Abstract
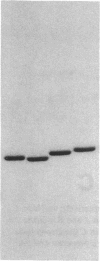
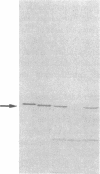
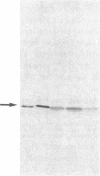
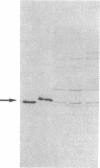
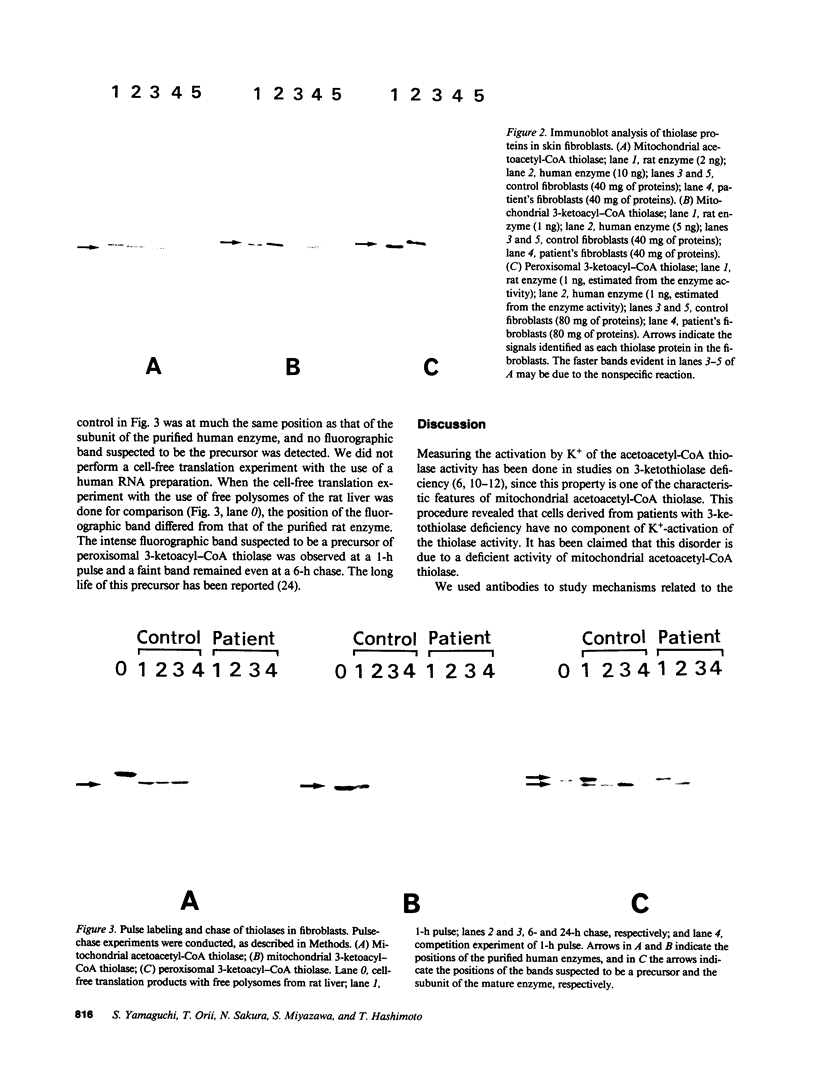
The etiology of 3-ketothiolase deficiency has been attributed to a defect of mitochondrial acetoacetyl-CoA thiolase because the acetoacetyl-CoA thiolase activity in related materials is not activated by K+, a property characteristic for this enzyme. We studied the enzyme protein and the biosynthesis of mitochondrial acetoacetyl-CoA thiolase, using cultured skin fibroblasts from a 5-yr-old boy with 3-ketothiolase deficiency. The following results were obtained. (a) Activation of acetoacetyl-CoA thiolase activity by K+ was nil; (b) The enzyme activity was not affected by treatment with the antibody against mitochondrial acetoacetyl-CoA thiolase; (c) A signal for mitochondrial acetoacetyl-CoA thiolase protein was not detected in the immunoblot analysis; and (d) Pulse-chase experiments of skin fibroblasts, using [35S]methionine, revealed no incorporation of radioactivity into this enzyme. Therefore, fibroblasts from this patient lacked mitochondrial acetoacetyl-CoA thiolase protein due to a defect in its biosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. J., Littlewood J. M., MacDonald A., Pollitt R. J., Thompson J. A case of beta-ketothiolase deficiency. J Inherit Metab Dis. 1983;6(4):157–157. doi: 10.1007/BF02310871. [DOI] [PubMed] [Google Scholar]
- Daum R. S., Lamm P. H., Mamer O. A., Scriver C. R. A "new" disorder of isoleucine catabolism. Lancet. 1971 Dec 11;2(7737):1289–1290. doi: 10.1016/s0140-6736(71)90605-2. [DOI] [PubMed] [Google Scholar]
- Daum R. S., Scriver C. R., Mamer O. A., Delvin E., Lamm P., Goldman H. An inherited disorder of isoleucine catabolism causing accumulation of alpha-methylacetoacetate and alpha-methyl-beta -hydroxybutyrate, and intermittent metabolic acidosis. Pediatr Res. 1973 Mar;7(3):149–160. doi: 10.1203/00006450-197303000-00007. [DOI] [PubMed] [Google Scholar]
- Gompertz D., Saudubray J. M., Charpentier C., Bartlett K., Goodey P. A., Draffan G. H. A defect in l-isoleucine metabolism associated with alpha-methyl-beta-hydroxybutyric and alpha-methylacetoacetic aciduria: quantitative in vivo and in vitro studies. Clin Chim Acta. 1974 Dec 17;57(3):269–281. doi: 10.1016/0009-8981(74)90407-0. [DOI] [PubMed] [Google Scholar]
- Halvorsen S., Stokke O., Jellum E. A variant form of 2-methyl-3-hydroxybutyric and 2-methylacetoacetic aciduria. Acta Paediatr Scand. 1979 Jan;68(1):123–128. doi: 10.1111/j.1651-2227.1979.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Keating J. P. Beta-ketothiolase deficiency as a cause of the "ketotic hyperglycinemia syndrome". Pediatrics. 1974 Feb;53(2):221–225. [PubMed] [Google Scholar]
- Hiyama K., Sakura N., Matsumoto T., Kuhara T. Deficient beta-ketothiolase activity in leukocytes from a patient with 2-methylacetoacetic aciduria. Clin Chim Acta. 1986 Mar 16;155(2):189–194. doi: 10.1016/0009-8981(86)90283-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Middleton B., Bartlett K., Romanos A., Gomez Vazquez J., Conde C., Cannon R. A., Lipson M., Sweetman L., Nyhan W. L. 3-Ketothiolase deficiency. Eur J Pediatr. 1986 Apr;144(6):586–589. doi: 10.1007/BF00496042. [DOI] [PubMed] [Google Scholar]
- Middleton B., Bartlett K. The synthesis and characterisation of 2-methylacetoacetyl coenzyme A and its use in the identification of the site of the defect in 2-methylacetoacetic and 2-methyl-3-hydroxybutyric aciduria. Clin Chim Acta. 1983 Mar 14;128(2-3):291–305. doi: 10.1016/0009-8981(83)90329-7. [DOI] [PubMed] [Google Scholar]
- Middleton B., Gray R. G., Bennett M. J. Two cases of beta-ketothiolase deficiency: a comparison. J Inherit Metab Dis. 1984;7 (Suppl 2):131–132. [PubMed] [Google Scholar]
- Middleton B. The kinetic mechanism and properties of the cytoplasmic acetoacetyl-coenzyme A thiolase from rat liver. Biochem J. 1974 Apr;139(1):109–121. doi: 10.1042/bj1390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B. The oxoacyl-coenzyme A thiolases of animal tissues. Biochem J. 1973 Apr;132(4):717–730. doi: 10.1042/bj1320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
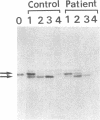
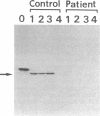
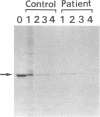
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur J Biochem. 1980 Feb;103(3):589–596. doi: 10.1111/j.1432-1033.1980.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Ozasa H., Furuta S., Miyazawa S., Osumi T., Hashimoto T., Mori M., Miura S., Tatibana M. Biosynthesis of enzymes of rat-liver mitochondrial beta-oxidation. Eur J Biochem. 1984 Nov 2;144(3):453–458. doi: 10.1111/j.1432-1033.1984.tb08487.x. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Sherwood W. G., Taylor J., Balfe J. W., Mamer O. A. Acetoacetyl CoA thiolase deficiency: a cause of severe ketoacidosis in infancy simulating salicylism. J Pediatr. 1979 Aug;95(2):228–233. doi: 10.1016/s0022-3476(79)80656-3. [DOI] [PubMed] [Google Scholar]
- Schram A. W., Strijland A., Hashimoto T., Wanders R. J., Schutgens R. B., van den Bosch H., Tager J. M. Biosynthesis and maturation of peroxisomal beta-oxidation enzymes in fibroblasts in relation to the Zellweger syndrome and infantile Refsum disease. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6156–6158. doi: 10.1073/pnas.83.16.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens R. B., Middleton B., vd Blij J. F., Oorthuys J. W., Veder H. A., Vulsma T., Tegelaers W. H. Beta-ketothiolase deficiency in a family confirmed by in vitro enzymatic assays in fibroblasts. Eur J Pediatr. 1982 Sep;139(1):39–42. doi: 10.1007/BF00442077. [DOI] [PubMed] [Google Scholar]
- Tager J. M., Van der Beek W. A., Wanders R. J., Hashimoto T., Heymans H. S., Van den Bosch H., Schutgens R. B., Schram A. W. Peroxisomal beta-oxidation enzyme proteins in the Zellweger syndrome. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1269–1275. doi: 10.1016/0006-291x(85)90322-5. [DOI] [PubMed] [Google Scholar]
- Takiguchi M., Mori M., Tatibana M. A simple and rapid procedure for high-yield isolation of essentially undegraded free and membrane-bound polysomes from rat liver. J Biochem. 1985 May;97(5):1447–1459. doi: 10.1093/oxfordjournals.jbchem.a135199. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]