried out using a paired t test on the average reviewer score for each scar section; normality was established using the Shapiro-Wilk test (p = 0.28). Patient and Observer Scar Assessment Scale results were evaluated using a Wilcoxon signed rank test for both observer and subject assessments, and are represented graphically as mean values ± standard error of the mean. No adjustments were made for multiple hypothesis testing. A value of p < 0.05 was considered statistically significant for all comparisons.
RESULTS
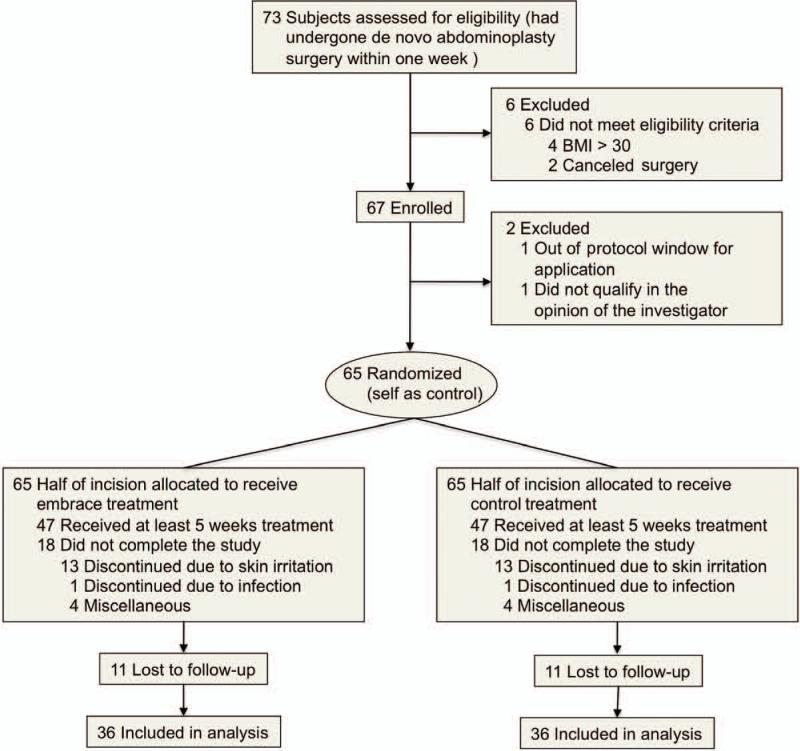
Between June of 2011 and May of 2012, a total of 67 subjects were initially enrolled in this study, with 36 subjects completing at least 5 weeks of dressing application and providing the final 12-month study photographs (Fig. 2). Procedures were performed by 12 plastic surgeons at 12 surgical centers in the United States. (See Table, Supplemental Digital Content 3, which shows clinical site distribution, http://links.lww.com/PRS/B70.) Subject ages ranged from 24 to 65 years, with an average age of 42.6 years, and 35 of the 36 participants providing the 12-month photograph results were female patients. Based on self-reported race and ethnicity data (using options defined by the investigator), 55.6 percent of subjects were white non-Hispanic, 19.4 percent were white Hispanic, 11.1 percent were black, 5.6 percent were Asian, and 8.3 percent were other (Table 1).
Fig. 2.
Flow diagram of clinical trial. Seventy-three subjects were initially assessed for eligibility, with four excluded because of a body mass index (BMI) in excess of 30 and two excluded after canceling surgery. In addition, two were excluded after enrollment but before treatment (one because of a body mass index out of range and the other as a result of missing the treatment window). A total of 65 subjects underwent randomization (self as control), with half of each incision allocated to receive either embrace or control treatment. Eighteen subjects did not complete the study, with 13 discontinuing because of skin irritation, one because of a wound-site infection, and four for miscellaneous reasons. An additional 11 subjects completed treatment but were subsequently lost to follow-up, resulting in 36 subjects included in the final analysis.
Table 1.
Subject Demographics
| Characteristic | Value (%) |
|---|---|
| Male-to-female ratio | 1:35 |
| Age, yr | |
| Mean ± SD | 42.6 ± 9.9 |
| Range | 24-65 |
| BMI | |
| Mean ± SD | 23.3 ± 2.8 |
| Range | 18.8-30.4 |
| Race | |
| White non-Hispanic | 20 (55.6) |
| White Hispanic | 7 (19.4) |
| Black | 4 (11.1) |
| Asian | 2 (5.6) |
| Other | 3 (8.3) |
| Fitzpatrick skin type score | |
| Mean ± SD | 20.2 ± 6.4 |
| Range | 8-31 |
| Type I | 0 (0) |
| Type II | 9 (25.0) |
| Type III | 21 (58.3) |
| Type IV | 4 (11.1) |
| Type V | 2 (5.6) |
BMI, body mass index.
Of the 31 subjects that did not complete the 12-month study exit, two were exited before treatment (one because of a body mass index out of range and the other as a result of missing the treatment window). Early termination occurred in an additional 18 subjects. Of these, 13 were related to irritation or a rash, one because of a wound-site infection, and four for miscellaneous reasons. An additional 11 subjects completed treatment but did not return for their 12-month follow-up.
Scar images were obtained at 6 and 12 months after the abdominoplasty procedure, and the embrace-treated and control-treated images for all subjects were evaluated independently in a blinded fashion by three board-certified plastic surgeons using the visual analogue scale (Figs. 3 and 4). That is, each image was graded not in comparison to the contralateral side but as a single image. The mean visual analogue scale score for treated scars (2.90) was significantly less than that of the control scars (3.29) at 12 months (difference, 0.39; 95 percent CI, 0.14 to 0.66; p = 0.027).
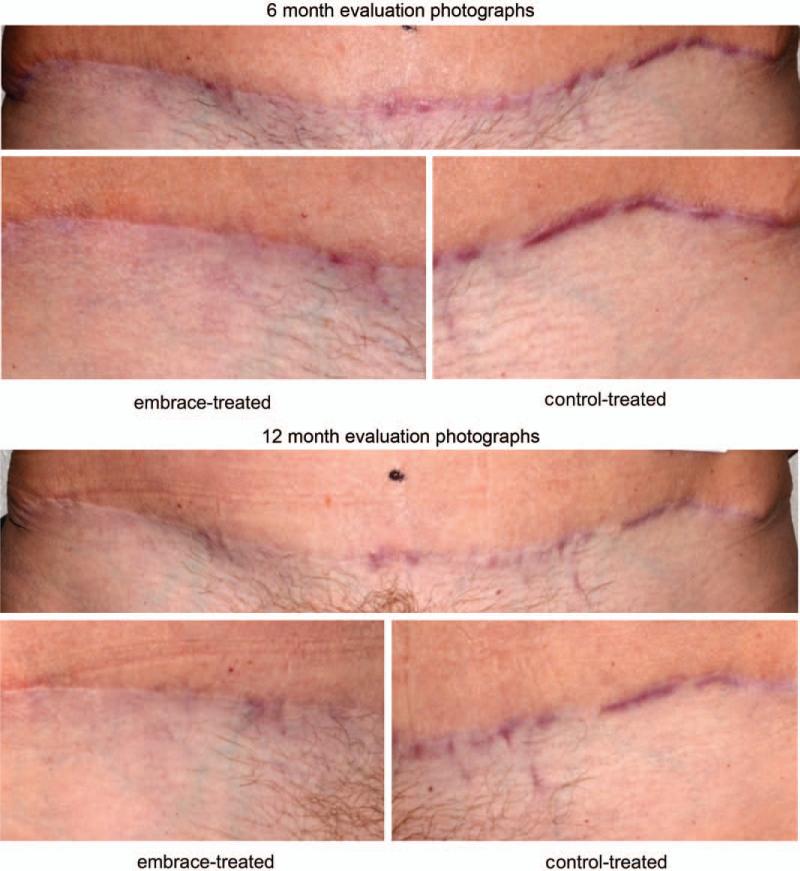
Fig. 3.
Photographic evaluation. (Above and second row) Patient at 6 months after abdominoplasty surgery. (Above) entire view of abdominoplasty scar. (Second row, left) The embrace-treated side and (second row, right) the control-treated side. The mean visual analogue scale scores for treated and control incisions were 2.13 and 6.87, respectively. Overall subject Patient and Observer Scar Assessment Scale scores were 5 for the treated and 10 for the control incision. Overall investigator Patient and Observer Scar Assessment Scale scores were 2 for the treated and 5 for the control incision. (Third row and below) Patient at 12 months following abdominoplasty surgery. (Third row) Entire view of abdominoplasty scar. (Below, left) The embrace-treated side and (below, right) the control-treated side. The mean visual analogue scale scores for treated and control incisions were 2.60 and 5.27, respectively. Overall subject Patient and Observer Scar Assessment Scale scores were 2 for the treated and 7 for the control incision. Overall investigator Patient and Observer Scar Assessment Scale scores were 2 for the treated and 6 for the control incision.
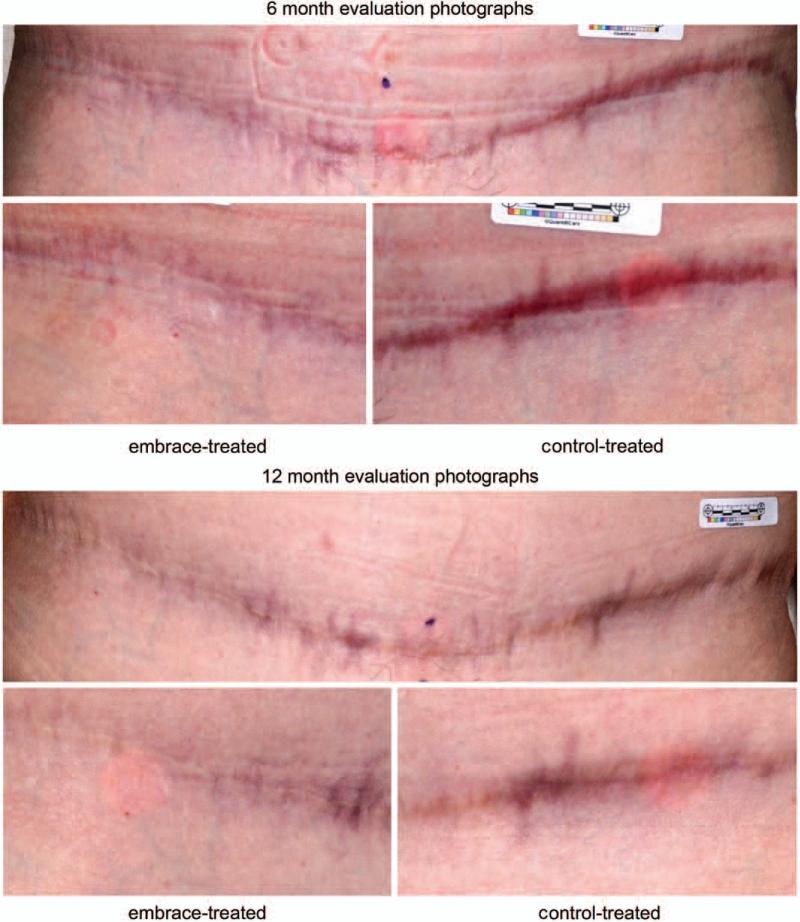
Fig. 4.
Photographic evaluation. (Above and second row) Patient at 6 months after abdominoplasty surgery. (Above) Entire view of abdominoplasty scar. (Second row, left) The embrace-treated side and (second row, right) the control-treated side. The mean visual analogue scale scores for treated and control incisions were 4.52 and 5.90, respectively. Overall subject Patient and Observer Scar Assessment Scale scores were 10 for the treated and 10 for the control incision. Overall investigator Patient and Observer Scar Assessment Scale scores were 2 for the treated and 4 for the control incision. (Third row and below) Patient at 12 months after abdominoplasty surgery. (Third row) Entire view of abdominoplasty scar. (Below, left) The embrace-treated side and (below, right) the control-treated side. The mean visual analogue scale scores for treated and control incisions were 2.53 and 4.40, respectively. Overall subject Patient and Observer Scar Assessment Scale scores were 4 for the treated and 10 for the control incision. Overall investigator Patient and Observer Scar Assessment Scale scores were 1 for the treated and 4 for the control incision.
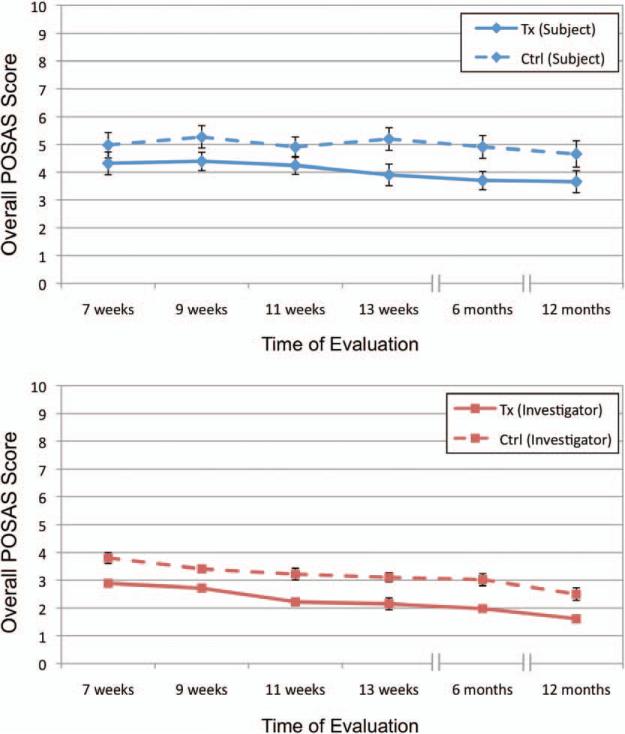
In addition to the objective visual analogue scale photographic analysis, treating physicians and subjects were asked to evaluate each scar using the Patient and Observer Scar Assessment Scale system. This evaluation is of importance because the treating physician uses three-dimensional examination and palpation to grade the scar. Similarly, subjects grade the scars based on real-life visual and topographic information. Investigators and subjects each rated embrace-treated and control-treated scars on seven categories using the Patient and Observer Scar Assessment Scale at 7, 9, 11, and 13 weeks, and at 6 and 12 months (Table 2). Investigators found that at 12 months, the embrace-treated scars demonstrated signifi-cant improvements in pigmentation (p < 0.001), pliability (p < 0.001), relief/roughness (p = 0.006), and vascularity (p = 0.004), in addition to overall opinion (p < 0.001). Subjects rated embrace-treated scars significantly better according to irregularity (p = 0.03), thickness (p = 0.01), stiffness (p = 0.002), and overall opinion (p = 0.02).
Table 2.
Patient and Observer Scar Assessment Scale Evaluation at 12 Months
| Treatment | Control | p | |
|---|---|---|---|
| Subject evaluation | |||
| Color | 3.80 | 4.31 | 0.10 |
| Irregular | 3.46 | 4.29 | 0.03* |
| Itching | 1.40 | 1.29 | 0.68 |
| Painful | 1.00 | 1.06 | 0.22 |
| Thickness | 2.86 | 3.97 | 0.01* |
| Stiffness | 2.63 | 3.77 | 0.003† |
| Overall opinion | 3.66 | 4.66 | 0.02* |
| Investigator evaluation | |||
| Pigmentation | 1.61 | 2.69 | <0.001‡ |
| Pliability | 1.67 | 2.25 | <0.001‡ |
| Relief | 2.56 | 3.11 | 0.006† |
| Surface area | 2.92 | 2.81 | 0.90 |
| Thickness | 3.36 | 3.50 | 0.39 |
| Vascularity | 1.67 | 2.25 | 0.004† |
| Overall opinion | 1.61 | 2.50 | <0.001‡ |
p < 0.05.
p < 0.01.
p < 0.001.
The evaluations at multiple time points allowed comparison of the difference between the embrace-treated incisions and the control-treated incisions over time. Both subjects and investigators noted significant improvements in their overall opinion as early as 9 weeks postoperatively (p = 0.02 and p < 0.001, respectively). Figure 5 shows that, in the opinions of the treating surgeons and subjects, the difference remained consistent over time. This is powerful, as it was important to know that any advantage at the end of treatment is not dissipated over a larger sample of treatment weeks. In addition, the overall Patient and Observer Scar Assessment Scale scores for both investigators and subjects decreased monotonically from 9 weeks to 12 months for embrace-treated scars.
Fig. 5.
Evolution of scar appearance over time. Graph illustrating overall scar appearance as measured by (above) subjects and (below) investigators using the Patient and Observer Scar Assessment Scale (POSAS) at all recorded time points. Error bars represent standard error of the mean. Tx, treatment; Ctrl, control.
To determine whether the embrace device exhibited differential efficacy according to certain subject variables, we constructed a linear model to evaluate whether age, race, body mass index, or Fitzpatrick skin type were predictive of 12-month visual analogue scale scores. We found no significant contribution according to age (p = 0.36), body mass index (p = 0.73), Fitzpatrick skin type (p = 0.47), or race (p = 0.48, p = 0.38, p = 0.74, p = 0.86, and p = 0.88 for white, Hispanic, black, Asian, and other, respectively). These results suggest that the effect of the embrace device is not specific to one or more subpopulations identifiable by these variables.
Many patients believe that they heal with “bad scars.” In contrast, very few patients believe that they heal with only a fine scar that is hardly visible. We examined whether the embrace device would be more effective in reducing scarring for patients who healed with scars that were rated worse by the investigator (surgeon) on the Patient and Observer Scar Assessment Scale. Importantly, in these subjects, the difference in Patient and Observer Scar Assessment Scale score was more dramatic both clinically and statistically (embrace-treated, 2.36; control-treated, 4.79; difference, 2.43; p < 0.001).
Furthermore, we evaluated whether subjects who dropped out of the study may have been those preinclined to experience less improvement with the embrace device, thus confounding our final evaluation. As 9 weeks was the first statistically significant time point for overall subject evaluation with the Patient and Observer Scar Assessment Scale, we used these data to compare the ratings of those subjects that did return for final evaluation at 12 months and those that did not. We found no statistically significant difference between absolute assessments of the treated incision between these groups at the 9-week time point (p = 0.32). In addition, at the 9 week assessment, when we compared the deltas (differences between overall assessment of treated side versus control side), we found no statistically significant difference between those patients who did or did not return for final evaluation (p = 0.28). These data suggest that early dropouts did not play a strong role in confounding our analysis.
Subjects were asked to rate their general satisfaction by answering three questions regarding their study participation. They were asked to compare the embrace-treated side with the control-treated side with regard to the minimization of scarring of their incision. Seventy-one percent of subjects (71.4 percent) indicated they were either “satisfied” or “very satisfied,” with no subjects selecting “dissatisfied” or “very dissatisfied.” (See Table, Supplemental Digital Content 4, which shows subject exit questions I, http://links.lww.com/PRS/B71.) Second, they were asked how likely they would be to recommend the embrace treatment to a friend. Seventy-four percent of subjects (74.3 percent) indicated they were either “likely” or “very likely” to recommend the treatment. (See Table, Supplemental Digital Content 5, which shows subject exit questions II, http://links.lww.com/PRS/B72.) Third, they were asked, if they were to have another procedure that might leave a scar, how likely would they be to use the embrace treatment again. Seventy-seven of subjects (77.2 percent) indicated they were “likely” or “very likely” to use the embrace treatment in a subsequent procedure.
Because the embrace device is a topical treatment with no active chemical or biological agents, we did not expect to see any serious adverse events, and in fact none were reported. Skin irritation sufficient to terminate treatment occurred in 13 subjects, one subject developed a wound-site infection leading to withdrawal from the study, and one experienced an allergic reaction. An additional 59 nonserious adverse events were reported and officially designated as “other,” including mild irritation, erythema, and rash. No serious adverse events were reported.
DISCUSSION
To our knowledge, this study represents the first pivotal level I evidence for postoperative scar reduction. Our data strongly support that the embrace device significantly reduces scarring after excisional wound closure.
The embrace device has a mechanism of action that is based on surgical principles currently used to minimize scarring. During an operation, surgeons strive to make incisions that follow the relaxed tension lines on the body, so-called Langer lines. This strategy is used because tension is well known to increase scarring. The embrace device is designed to shield the healing incision from the natural tension that is inherent in any break in skin that must be pulled together to close a wound. Previous preclinical and first-in-human data initially demonstrated that this mechanism of action was effective in scar mitigation in both pigs and humans.21 The impact of offloading tension is attractive when compared with targeting a single gene or protein for minimizing scarring, as hundreds or perhaps thousands of genes modulate in response to mechanical forces during wound repair.17
Abdominoplasty incisions were selected for this trial for several reasons. First, the wounds are closed under considerable tension, which presents the most challenging test of a product intended to modulate the mechanical environment of a healing wound. One could postulate that if the device was effective in shielding the healing wound under this challenging mechanical environment, it would be effective in incisions closed under less strain. Second, the length of the incision is ideal for a randomized controlled design so that each subject can serve as his or her own control. This is important, as it allows for the most rigorous direct comparison of two treatment arms on the same subject and completely avoids subject-to-subject variability as a confounding factor when comparing two treatment types. Furthermore, because of the high-tension closure, abdominoplasty scars are well known to be wide and surgeons have developed their own individual postoperative approaches for scar mitigation. It is important to emphasize that the surgeons participating in this trial were asked to use their optimal treatment to mitigate scars as the control treatment, making this in essence a comparative effectiveness study. Thus, based on a very challenging indication and compared to each surgeon's optimal treatment regimen, this study strongly supports the use of the embrace device to effectively mitigate scarring postoperatively.
Endpoint analysis included evaluation of scars using both a blinded analysis by visual analogue scale scoring by three independent board-certified plastic surgeons and Patient and Observer Scar Assessment Scale scoring from both the treating surgeon and the subject at 12 months after abdominoplasty. The choice of the 12-month endpoint allows time for the majority of repair and remodeling to occur. At shorter endpoints such as 3 months, scar remodeling is not complete. The visual analogue scale is an established method for scar analysis and has been used in numerous other scar trials.22 However, the visual analogue scale only allows a two-dimensional assessment of the wound. Despite this limitation, our visual analogue scale data showed a significant difference between embrace-treated and control-treated sides. The Patient and Observer Scar Assessment Scale data from both treating surgeons and subjects include three-dimensional assessment and palpation, and provide what may be a more comprehensive and relevant assessment of the result. The treating surgeon's assessment of pigmentation, pliability, relief, vascularity, and overall opinion shared a highly significant difference in favor of embrace treatment. Similarly, the subject's assessment of irregular contour, thickness, stiffness, and overall opinion of the scar was highly significant in favor of the embrace-treated scar.
It was also important to follow the Patient and Observer Scar Assessment Scale data from both subjects and investigators over time to account for scar remodeling and maturation. The overall Patient and Observer Scar Assessment Scale scores for both decreased monotonically from 9 weeks to 12 months for embrace-treated scars. Alternatively viewed, this means that the opinion is more favorable on the embrace-treated side of the incision at 1 year than it was at 9 weeks. It is worth emphasizing this, because wound repair is a complex biological process that goes through an ordered series of events involving cell migration, matrix deposition, and turnover. Furthermore, after 8 to 12 weeks, the remodeling portion of the wound can take up to 1 year or even longer; thus, it was important to capture the opinion of the treating surgeon, and the subject, over the 1-year time frame.
In contrast to other technologies that specifically examined only hypertrophic or keloid scars, in the present study, we examined the entire spectrum of human scar formation following surgery. As expected, the results of this trial were significantly more dramatic (reducing scarring) in the patients with the worst scars as quantified by the surgeon (treating physician).
CONCLUSIONS
In conclusion, the results of this randomized controlled trial strongly support that the embrace device minimizes scarring in a challenging surgical procedure over a 1-year time period. Furthermore, the differences were significant between embrace-treated and control-treated groups when analyzed by blinded reviewers using visual analogue scale photographic assessment and/or treating physicians and patients using the Patient and Observer Scar Assessment Scale.
Supplementary Material
Footnotes
This trial is registered under the name “A Study of a Novel Silicone Dressing to Minimize Scar Formation (REFINE),” ClinicalTrials.gov identification number NCT01399099 (http://clinicaltrials.gov/show/NCT01399099).
CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, II.
Disclosure:
Michael T. Longaker, M.D., M.B.A., and Geoffrey C. Gurtner, M.D., are founders of, have equity positions in, and serve on the board of Neodyne Biosciences, Inc., which provided the devices used in the study and supported the clinical trial, and Bill Beasley, B.S., is an employee and officer of the company. Christy Cowley, M.P.H., and Peggy McLaughlin, B.S., are consultants to Neodyne Biosciences, Inc., and have equity positions in the company. Rod J. Rohrich, M.D., Lauren Greenberg, M.D., Heather Furnas, M.D., Robert Wald, M.D., Vivek Bansal, M.D., Hisham Seify, M.D., Anthony Tran, M.D., Jane Weston, M.D., Joshua M. Korman, M.D., Rodney Chan, M.D., David Kaufman, M.D., Vipul R. Dev, M.D., and Joseph A. Mele, M.D., received clinical research grant support for participation in the study. Michael T. Longaker, M.D., M.B.A., analyzed the data and wrote this article while on sabbatical from Stanford University. Rod J. Rohrich, M.D., received clinical research grant support for participation in the study; he also receives instrument royalties from Micrins Instruments and book royalties from Quality Medical Publishing.
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal's Web site (www.PRSJournal.com).
Contributor Information
Michael T. Longaker, Division of Plastic and Reconstructive Surgery Department of Surgery, Stanford University Hagey Laboratory for Pediatric Regenerative Medicine 257 Campus Drive West Stanford, Calif. 94305-5148 longaker@stanford.edu
Geoffrey C. Gurtner, Division of Plastic and Reconstructive Surgery Department of Surgery, Stanford University Hagey Laboratory for Pediatric Regenerative Medicine 257 Campus Drive West Stanford, Calif. 94305-5148 ggurtner@stanford.edu
REFERENCES
- 1.MedMarket Diligence . Established and Emerging Products, Technologies and Markets in the U.S., Europe, Japan and Rest of the World. MedMarket Diligence; Foothill Ranch, Calif: 2005. [Google Scholar]
- 2.Stuart M. Start-ups, strategics help mend wound repair. Start Up. 2013 May; [Google Scholar]
- 3.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 4.Cullen KA, Hall MJ, Golosinkiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11:1–25. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention [June 6, 2013];National hospital discharge survey: 2010 table. Procedures by selected patient characteristics—Number by procedure category and age. 2010 Available at: http://www.cdc.gov/nchs/data/ nhds/4procedures/2010pro4_numberprocedureage.pdf.
- 6.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 7.Singer AJ, Hollander JE, Quinn JV. Evaluation and management of traumatic lacerations. N Engl J Med. 1997;337:1142–1148. doi: 10.1056/NEJM199710163371607. [DOI] [PubMed] [Google Scholar]
- 8.Maher SF, Dorko L, Saliga S. Linear scar reduction using silicone gel sheets in individuals with normal healing. J Wound Care. 2012;21:602, 604–606, 608–609. doi: 10.12968/jowc.2012.21.12.602. [DOI] [PubMed] [Google Scholar]
- 9.Saulis AS, Mogford JH, Mustoe TA. Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg. 2002;110:177–183. doi: 10.1097/00006534-200207000-00029. discussion 184–186. [DOI] [PubMed] [Google Scholar]
- 10.Gold MH, Foster TD, Adair MA, Burlison K, Lewis T. Prevention of hypertrophic scars and keloids by the prophylactic use of topical silicone gel sheets following a surgical procedure in an office setting. Dermatol Surg. 2001;27:641–644. doi: 10.1046/j.1524-4725.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 11.Flynn TC, Coleman WP. Topical revitalization of body skin. J Eur Acad Dermatol Venereol. 2000;14:280–284. doi: 10.1046/j.1468-3083.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel: A new treatment for hypertrophic scars. Surgery. 1989;106:781–786. discussion 786–787. [PubMed] [Google Scholar]
- 13.Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel for the prevention and treatment of hypertrophic scar. Arch Surg. 1991;126:499–504. doi: 10.1001/archsurg.1991.01410280103016. [DOI] [PubMed] [Google Scholar]
- 14.Mustoe TA, Cooter RD, Gold MH, et al. International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2013;9:CD003826. doi: 10.1002/14651858.CD003826.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renovo. Justive EU. [March 7, 2013];Phase 3 Trial Results. Available at: http://www.renovo.com/en/news/juvista-eu-phase-3-trial-results.
- 17.Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg. 2005;116:514–522. doi: 10.1097/01.prs.0000172982.43599.d6. [DOI] [PubMed] [Google Scholar]
- 19.Truong PT, Lee JC, Soer B, Gaul CA, Olivotto IA. Reliability and validity testing of the Patient and Observer Scar Assessment Scale in evaluating linear scars after breast cancer surgery. Plast Reconstr Surg. 2007;119:487–494. doi: 10.1097/01.prs.0000252949.77525.bc. [DOI] [PubMed] [Google Scholar]
- 20.Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: A suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118:909–918. doi: 10.1097/01.prs.0000232378.88776.b0. [DOI] [PubMed] [Google Scholar]
- 21.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: Large animal and phase I studies. Ann Surg. 2011;254:217–225. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 22.Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.