Abstract
Objective
To compare outcomes for patients with hepatocellular carcinoma (HCC) treated with either liver resection or transplantation.
Methods
A retrospective, single institution analysis of 413 HCC patients from 1999–2009.
Results
413 patients with HCC underwent surgical resection (n=106), transplantation (n=270), or were listed without receiving transplantation (n=37). Excluding transplanted patients with incidental tumors (n=50), 257 patients with suspected HCC were listed with the intent to transplant (ITT). The median diameter of the largest tumor by radiography was 6.0 cm in resected, 3.0 cm in transplanted, and 3.4 cm in the listed-but-not-transplanted patients. Median time to transplant was 48 days. Recurrence rates were 19.8% for resection and 12.1% for all ITT patients. Overall, patient survival for resection vs. ITT patients was similar (5-year survival of 53.0% vs. 52.0%, NS). However, for HCC patients with MELD scores <10 and who radiologically met Milan or UCSF criteria, 1-year and 5-year survival rates were significantly improved in resected patients. For patients with MELD <10 and who met Milan criteria, 1-year and 5-year survival were 92.0% and 63.0% for resection (n=26) vs. 83.0% and 41.0% for ITT (n=73, p=0.036). For those with MELD <10 and met UCSF criteria, 1-year and 5-year survival was 94.0% and 62.0% for resection (n=33) vs. 81.0% and 40.0% for ITT (n=78, p=0.027).
Conclusions
Among known HCC patients with preserved liver function, resection was associated with superior patient survival versus transplantation. These results suggest surgical resection should remain the first line therapy for patients with HCC and compensated liver function who are candidates for resection.
Keywords: liver, resection, cancer, transplantation, outcomes
INTRODUCTION
World wide, hepatocellular carcinoma (HCC) has been estimated to be the third most common cause of cancer-related death (1–3). In vast regions of the world including sub-Saharan Africa and East-Asia, HCC is the most common cause of cancer associated mortality surpassing gastric and lung cancers in incidence and mortality. The incidence of HCC remains far lower in the United States and Europe but has dramatically increased in the past several decades (4). The increase in HCC prevalence is anticipated to continue in both the United States and Europe over the next several decades, primarily due to the Hepatitis C virus and to a lesser extent due to emigration from endemic regions, non-alcoholic steatohepatitis (NASH) and the spread of the Hepatitis B virus (2, 4–6).
The best potential curative therapies available to treat patients who develop HCC are liver resection or transplantation (7–9). Unfortunately, due to intrinsic liver dysfunction (limiting resection), and lack of liver donor availability (limiting transplant), and late detection (limiting both), only a small subset of patients are candidates for curative therapies (10, 11). Increasingly, a role for hepatic ablative therapies has been recognized, but such therapies in Western series have not been universally associated with equivalent patient outcomes (12–14). Nonetheless, determination of which curative intent therapies to provide patients remain poorly defined (15). Although outstanding outcomes have been observed with the use of ablative techniques at select centers, particularly for tumors under 3 cm, most studies report resection or transplantation as superior therapies in the management of HCC. Thus, ablative therapies have generally been restricted as a bridge therapy prior to transplantation, or as palliative therapy for patients who are not candidates for either resection or transplantation (12–14). Similarly, due to restrictions of size on candidacy for transplantation (those who satisfy the Milan or UCSF Criteria) and intrinsic regenerative abnormalities from sequelae of cirrhosis (limiting the ability to provide resective therapies), many patients are potential candidates for only resection or transplantation respectively (16).
Several studies have compared outcomes for patients with HCC treated with various curative-intent therapies (17–26). The majority of studies have mainly limited their analysis to patients satisfying current transplantation guidelines by size parameters. Those data have generally observed equivalent overall outcomes for patients who have been treated with either resection or transplantation. To better define the relative outcomes for patients who theoretically might be candidates for either resection or transplantation, we compared outcomes from a single institution that actively practices both resective and transplantation approaches in the management of HCC.
METHODS
The study was approved by the Institutional Review Boards of the University of Miami Hospital and Clinics and Jackson Memorial Hospital. The Tumor Registries of the University of Miami Hospital, Sylvester Cancer Center and Jackson Memorial Hospital were examined for prospectively collected HCC patients treated since 1999. This patient series was queried for primary liver malignancies using ICD-9 code 155.0. All data were secondarily confirmed by review of the medical records including, initial clinic notes, radiographic reports, operative and pathology reports as well as clinical progress notes. Patients with primary HCC were included and those with other primary liver tumors were excluded from the analysis. The University of Miami/Jackson Memorial Hospital system has also prospectively collected an HCC registry for patients who were considered for transplantation beginning in 2001. This data set was queried to identify patients with HCC who were listed and/or underwent liver transplantation for HCC. Merging of these datasets identified the patient pool for evaluation in this study. Among all HCC patients identified, this study examined outcomes for patients who were provided curative-intent treatment for HCC.
Patients who underwent hepatic resection from 1999–2009 or underwent liver transplantation from 2001–2009 were examined. Overall, 413 patients with HCC treated with either resection or liver transplantation were identified. All patients were stratified by treatment strategies that included resection, incidental transplant (HCC noted on final pathology report), non-incidental transplant (known HCC prior to transplantation), and listed but not transplanted (known HCC but donor organ not available). Those patients with non-incidental transplants plus those who were listed with known HCC but not transplanted were defined as the Intent to Transplant group (ITT).
MELD scores were prospectively collected in the transplantation cohort and were determined retrospectively in most resected candidates. Tumor sizes were determined by radiographic imaging, including computerized tomography, magnetic resonance, or ultrasound. Patients with missing data were excluded from each respective analysis. Survival was determined by the tumor registries and independently verified during data collection with the examination of the National Social Security Death Index. Patients lost to follow-up were censored at the time of last contact or confirmed date of death. Survival was calculated from the time of initial diagnosis to the date of last contact or death. Recurrence free survival analysis was calculated from the date of initial resection or transplantation to the date of documented recurrence of disease or death.
All patients were also stratified by MELD scores and radiographic size: Milan (one lesion smaller than 5 cm, or up to 3 lesions smaller than 3 cm,), or UCSF (single lesion ≤ 6.5 cm, multiple lesions ≤ 3 cm, largest tumor diameter if multiple ≤ 4.5 cm, total tumor diameter if multiple ≤ 8 cm) (27–29). In order to evaluate the effects of intrinsic liver function as well as tumor size, subgroup analysis was performed with patient sub-categorization as either meeting Milan and MELD < 10 or UCSF and MELD < 10. Rates of recurrences were analyzed for the various resective procedures (wedge/partial, formal right, extended right, formal left, and extended left) and their margin status (positive or negative). The subgroup of resected patients requiring secondary procedures was also examined.
Statistical analysis was performed with SPSS version 18.0 (PASW), released July 30th 2009 (IBM Corporation, Somers NY). Chi-square test was used for categorical variables and one-way ANOVA for age, MELD score and radiological size. Survivals were analyzed for the entire cohort of patients and for each subgroup that met size or MELD criteria. Survival curves were performed by Kaplan Meier method and the Log-Rank (Mantel Cox) test was used for survival comparisons. Survivals were expressed as median, 1-yr, 5-yr and 7-yr percentages. Statistical significance was defined as p < 0.05.
RESULTS
Patient and tumor demographics for the cohort
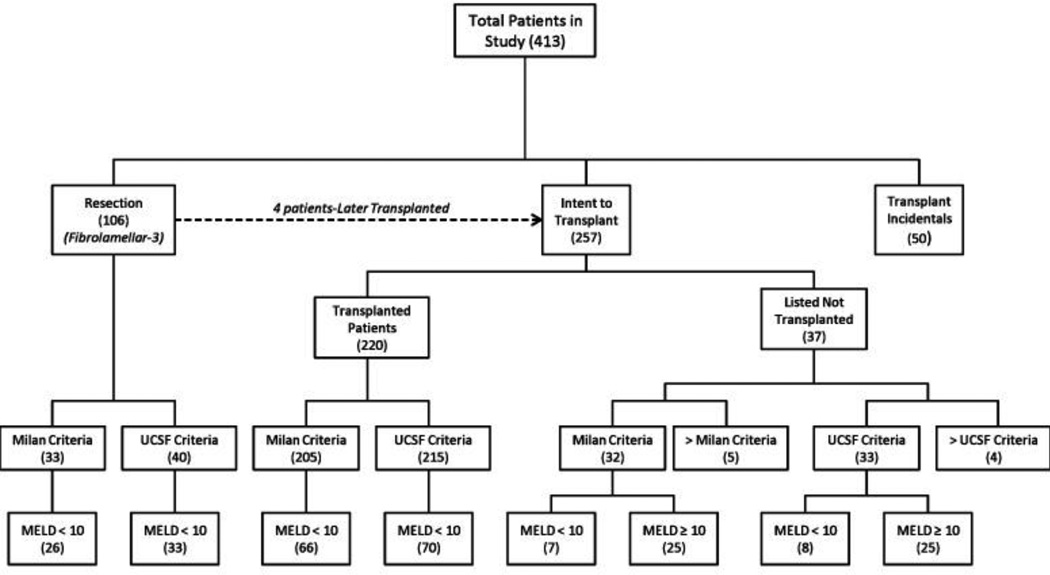
Overall, we identified over 1,400 patients who were evaluated for the treatment of HCC at The University of Miami/Jackson Memorial Hospital. Of these, 413 patients with HCC underwent surgical resection (n=106), transplantation (n=270), or were listed without receiving transplantation (n=37) (Table 1, Figure 1).
Table 1.
Patient Demographics
| Total | Resection | ITT - Intention To Treat (A + B) |
(A) Transplants (Non- Incidentals) |
(B) Listed Not Transplanted |
(C) Transplants (Incidental) |
Transplants (Total) (A+B+C) |
Significance* Resection vs. ITT |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Total Pts |
N | % Group |
N | % Group |
N | % Group |
N | % Group |
N | % Group |
N | % Group | (P-Value) | |
| Number of Patients | 413 | 106 | 25.7 | 257 | 62.2 | 220 | 53.3 | 37 | 9 | 50 | 12.1 | 307 | 74.3 | ||
| Sex | |||||||||||||||
| Male | 302 | 73.1 | 72 | 67.9 | 193 | 75.1 | 164 | 74.5 | 29 | 78.4 | 37 | 74 | 230 | 74.9 | 0.1933 |
| Female | 111 | 26.9 | 34 | 32.1 | 64 | 24.9 | 56 | 25.5 | 8 | 21.6 | 13 | 26 | 77 | 25.1 | |
| Age at Diagnosis | |||||||||||||||
| Mean ± S.D. | 58.29 ± 10.908 | 59.06 ± 15.92 | 57.37 ± 8.48 | 57.3 ± 8.3 | 58.1 ± 9.54 | 61.3 ± 8.17 | 58 ± 8.54 | 0.193 | |||||||
| Median | 58 | 62 | 57 | 57 | 57 | 63 | 57 | ||||||||
| Range | 4.0 – 82 | 6.0 – 82 | 4.0 – 77 | 4.0 – 77 | 26 – 74 | 42 – 76 | 4.0 – 77 | ||||||||
| Liver Disease | |||||||||||||||
| Hepatitis B | 25 | 6.1 | 11 | 10.4 | 12 | 4.7 | 12 | 5.5 | 0 | 0 | 2 | 4 | 14 | 4.6 | 0.0001 |
| Hepatitis C | 248 | 60 | 30 | 28.3 | 191 | 74.3 | 164 | 74.5 | 27 | 73 | 27 | 54 | 218 | 71 | |
| Hepatitis B+C | 5 | 1.2 | 2 | 1.9 | 3 | 1.2 | 3 | 1.4 | 0 | 0 | 0 | 0 | 3 | 1 | |
| No Hepatitis | 135 | 32.7 | 63 | 59.4 | 51 | 19.8 | 41 | 18.6 | 10 | 27 | 21 | 42 | 72 | 23.5 | |
| MELD Score | |||||||||||||||
| Mean ± S.D. | 12.18 ± 6.59 | 7.3 ± 2.76 | 12.89 ± 6.28 | 13 ± 6.53 | 12.2 ± 4.53 | 18.9 ± 6.57 | 13.87 ± 6.69 | 0.0001 | |||||||
| Median | 11 | 6 | 11 | 11.5 | 11 | 17 | 12 | ||||||||
| Mode | 6 | 6 | 7 | 7 | 9 | 16 | 13 | ||||||||
| Max Radiological Size | |||||||||||||||
| Mean ± S.D. | 3.56 ± 2.36 | 6.08 ± 3.78 | 3.02 ± 1.46 | 2.95 ± 1.34 | 3.42 ± 1.97 | - | 3.02 ± 1.46 | 0.0001 | |||||||
| Median | 3 | 5 | 2.8 | 2.8 | 3 | - | 2.8 | ||||||||
| Range | 0 – 19 | 1.8 – 19 | 0.7 – 10 | 0.7 – 8.5 | 1.0 – 10 | - | 0 – 10 | ||||||||
Comparisons between Resection and Intent To Treat Transplant via chi-squared and students T-Test,
Bold: Percent Total Pts.
Non-Bold: Percent of Group.
Figure 1.
Patient stratification by Milan, UCSF criteria and MELD score.
Median age of the cohort of 413 patients was 58 years with a 3:1 male predominance (Table 1). There were no significant differences in age or sex ratios between resection and ITT patient groups. A significantly higher fraction of ITT patients as compared to those who underwent resection had viral hepatitis, particularly hepatitis C (74.3%, 28.3 %, respectively, P=0.0001) and overall a significantly increased median MELD score (11 vs. 6 respectively, P=0.0001). Mean radiographic tumor size or the largest tumor in cases of multifocal disease was considerably larger in the resection group (6 cm, 3.0 cm respectively, P=0.0001).
Tumor characteristics for the cohort were analyzed in Table 2. Overall, the ITT group as compared to the resection group had considerably more right hepatic lesions (77.8%, 59.4% respectively), bilateral lesions (10.4%, 5.4% respectively), and multifocal disease (48.2%, 15.1%, respectively). Median pathological sizes were significantly greater in the resection group than the ITT group (6.0 cm vs. 3.0 cm, respectively, P<0.0001). Similar differences were noted on final pathological measurements although, pathological sizes were slightly greater than radiographic sizes particularly in the transplantation group, likely due to the delay between imaging and definitive therapy. Tumor morphology was similar between the resection and ITT groups with the majority being low grade (73.6%), representing well or moderately differentiated tumors. Most tumors in the resection and ITT groups did not demonstrate lymphatic (92.5%, 59.9% respectively), or vascular invasion (70.8%, 59.9% respectively). Overall, recurrences were noted in 13.3% of the entire cohort, with the majority of these occurring within the liver or lung. Overall, recurrences were documented in 19.8% of the resection group and 12.1% of the ITT group with no significant difference between the two groups (P=0.335). Of note, four patients had fibrolamellar HCC. Also, no evidence for fibrosis or cirrhosis was noted in 35 of 106 (33%) patients who underwent resection. All patients who underwent transplantation had fibrosis or cirrhosis.
Table 2.
Tumor Characteristics
| Total | Resection | ITT - Intention To Treat (A + B) |
(A) Transplants (Non-Incidentals) |
(B) Listed Not Transplanted |
(C) Transplants (Incidental) |
Transplants (Total) (A+B+C) |
Significance* Resection vs. ITT |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Total Pts |
N | % Group |
N | % Group |
N | % Group | N | % Group |
N | % Group |
N | % Group | P - Value | |
| Total Patients | 413 | 106 | 25.7 | 257 | 62.2 | 220 | 53.3 | 37 | 9 | 50 | 12.1 | 307 | 74.3 | ||
| Affected Lobe | |||||||||||||||
| Right | 297 | 71.9 | 63 | 59.4 | 200 | 77.8 | 177 | 80.5 | 23 | 62.2 | 34 | 68 | 234 | 76.2 | 0.005 |
| Left | 80 | 19.4 | 30 | 28.3 | 41 | 16 | 31 | 14.1 | 10 | 27 | 9 | 18 | 50 | 16.3 | |
| Bilateral | 32 | 7.7 | 11 | 10.4 | 14 | 5.4 | 10 | 4.5 | 4 | 10.8 | 7 | 14 | 21 | 6.8 | |
| N/A | 4 | 1 | 2 | 1.9 | 2 | 0.8 | 2 | 0.9 | 0 | 0 | 0 | 0 | 2 | 0.7 | |
| Focality | |||||||||||||||
| Single | 239 | 57.9 | 77 | 72.6 | 131 | 51 | 105 | 47.7 | 26 | 70.3 | 31 | 62 | 162 | 52.8 | 0.0001 |
| Multifocal | 159 | 38.5 | 16 | 15.1 | 124 | 48.2 | 113 | 51.4 | 11 | 29.7 | 19 | 38 | 143 | 46.6 | |
| N/A | 15 | 3.6 | 13 | 12.3 | 2 | 0.8 | 2 | 0.6 | 0 | 0 | 0 | 0 | 2 | 0.7 | |
| Path largest Size | |||||||||||||||
| Mean ± S.D. | 3.91 ± 3.1 | 7.02 ± 4.53 | 3.12 ± 1.53 | 3.13 ± 1.53 | N/A | 2.21 ± 1.49 | 2.96 ± 1.56 | < 0.0001 | |||||||
| Median | 3 | 6 | 3 | 3 | N/A | 2 | 2.9 | ||||||||
| Range | 0.2 – 26 | 1.5 – 26 | 0.2 – 11 | 0.2 – 11 | N/A | 0.5 – 9.0 | 0.2 – 11.0 | ||||||||
| Path Morphology | |||||||||||||||
| High Grade: (Poor Diff) | 45 | 10.9 | 17 | 16 | 26 | 10.1 | 26 | 11.8 | N/A | - | 2 | 4 | 28 | 9.1 | 0.0956 |
| Low Grade: (Well/Mod Diff) | 304 | 73.6 | 76 | 71.7 | 180 | 70 | 180 | 81.8 | N/A | - | 48 | 96 | 228 | 74.3 | |
| N/A | 64 | 15.5 | 13 | 12.3 | 51 | 19.8 | 14 | 6.4 | 37 | - | 0 | 0 | 51 | 16.6 | |
| Lymphatic Invasion | |||||||||||||||
| Yes | 83 | 20.1 | 8 | 7.5 | 66 | 25.7 | 66 | 30 | N/A | - | 9 | 18 | 75 | 24.4 | < 0.0001 |
| No | 293 | 70.9 | 98 | 92.5 | 154 | 59.9 | 154 | 70 | N/A | - | 41 | 82 | 195 | 63.5 | |
| N/A | 37 | 9 | 0 | 0 | 37 | 14.4 | 0 | 0 | 37 | - | 0 | 0 | 37 | 12.1 | |
| Vascular Invasion | |||||||||||||||
| Yes | 106 | 25.7 | 31 | 29.2 | 66 | 25.7 | 66 | 30 | N/A | - | 9 | 18 | 75 | 24.4 | 0.0002 |
| No | 270 | 65.4 | 75 | 70.8 | 154 | 59.9 | 154 | 70 | N/A | - | 41 | 82 | 195 | 63.5 | |
| N/A | 37 | 9 | 0 | 0 | 37 | 14.4 | 0 | 0 | 37 | - | 0 | 0 | 37 | 12.1 | |
| Recurrences | |||||||||||||||
| Total | 55 | 13.3 | 21 | 19.8 | 31 | 12.1 | 31 | 14.1 | N/A | - | 3 | 6 | 34 | 11.1 | 0.3354 |
| Liver Recurrence | 30 | 7.3 | 15 | 14.2 | 16 | 6.2 | 16 | 7.3 | N/A | - | 1 | 2 | 17 | 5.5 | |
| Metastasis | 20 | 4.8 | 5 | 4.7 | 13 | 5.1 | 13 | 5.9 | N/A | - | 2 | 4 | 15 | 4.9 | |
| Needle Tract | 3 | 0.7 | 1 | 1 | 2 | 0.8 | 2 | 0.9 | N/A | - | 0 | 0 | 2 | 0.7 | |
Comparisons between Resection and Intent To Treat Transplant via chi-squared and students T-Test
N/A: Not Available.
Bold: Percent Total Pts
Non-Bold: Percent of Group.
Treatment by size and liver function
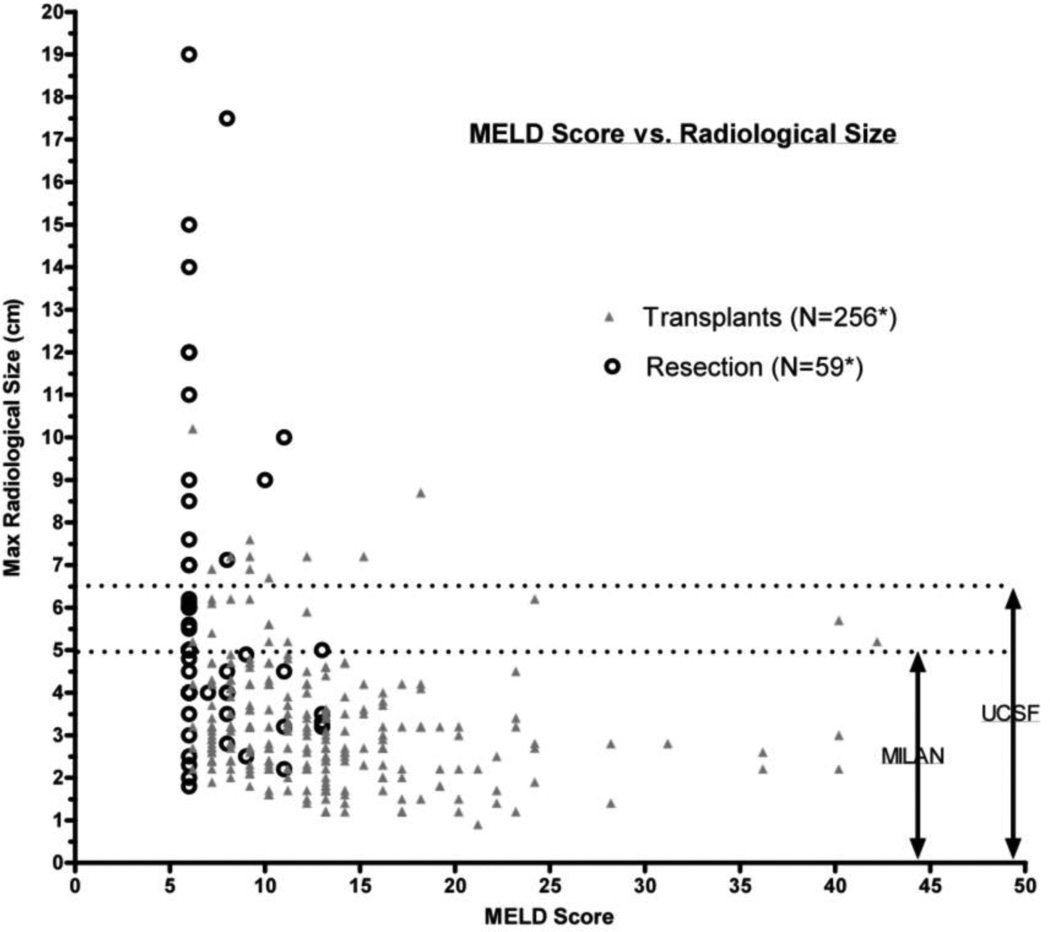
Radiographic tumor size and MELD scores were used to compare which patients underwent resection or transplantation (Figure 2). As noted in figure 2, few resections were performed for patients with MELD scores above 12–13 and few liver transplants were performed in patients with tumors > 6.5 cm. Thus, overlap of patients receiving liver resection or transplantation was observed primarily for tumors < 6.5 cm and MELD scores under 13.
Figure 2.
Radiographic tumor size versus MELD score for resection and intent to transplant patients.
Table 3 displays the number of patients in each subgroup satisfying Milan (<5 cm), or UCSF criteria (<6.5 cm) and having MELD scores < or ≥ 10. The majority of patients in the resection groups had biological MELD scores of < 10 (84.9%) and in the ITT group the majority of patients (67.3%) had a MELD score ≥ 10. Resection and ITT patients who met Milan criteria and had MELD scores of < 10, were 31.1% and 30.4% of their respective groups. Resection and ITT patients who satisfied UCSF criteria and had MELD scores of < 10 were, 24.5% and 28.4% of their respective groups.
Table 3.
Patient Stratification.
| Total | Resection | ITT - Intention To Treat (A + B) |
(A) Transplants (Non-Incidentals) |
(B) Listed Not Transplanted |
(C) Transplants (Incidental) |
Transplants (Total) (A+B+C) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Total Pts |
N | % Group |
N | % Group |
N | % Group | N | % Group |
N | % Group |
N | % Group |
|
| Total Patients | 413 | 106 | 25.7 | 257 | 62.2 | 220 | 53.3 | 37 | 9 | 50 | 12.1 | 307 | 74.3 | |
| MELD < 10 | 174 | 42.1 | 90 | 84.9 | 84 | 32.7 | 72 | 32.7 | 12 | 32.4 | 1 | 0.2 | 85 | 27.7 |
| MELD ≥ 10 | 239 | 57.9 | 16 | 15.1 | 173 | 67.3 | 148 | 67.3 | 25 | 67.6 | 49 | 11.9 | 222 | 72.3 |
| UCSF Criteria (<6.5 cm) | ||||||||||||||
| Total | 288 | 69.7 | 40 | 37.7 | 248 | 96.5 | 215 | 97.7 | 33 | 89.2 | - | - | 248 | 80.8 |
| MELD < 10 | 111 | 26.9 | 33 | 31.1 | 78 | 30.4 | 70 | 31.8 | 8 | 21.6 | - | - | 78 | 25.4 |
| MELD ≥ 10 | 177 | 42.9 | 7 | 6.6 | 170 | 66.1 | 145 | 65.9 | 25 | 67.6 | - | - | 170 | 55.4 |
| Milan Criteria (<5 cm) | ||||||||||||||
| Total | 270 | 65.4 | 33 | 31.1 | 237 | 92.2 | 205 | 93.2 | 32 | 86.5 | - | - | 237 | 77.2 |
| MELD < 10 | 99 | 24 | 26 | 24.5 | 73 | 28.4 | 66 | 30 | 7 | 18.9 | - | - | 73 | 23.8 |
| MELD ≥ 10 | 171 | 41.4 | 7 | 6.6 | 164 | 63.8 | 139 | 63.2 | 25 | 67.6 | - | - | 164 | 53.4 |
Bold: Percent Total Pts.
Non-Bold: Percent of Group.
Degree of resection and incidence of recurrence by margin status
Table 4 summarizes the treatment strategies used in the 106 patients who underwent hepatic resection. Techniques of liver resection and parenchymal transection varied widely depending upon the operative surgeon. Overall, the majority of resections were partial hepatic lobectomies (55.7%). Right hepatic lobectomies were performed in 31.1% and left lobectomies in 10.4% of patients. Resection margins on final pathology were reported as positive in 17.9% of patients. Recurrences and local failures were noted in 5 of 19, or 26.3% of positive-margin resections and in 16 of 87, or 18.4% of margin-negative resections and the difference between groups was not significant (p = 0.525). Overall, 4 patients who underwent hepatic resection subsequently underwent liver transplantation. Similarly, 10 patients underwent subsequent repeat hepatic resection for recurrence or residual disease. Those patients requiring repeat resections of residual or recurrent disease had a median survival of 49 months and five-year survival of 60% from first resection and 31.2 months and 20% respectively from subsequent resection. The 4 patients who were later transplanted had a median and five-year survival of 48.5 months and 25% respectively following resection and 35.8 months and 25% respectively following subsequent transplantation. One patient required a second liver transplant and died five months following a second liver transplantation.
Table 4.
Type of Surgery with Margin Status and Requirements for Secondary Procedures.
| Total | Positive Margin | Negative Margin | Recurrence | Secondary Procedures | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Total Pts |
N | % Total Pts |
N | % Total Pts |
+ Margin |
% Total Pts |
− Margin |
% Total Pts |
Later Transplanted |
% Total Pts |
Re- Resected |
% Total Pts |
|
| Total Patients | 106 | 19 | 17.9 | 87 | 82.1 | 5 | 4.7 | 16 | 15.1 | 4 | 3.8 | 10 | 9.4 | |
| Wedge/Partial Lobectomy | 56 | 55.7 | 9 | 8.5 | 47 | 44.3 | 2 | 1.9 | 8 | 7.5 | 2 | 1.9 | 3 | 2.8 |
| Formal Right Lobectomy | 26 | 24.5 | 4 | 3.8 | 22 | 20.8 | 2 | 1.9 | 4 | 3.8 | 1 | 0.9 | 5 | 4.7 |
| Extended Right Lobectomy | 7 | 6.6 | 0 | 0 | 7 | 6.6 | 0 | 0 | 2 | 1.9 | 1 | 0.9 | 1 | 0.9 |
| Formal Left Lobectomy | 6 | 5.7 | 3 | 2.8 | 3 | 2.8 | 0 | 0 | 1 | 0.9 | 0 | 0 | 1 | 0.9 |
| Extended Left Lobectomy | 5 | 4.7 | 2 | 1.9 | 3 | 2.8 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 6 | 5.7 | 1 | 0.9 | 5 | 4.7 | 0 | 0 | 1 | 0.9 | 0 | 0 | 0 | 0 |
Resection versus hepatic transplantation
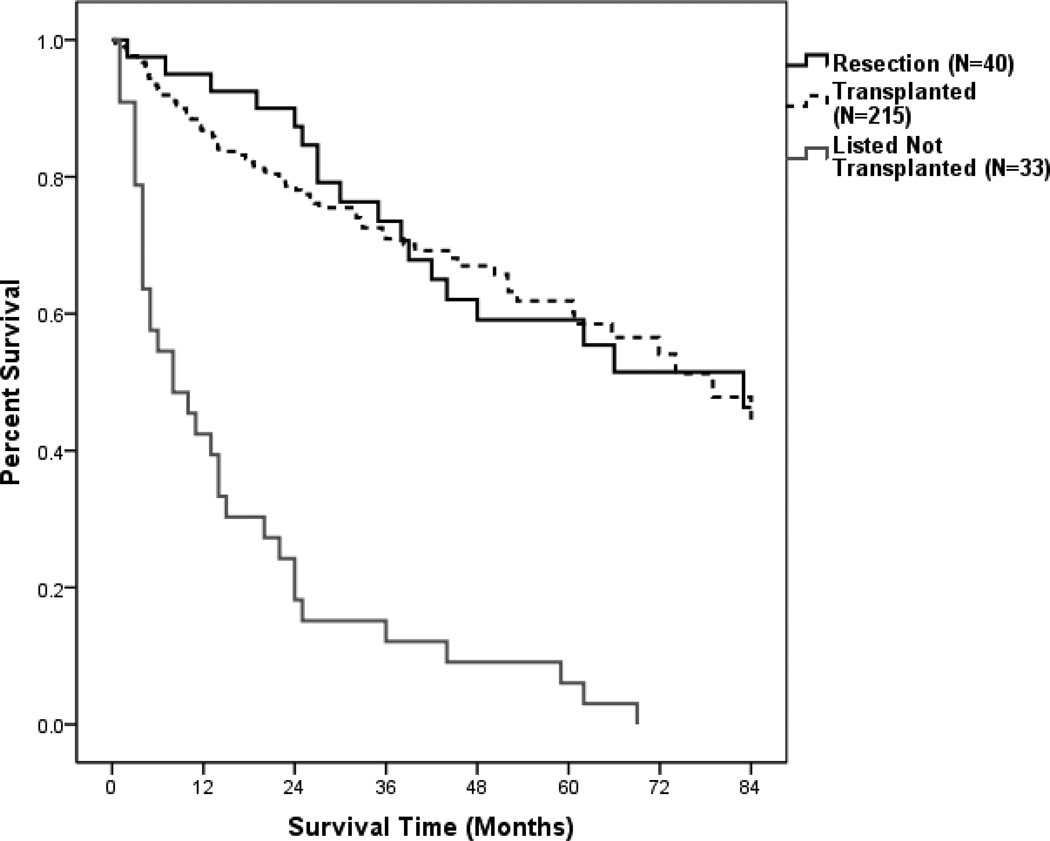
Based upon the distribution of therapies as a function of MELD score and radiographic tumor size, few patients were potential candidates for either hepatic resection or transplantation. Specifically, patients with HCC were generally not considered candidates for liver transplantation if the tumor exceeded Milan criteria or UCSF criteria (single lesion ≤ 6.5 cm, multiple lesions ≤ 3 cm, largest tumor diameter if multiple ≤ 4.5 cm, total tumor diameter if multiple ≤ 8 cm, no extrahepatic or vascular invasion) (6). Use of hepatic resection was generally limited to patients with relatively preserved liver synthetic function, without ascites and a sufficiently large hepatic remnant following resection in order to prevent death from liver failure (30, 31). Upon initial analysis, when comparing resection versus transplantation we found no significant difference between the two groups. However, all patients who were listed but not transplanted were dead at less than 6 years from listing (Table 5, Figure 3).
Table 5.
Overall Survivals (1,5,7 yrs)
| Median Survival |
Overall Survival (%)a | P-Valueb | Comparisons | ||||
|---|---|---|---|---|---|---|---|
| 1yr | 5yr | 7yr | |||||
| 1-All Patients (N=413) | 66 | 83 | 53 | 44 | |||
| All Sizes | 2-Resection (N=106) | 66 | 88 | 53 | 46 | ||
| All MELD | 3-Non-incidendtal transplant (N=220) | 79 | 87 | 62 | 49 | 0.411 | 2 vs. 3 |
| 4-Listed Not Transplanted (N =37) | 10 | 46 | 5 | 0 | <0.0001 | 2 vs. 4 | |
| 5-Intention to Treat (N=257) | 61 | 81 | 52 | 38 | 0.307 | 2 vs. 5 | |
| 1-All Patients (N=270) | 62 | 82 | 54 | 40 | |||
| 2-Resection (N=33) | 83 | 94 | 59 | 46 | |||
| Milan (<5 cm) | 3-Non-incidendtal transplant (N=205) | 79 | 87 | 63 | 49 | 0.913 | 2 vs. 3 |
| All MELD | 4-Listed Not Transplanted (N =32) | 8 | 44 | 6 | 0 | <0.0001 | 2 vs. 4 |
| 5-Intention to Treat (N=237) | 62 | 81 | 53 | 39 | 0.238 | 2 vs. 5 | |
| 1-All Patients (N=99) | 59 | 85 | 49 | 30 | |||
| Milan (<5 cm) | 2-Resection (N=26) | 83 | 92 | 63 | 47 | ||
| MELD < 10 | 3-Non-incidendtal transplant (N=66) | 52 | 87 | 46 | 21 | 0.11 | 2 vs. 3 |
| 4-Listed Not Transplanted (N =7) | 4 | 43 | 14 | 0 | <0.0001 | 2 vs. 4 | |
| 5-Intention to Treat (N=73) | 50 | 83 | 41 | 16 | 0.036 | 2 vs. 5 | |
| 1-All Patients (N=171) | 74 | 81 | 57 | 47 | |||
| Milan (<5 cm) | 2-Resection (N=7) | 44 | 100 | 40 | 40 | ||
| MELD ≥ 10 | 3-Non-incidendtal transplant (N=139) | N/A | 85 | 70 | 60 | 0.593 | 2 vs. 3 |
| 4-Listed Not Transplanted (N =25) | 10 | 44 | 4 | 0 | <0.0001 | 2 vs. 4 | |
| 5-Intention to Treat (N=164) | 74 | 80 | 58 | 48 | 0.854 | 2 vs. 5 | |
| 1-All Patients (N=288) | 62 | 82 | 53 | 40 | |||
| UCSF (<6.5 cm) | 2-Resection (N=40) | 83 | 95 | 59 | 46 | ||
| ALL MELD | 3-Non-incidendtal transplant (N=215) | 79 | 86 | 62 | 48 | 0.901 | 2 vs. 3 |
| 4-Listed Not Transplanted (N =33) | 8 | 42 | 6 | 0 | <0.0001 | 2 vs. 4 | |
| 5-Intention to Treat (N=248) | 61 | 80 | 52 | 38 | 0.204 | 2 vs. 5 | |
| 1-All Patients (N=111) | 59 | 85 | 49 | 32 | |||
| UCSF (<6.5 cm) | 2-Resection (N=33) | 83 | 94 | 62 | 47 | ||
| MELD < 10 | 3-Non-incidendtal transplant (N=70) | 52 | 86 | 45 | 21 | 0.111 | 2 vs. 3 |
| 4-Listed Not Transplanted (N =8) | 4 | 38 | 13 | 0 | <0.0001 | 2 vs. 4 | |
| 5-Intention to Treat (N=78) | 50 | 81 | 40 | 16 | 0.027 | 2 vs. 5 | |
| 1-All Patients (N=177) | 72 | 81 | 56 | 47 | |||
| UCSF (<6.5 cm) | 2-Resection (N=7) | 44 | 100 | 40 | 40 | ||
| MELD ≥ 10 | 3-Non-incidendtal transplant (N=145) | N/A | 86 | 68 | 59 | 0.635 | 2 vs. 3 |
| 4-Listed Not Transplanted (N =25) | 10 | 44 | 4 | 0 | 0.001 | 2 vs. 4 | |
| 5-Intention to Treat (N=170) | 74 | 80 | 57 | 47 | 0.84 | 2 vs. 5 | |
Overall Survival determined by Kaplan Meier Method.
Comparisons by Log-Rank (Mantel Cox) Test.
Figure 3.
Overall Percent Survival for patients satisfying current UNOS criteria (Milan Criteria) comparing hepatic resection versus non-incidental transplant and listed but not transplanted patients.
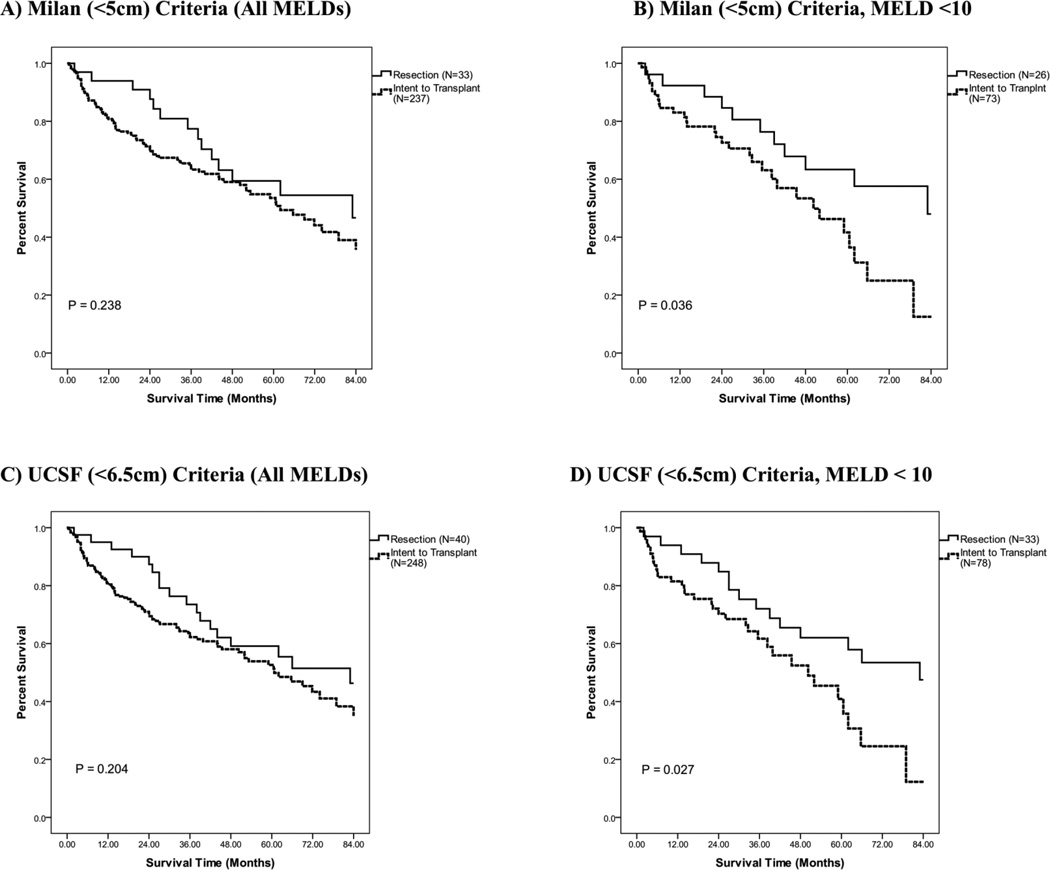
Given our objective was to understand the relative benefit of resection versus transplantation, we first focused on patients whose tumor size met Milan or UCSF criteria (270 and 288 patients respectively, Table 5). First, univariate analysis demonstrated no significant difference in survival for patients who met Milan criteria between the resection and ITT groups. Similarly, univariate analysis demonstrated there was no significant difference in survival for tumors that met either Milan or UCSF criteria between the resection and ITT groups. (Table 5, Figures 4A, 4C). Of note, however, a trend for increased survival for patients in the first 2-years was observed in the resection group although not statistically significant (Resection vs. ITT: met Milan, p = 0.146, met UCSF, p = 0.089.). This decreased short-term survival with transplantation was largely attributable to patients who were listed but not transplanted as they demonstrated poor long-term survival.
Figure 4.
Resection versus intent to transplant overall survival using the Kaplan-Meier method. (P-Values via Log Rank (Mantel Cox) Method. A) Restricted to patients meeting the Milan criteria. B) Patients meeting the Milan criteria with MELD score below 10. C) Restricted to patients meeting the UCSF criteria. D) Patients meeting the UCSF criteria with MELD score below 10.
We therefore next evaluated if there was a survival difference between the resection and ITT groups for those patients that met Milan or UCSF criteria and demonstrated lesser degrees of liver dysfunction (32). Specifically, a MELD score of < 10 is associated with a one year survival rate of 95%, when excluding the presence of a HCC (32). Hepatic transplantation is known to have a one-year survival rate in the range of 85–90%, with mortality related to both technical challenges of transplantation as well as problems related to immunosuppression (33). We posited therefore, that for HCC patients with a MELD score under 10 improved survival rates might be observed with hepatic resection.
Median, overall one, five, and seven year survivals were calculated for all patients with their respective treatment strategies and for those resection and ITT patients subcategorized for met MILAN or UCSF criteria and specified MELD scores (< 10, ≥ 10) (Table 5). There was a statistically improved survival in the resection group as compared to the ITT group for those patients who met MILAN or UCSF criteria and had a MELD score under 10 (Table 5, Figure 4B, 4D). However, no statistically significant difference in survival was observed when those who were listed but not transplanted were excluded from the analysis (Table 5). Table 5 also reports additional survivals by various categorization and treatment strategies.
Receipt of liver transplant is associated with superior late disease-free survival versus resection
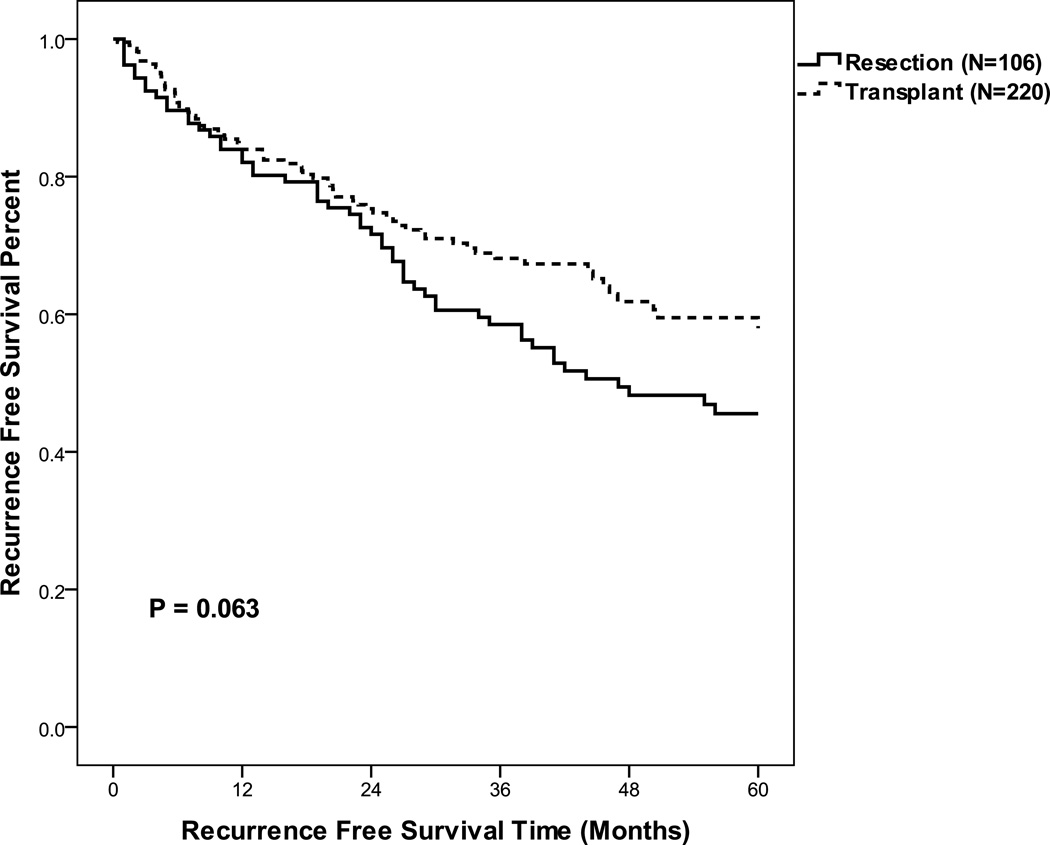
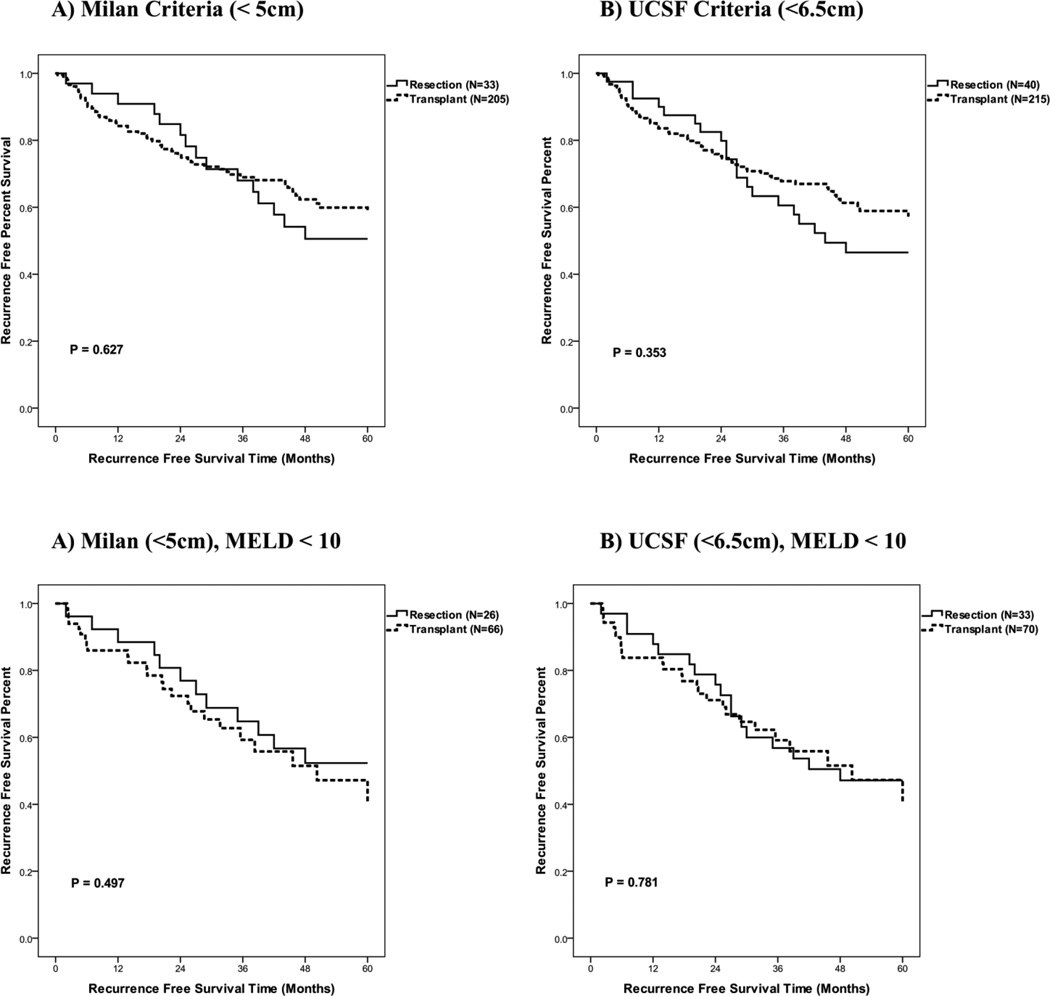
We next determined recurrence free survival rates for the resection and transplantation groups. As listed but never transplanted patients never were rendered free of disease, these patients were excluded form this analysis. Upon our initial analysis of all patients, without stratification for size and MELD < 10, we noted a trend for an increased 5-year recurrence free survival in those patients who underwent transplantation as compared to resection (60%, 45% respectively, p = 0.063) (Table 6, Figure 5). When evaluating patients who met Milan or UCSF criteria, transplanted patients as compared to resected patients had a decreased 1-yr but increased 5-yr RFS (Table 6, Figure 6). However, when evaluating patients who met Milan or UCSF criteria, with a MELD < 10, RFS appeared to be equivalent between the transplanted and resected groups (Table 6, Figure 5). These data suggest that more advanced MELD scores were associated with increased tumor recurrence risk in the resection group.
Table 6.
Recurrence Free Survival Resection vs. Transplant (Non-Incidental).
| Median | 1 yr | 5 yr | Significance P-Value |
||
|---|---|---|---|---|---|
| All Sizes | Resection (N=106) | 47 | 84 | 45 | 0.063 |
| All MELD | Transplant (N=220) | > 60 | 84 | 60 | |
| Milan (<5 cm) | Resection (N=33) | > 60 | 94 | 50 | 0.627 |
| All MELD | Transplant (N=205) | > 60 | 84 | 60 | |
| Milan (<5 cm) | Resection (N=26) | > 60 | 92 | 52 | 0.497 |
| MELD < 10 | Transplant (N=66) | 50 | 85 | 46 | |
| UCSF (<6.5 cm) | Resection (N=40) | 44 | 93 | 46 | 0.353 |
| ALL MELD | Transplant (N=215) | > 60 | 84 | 59 | |
| UCSF (<6.5 cm) | Resection (N=33) | 48 | 91 | 47 | 0.781 |
| MELD < 10 | Transplant (N=70) | 50 | 83 | 46 |
Figure 5.
Recurrence free survival for all hepatic resection and transplant patients excluding incidentally identified hepatocellular carcinomas.
Figure 6.
Recurrence free survival for hepatic resection and transplant patients excluding incidentally identified hepatocellular carcinomas by tumor size for all MELD or MELD under 10. A) Milan Criteria, B) UCSF Criteria, C) Milan Criteria with MELD <10 and D) UCSF Criteria with MELD <10.
DISCUSSION
Liver resection, radiofrequency ablation and transplantation are recognized as effective palliative and potentially curative therapies for patients who develop HCC. However, the optimal therapeutic approach for HCC remains undefined. Recent work from Pawlik and coworkers has demonstrated that choice of HCC treatment is somewhat more strongly related to surgeon specialty than to certain clinical factors (34). The University of Miami / Jackson Memorial Medical Center treats a large number of patients with HCC using both hepatic resection and transplantation approaches. We sought to evaluate patient outcomes for HCC retrospectively with the goal of comparing outcomes of resection versus transplantation in patients who were initially candidates for either approach. We also specifically sought to evaluate the impact of size and MELD score on overall survival and recurrence free survival between the two treatment strategies.
We compared outcomes utilizing a retrospective, intent-to-treat analysis. We excluded patients with HCC discovered incidentally at pathological examination of the excised liver, because such transplantation was not intentionally performed for HCC and is thus not applicable to defining treatment for known HCC.
In the first analysis, we included all patients who were listed for transplantation, irrespective of whether the patient ultimately received liver transplantation. This analysis is the most inclusive and reflects the risks and mortalities associated with listing patients for transplantation in a setting where donor livers are limiting. Within that cohort, we observed a trend for increased overall one and two-year survival rates in the resection group. We also observed equivalent overall five-year survival rates for patients treated with either resection or attempt at transplantation for HCC (Table 5, Figure 4). In the focused analysis of the subset of patients with relatively preserved liver function (MELD < 10) who were potential candidates for either therapy (i.e., they met Milan or UCSF criteria), improved overall survival was observed for surgical resection versus transplantation (Table 5, Figures 4 B, C).
To focus on clinical outcomes relating to surgery rather than to graft availability, we then eliminated from our analysis patients who were listed but not transplanted. Among patients who met Milan or UCSF criteria, we observed diminished one-year overall survival and recurrence-free-survival for liver transplantation versus resection (Table 6, Figure 6A, B). This was due to decreased overall survival in the acute period following transplantation. Such acute deaths are typically from technical challenges associated with transplantation and/or immunosuppression rather than recurrence of disease. Nevertheless, at five years, there was increased recurrence-free-survival in patients who underwent liver transplantation rather than resection. Limiting the analysis to patients who had preserved liver function (i.e., MELD < 10) and met Milan or UCSF criteria for transplantation, we observed no difference in five-year recurrence-free-survival for resection versus liver transplantation.
One intepretation of these data is that for patients who are candidates for either therapy, liver resection can result in equivalent long-term survival without the risks of organ non-availability or the acute postoperative complications associated with transplantation and immunosuppression. Amongst patients who survive the transplantation procedure beyond the first perioperative year, however, liver transplantation resulted in greater recurrence-free survival, potentially due to the replacement of the cancer-prone organ.
To date, a number of series have attempted to compare outcomes for HCC patients who were potentially candidates for either resection or transplantation. A recent review by Rahbari and coworkers from Heidelberg (6) reviewed nine such studies (17–25). Among those studies, most identified no significant difference in patient survival rates. Only in four studies were patients who failed to obtain a new liver organs considered in the comparisons. Thus, the results of the other studies might exclude the mortalities resulting from organ non-availability. Such an analysis would fail to reflect the realities facing the surgeon and patient choosing transplantation over resection. Furthermore, only the study by Facciuto et al. (n=161 patients) specifically excluded patients found to have HCC incidental to liver transplantation performed for other indications (23). Such patients would not accurately reflect the true patient pool under consideration here because inclusion of such patients would artificially increase outcomes related to transplantation for HCC.
For these reasons, the series reported here with its 413 patients is among the largest to examine the benefits of resection versus transplantation in patients that may be candidates for both treatment strategies. This study is also the first study to evaluate survival outcomes for such patients with relatively preserved liver function (MELD <10). MELD scores under 10 are generally associated with normal or well-compensated liver function and this cohort in general carries a 98–99% three-month survival rate and 95% one-year survival rate in the absence of HCC (32). Some patients with MELD scores under 10 may have ascites, however, rendering them poor candidates for hepatic resection. Our finding of improved survival with resection for HCC among the patient cohort with tumors that met Milan or UCSF criteria and MELD <10 is of particular interest, but certainly needs confirmation in non-overlapping data sets (28).
Nonetheless, based upon this retrospective series, if a patient with preserved liver function has a HCC and is a candidate for hepatic resection, this series supports superiority of resection. Moreover, the number of patients requiring re-resections or transplantation following a recurrence were limited (10 and 4 respectively), however this series indicates that both strategies can provide favorable survival outcomes and that repeat therapies can be associated with long-term survival.
There are certainly limitations of this dataset and our analysis. The analysis is retrospective and thus limited by the data collected and by the lack of patient randomization. One consequence of this is that recurrence in patients treated with resection might be under-reported because those patients did not undergo the very close post-operative follow-up screening that transplantation patients are given. As well, our analysis assumes that patients with small liver tumors in the MELD < 10 group were candidates for either therapy, however almost certainly some were actually not candidates for resection, e.g. due to the presence of ascites or tumor multifocality such that a margin-negative resection with a sufficient hepatic remnant would be impossible. Similarly, while 100% of the transplanted patients had hepatic fibrosis or cirrhosis, 28% of the resection group had minimal or no evidence for hepatic fibrosis or cirrhosis. Such patients likely have better prognosis cancers and may have contributed to the relatively low recurrence rate observed in the resection group (17–25). Furthermore, while we identified all patients with intent-to-transplant, we do not know how many patients were treated with intent-to-resect. All patients who underwent resection were included, but we do not know how many patients who were believed to be candidates for resection pre-operatively were later found to be poor candidates at the time of exploration and treated with ablative therapies or even later transplanted. Multifocal tumors were more frequently encountered in the transplantation group this may have biased outcomes in favor of hepatic resection. Finally, a much larger fraction of patients with HCC and cirrhosis from Hepatitis C underwent transplantation. Outcomes for HCC patients with hepatitis C have been noted to be worse than HCC arising from hepatitis B (17–25), potentially disproportionately decreasing survival within the transplantation group. Such limitations might bias the analysis in favor of resection. Conversely, the considerably larger tumor size noted in the resection group might bias the results in favor of transplantation.
This study does not address how to integrate ablative therapies such as radiofrequency ablation into the treatment algorithm for patients with HCC. Certainly, many centers have developed excellent local tumor control with such therapies, particularly for tumors under 2 or 3 cm. Identifying patients who might have been treated equally effectively with radiofrequency ablation is of critical interest. If equivalent local tumor control rates for HCC could be obtained, ablation might become first line therapy due to its reduced invasiveness and increased preservation of liver mass. In this series, RFA and other ablative therapies were reserved for patients deemed not candidates for resection or used in lieu of transplantation with transplantation reserved for a later date. Also, although it is now used extensively as a bridge to transplantation in the current practice at University of Miami/Jackson Medical Center, during the period analyzed radiofrequency ablation was not universally applied to all listed patients with known HCC tumors. Also although molecular insights are being made in the pathogenesis of HCC, no clearly effective adjuvant therapies currently exist (35–40).
Of course, determining the optimal therapy for HCC will require prospective, randomized clinical trials across multiple institutions. Acknowledging the limitations of this study, however, several hypotheses and a potential framework for clinical treatment guidelines can be drawn from our analysis. Currently, most transplantation guidelines suggest that resection should be performed in patients who are candidates for either approach (6). Consistent with those guidelines, our data support the use of liver resection as primary therapy for patients who develop HCC, if the patients are candidates for either hepatic resection or transplantation. In patients with preserved liver function who also meet current transplantation guidelines, the use of resection is superior for patient survival due to limited organ availability and transplantation-associated morbidity and mortality, although the cancer cure rate for the subset of patients who do receive a liver might be equivalent or better. In our cohort, the average waiting time to transplantation was 48 days. In other settings, reduced organ availability and longer wait times would necessarily be associated with increased mortality and disease progression. Our data balanced against the current, chronic shortage of available livers for transplantation supports the use of resection as primary therapy for HCC patients who are candidates for resection, particularly in those with preserved liver function. As such, transplantation could be reserved for the resection candidate group as salvage therapy following recurrence. This treatment strategy will not only preserve quality of life, but may improve survival, while at the same time reducing demand for available donor livers.
Acknowledgments
Funding sources:
This work was supported by the following grants: James and Esther King Tobacco Grant from the State of Florida 10KG06(L.G.K.), R01 GM092758-01A1S1 (F.E.P.), R01 CA122596 (T.A.Z.) and R01 GM092758 (T.A.Z.), Women's Cancer Association of the University of Miami (T.A.Z.), Wendy Will Case Cancer Fund (T.A.Z.).
REFERENCES
- 1.World Health Organization. [Accessed May 1, 2011]; Website: http://www.who.int/mediacentre/factsheets/fs297/en/index.html.
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5) Suppl 1:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5) Suppl 1:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Bruno S, Silini E, Crosignani A, Borzio F, Leandro G, Bono F, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology. 1997;25(3):754–758. doi: 10.1002/hep.510250344. [DOI] [PubMed] [Google Scholar]
- 5.McKillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006;136(1):125–135. doi: 10.1016/j.jss.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Rahbari NN, Mehrabi A, Mollberg NM, Muller SA, Koch M, Buchler MW, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253(3):453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 7.Earle SA, Perez EA, Gutierrez JC, Sleeman D, Livingstone AS, Franceschi D, et al. Hepatectomy enables prolonged survival in select patients with isolated noncolorectal liver metastasis. J Am Coll Surg. 2006;203(4):436–446. doi: 10.1016/j.jamcollsurg.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Levi DM, Tzakis AG, Martin P, Nishida S, Island E, Moon J, et al. Liver transplantation for hepatocellular carcinoma in the model for end-stage liver disease era. J Am Coll Surg. 2010;210(5):727–734. 35–36. doi: 10.1016/j.jamcollsurg.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen KT, Geller DA. Laparoscopic liver resection--current update. Surg Clin North Am. 2010;90(4):749–760. doi: 10.1016/j.suc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Liang HH, Chen MS, Peng ZW, Zhang YJ, Zhang YQ, Li JQ, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15(12):3484–3493. doi: 10.1245/s10434-008-0076-y. [DOI] [PubMed] [Google Scholar]
- 11.Raut CP, Izzo F, Marra P, Ellis LM, Vauthey JN, Cremona F, et al. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005;12(8):616–628. doi: 10.1245/ASO.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145(12):1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 13.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 15.Shabahang M, Franceschi D, Yamashiki N, Reddy R, Pappas PA, Aviles K, et al. Comparison of hepatic resection and hepatic transplantation in the treatment of hepatocellular carcinoma among cirrhotic patients. Ann Surg Oncol. 2002;9(9):881–886. doi: 10.1007/BF02557525. [DOI] [PubMed] [Google Scholar]
- 16.Koniaris LG, McKillop IH, Schwartz SI, Zimmers TA. Liver regeneration. J Am Coll Surg. 2003;197(4):634–659. doi: 10.1016/S1072-7515(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 17.Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250(5):738–746. doi: 10.1097/SLA.0b013e3181bd582b. [DOI] [PubMed] [Google Scholar]
- 18.Figueras J, Jaurrieta E, Valls C, Ramos E, Serrano T, Rafecas A, et al. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy. J Am Coll Surg. 2000;190(5):580–587. doi: 10.1016/s1072-7515(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 19.Bigourdan JM, Jaeck D, Meyer N, Meyer C, Oussoultzoglou E, Bachellier P, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl. 2003;9(5):513–520. doi: 10.1053/jlts.2003.50070. [DOI] [PubMed] [Google Scholar]
- 20.Margarit C, Escartin A, Castells L, Vargas V, Allende E, Bilbao I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11(10):1242–1251. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 21.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245(1):51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SA, Cleary SP, Tan JC, Wei AC, Gallinger S, Grant DR, et al. An analysis of resection vs transplantation for early hepatocellular carcinoma: defining the optimal therapy at a single institution. Ann Surg Oncol. 2007;14(9):2608–2614. doi: 10.1245/s10434-007-9443-3. [DOI] [PubMed] [Google Scholar]
- 23.Facciuto ME, Rochon C, Pandey M, Rodriguez-Davalos M, Samaniego S, Wolf DC, et al. Surgical dilemma: liver resection or liver transplantation for hepatocellular carcinoma and cirrhosis. Intention-to-treat analysis in patients within and outwith Milan criteria. HPB (Oxford) 2009;11(5):398–404. doi: 10.1111/j.1477-2574.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belghiti J, Cortes A, Abdalla EK, Regimbeau JM, Prakash K, Durand F, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238(6):885–892. doi: 10.1097/01.sla.0000098621.74851.65. discussion 92-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238(4):508–518. doi: 10.1097/01.sla.0000090449.87109.44. discussion 18-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy SK, Tsung A, Geller DA. Laparoscopic Liver Resection. World J Surg. 2010 doi: 10.1007/s00268-010-0906-5. [DOI] [PubMed] [Google Scholar]
- 27.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 28.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 29.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 30.Jin X, Zhang Z, Beer-Stolz D, Zimmers TA, Koniaris LG. Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology. 2007;46(3):802–812. doi: 10.1002/hep.21728. [DOI] [PubMed] [Google Scholar]
- 31.Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43(3):474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- 32.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 33.Goh A. An analysis of liver transplant survival rates from the UNOS registry. Clin Transpl. 2008:19–34. [PubMed] [Google Scholar]
- 34.Nathan H, Bridges JF, Schulick RD, Cameron AM, Hirose K, Edil BH, et al. Understanding surgical decision making in early hepatocellular carcinoma. J Clin Oncol. 2011;29(6):619–625. doi: 10.1200/JCO.2010.30.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jablonski EM, Mattocks MA, Sokolov E, Koniaris LG, Hughes FM, Jr, Fausto N, et al. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250(1):36–46. doi: 10.1016/j.canlet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran DM, Mattocks MA, Cahill PA, Koniaris LG, McKillop IH. Interleukin-6 mediates G(0)/G(1) growth arrest in hepatocellular carcinoma through a STAT 3-dependent pathway. J Surg Res. 2008;147(1):23–33. doi: 10.1016/j.jss.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran DM, Koniaris LG, Jablonski EM, Cahill PA, Halberstadt CR, McKillop IH. Microencapsulation of engineered cells to deliver sustained high circulating levels of interleukin-6 to study hepatocellular carcinoma progression. Cell Transplant. 2006;15(8–9):785–798. doi: 10.3727/000000006783981477. [DOI] [PubMed] [Google Scholar]
- 38.Zimmers TA, Jin X, Gutierrez JC, Acosta C, McKillop IH, Pierce RH, et al. Effect of in vivo loss of GDF-15 on hepatocellular carcinogenesis. J Cancer Res Clin Oncol. 2008;134(7):753–759. doi: 10.1007/s00432-007-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price JA, Kovach SJ, Johnson T, Koniaris LG, Cahill PA, Sitzmann JV, et al. Insulin-like growth factor I is a comitogen for hepatocyte growth factor in a rat model of hepatocellular carcinoma. Hepatology. 2002;36(5):1089–1097. doi: 10.1053/jhep.2002.36158. [DOI] [PubMed] [Google Scholar]
- 40.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49(3):821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]